Abstract
The present study was designed to investigate the whole plant of Pistacia integerrima Stewart in order to examine the pharmacological basis of the use of the plant in folk medicine for the treatment of infectious diseases and disorder. Phytochemical and pharmacological studies led to the isolation of a new triterpene pistagremic acid (3-methyl-7-(4,4,10,13,14-pentamethyl-3-2,3,4,5,6,7,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-oct-3-enoic). Pistagremic acid showed significant leishmanicidal activity (IC50: 6.71 ± 0.09 µM) against Leishmania major (DESTO) promastigotes in comparison to standard compound amphotericin B (IC50: 0.21 ± 0.06 µM).
Introduction
Pistacia chinensis var. integerrima Stewart ex. Brandis belongs to family AnacardiaceaCitation1. P. integerrima is an important medicinal plant and used as an herbal remedies for the treatments of different ailments such as anti-inflammatory, blood purifier, gastrointestinal disorders, cough, asthma, expectorant, fever, vomiting and diarrheaCitation2–5. Galls of P. integerrima are also used for the treatment of hepatitis and other liver disordersCitation6. P. integerrima is also used in oxidative stress and has potential to counter hyperuricemiaCitation7. Ground galls are aromatic, astringent, expectorant and has used in Indian traditional medicine for treatment of asthma, phthisis and other disorders of respiratory tract, dysentery, chronic bronchitis, hiccough, vomiting in children, skin diseases, psoriasis, fever and as appetizerCitation8. Furthermore its galls in combination with other herbal drugs are also used for the treatment of snake bite and scorpion stingCitation1. The literature revealed that gall’s oil of P. integerrima possesses CNS depressant activityCitation9. Discovery of new bioactive natural products based on ethanopharmacological investigations is a well established methodologyCitation10–14. So based on ethanopharmacological importance of P. integerrima in infectious diseases, we have made an effort to identify potential bioactive compound(s) responsible for its folk use.
Experimental
Plant material
Pistacia chinensis var. integerrima galls were collected from Toormang, Razagram area of district Dir, Khyber Pakhtunkhwa province of Pakistan during the month of February, 2010. The plant material was identified by plant taxonomist, Department of Botany, University of Peshawar, Pakistan where the voucher specimen (RF-895) was deposited in the herbarium.
Extraction and isolation
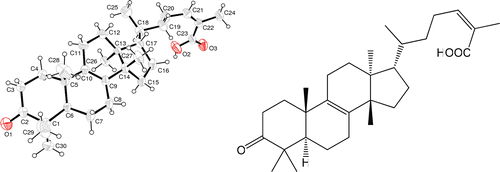
Dried and crushed galls of Pistacia chinensis var. integerrima (5 kg) were subjected to cold extraction with MeOH. The MeOH extract (400 g) was suspended in water and successively partitioned with hexane, CHCl3, EtOAc and BuOH. The CHCl3 fraction (10 g) was subjected to column chromatography on silica gel (Merck Silica gel 60 (0.063–0.200 mm), 5 × 60 cm). The column was first eluted with hexane-EtOAc (100:0 → 0:100) as solvent system. A total of 13 fractions, RF-1 to RF-13 were obtained based on TLC profiles. Fraction RF-4 obtained at (100:0 → 18:82) contained colorless crystals of various sizes and was separated from the solution by decantation. The crystals were washed with acetone for several times. To obtain pure and larger crystals, these crystals were re-grown from a mixture of hexane-acetone (4:1) and thus obtained a pure compound pistagremic acid (90 mg). Chemical structure of the isolated, new triterpene pistagremic acid i.e. 3-methyl-7-(4,4,10,13,14-pentamethyl-3-2,3,4,5,6,7,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta [a]phenanthren-17-yl)-oct-3-enoic acid was identified by X-ray crystallography as reported earlierCitation15.
Leishmanicidal activity
Leishmania major (DESTO) promastigotes were cultured at 22–25°C in RPMI-1640 (Sigma) as reported earlierCitation16. Medium was supplemented with 10% heat-inactivated (56°C for 30 min) fetal bovine serum (FBS). Promastigote culture in the logarithmic phase of growth was centrifuged at 2000 rpm for 10 min and washed with saline three times in the same condition. Parasites were diluted with fresh culture medium to a final density of 106 cells/ml. In a 96-well micro titer plate, 180 ml of medium was added in the first row and 100 ml of medium was added in others wells. Test sample (20 ml) was added in medium and serially diluted. 100 ml of parasite culture was added in all wells. One row was used for control (DMSO) which received medium while one for standard drugs (amphotericin B). Plate was incubated at 21–22°C for 72 h and the numbers of surviving parasites were counted microscopically in Neubauer chamberCitation17,Citation18. Results are the replicates of three different experiments. The 50% inhibitory concentrations (IC50) were calculated by EZ-Fit 5.03 Perrella Scientific Software.
Cytotoxicity assay
In vitro cytotoxicity assay was conducted using LCMK-2 monkey kidney epithelial cells and mice hepatocytesCitation19. The test samples were incubated for 24 h, and finally the cell viability was determined employing the MTT assay. For MTT assay, cells were maintained in RPMI-1640 medium (purchased from Gibco BRL) containing 10% FBS (also purchased from Gibco BRL), 110 μg/ml penicillin sodium salts, 2 mg/ml sodium bicarbonate solution, and 100 μg/ml streptomycin sulfate. Initial seeding of the 7.1 × 103 LCMK-2 cells and 8.6 × 103 mice hepatocytes, was conducted in 96-well plates. The cells were treated with test sample at various concentrations as well as with vehicle (0.2% DMSO) and then incubated for 48 h followed by performing MTT (3-[4, 5- dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay (Sigma Chemical Co., St. Louis, MO).
Results and discussion
Leishmaniasis is a group of prevalent diseases caused by protozoan parasites belonging to the genus Leishmania affecting 12 million people all over the worldCitation1. Leishmaniasis is transmitted by the Phlebotamine sand fly belonging to the genera Lutzomia Cutaneous leishmaniasis is caused by L. major. Cutaneous leishmaniasis, the most common form, is a complex disease with a wide spectrum of clinical manifestations. It is prevalent in tropical and subtropical countries including Iran, Afghanistan, Brazil, Peru, Saudi Arabia, Syria and Pakistan. It exists in the form of obligatory intracellular amastigotes found in the phagolysosomal compartment of mammalian macrophagesCitation20. New bioactive natural products are still immensely explored as new sources of drugs including anti-microbial agentsCitation21. In the current study we have reported triterpene from P. integerrima Stewart in order to scientifically explore its folk use in diseases including infections. Pistagremic acid was isolated as a new compound and showed substantial activity against omastigotes of L. major (IC50: 6.71 ± 0.09 µM) in comparison to standard compound amphotericin B (IC50: 0.21 ± 0.06 µM). However no considerable cytotoxicity was observed in case of MTT assay, which indicates its preliminary safety profile. This promising data shows potential of pistagremic acid to be further investigated and developed as potential lead compound for better treatment of cutaneous leishmaniasis caused by L. major.
Declaration of interest
The authors declare no interest.
References
- Anonymous, In the Wealth of India. A Dictionary of Indian Raw Materials and Industrial Products, Publication and Information Directorate, CSIR, New Delhi; 1998;8:120.
- Pant S, Samant SS. Ethanobotanical observation in the Mornaula Reserve Forest of Kumoun, West Himalaya, India Ethanobotanical Leaflets. 2010;14:193.
- Upadhye AS, Rajopadly AA. Pharmacogonostic and phytochemical evaluation of leaf galls of Kakadshringi used in Indian system of medicine. Journal of Scientific and Industrial Research 2010;69:700.
- Uddin G, Rauf A, Rehman T, Qaisar M. Phytochemical Screening of Pistacia chinensis var. integerrima. Middle-East Journal of Scientific Research 2011;7:5:707–711.
- Ahmad NS, Farman M, Najmi MH, Mian KB, Hasan A. Pharmacological basis for use of Pistacia integerrima leaves in hyperuricemia and gout. j Ethnopharmacol 2008;117:478–482.
- Ahmad NS, Waheed A, Farman M, Qayyum A. Analgesic and anti-inflammatory effects of Pistacia integerrima extracts in mice. J Ethnopharmacol 2010;129:250–253.
- Chopra RN, Chopra IC, Handa KL, Kapoor LD. In Indigenous Drugs of India; 2 ed.; Academic Publishers: Delhi 1982;377.
- Ansari SH, Ali M, Qadry JS. Essential oils of Pistacia integerrima galls and their effects on the central nervous system. International Journal of Pharmacognosy 1993;31:89–95.
- Ansari SH, Ali M, Qadry JS, Siddiqui N. Update Ayurveda 1994a;94:73.
- Kolar D, Wimmerova L, Kadek R. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of Berberis vulgaris. Phytopharmacol 2010;1:7–11.
- White G, Bourbonnais-Spear N, Garner F. Antibacterial constituents from Uncaria tomentosa. Phytopharmacol 2011;1:16–19.
- Song MJ, Lee SM, Kim DK. Antidiabetic effect of Chenopodium ambrosioides. Phytopharmacol 2011;1:12–15.
- Johns T, Windust A, Jurgens T, Mansor SM. Antimalarial alkaloids isolated from Annona squamosa. Phytopharmacol 2011;1:49–53.
- Araruna K, Carlos B. Anti-inflammatory activities of triterpene lactones from Lactuca sativa. Phytopharmacol, 2010;1:1–6.
- Arfan M, Rauf A, Tahir MN, Ali M, Uddin G. 3-Methyl-6-(4,4,10,13,14-pentamethyl-3-oxo 2,3,4,5,6,7,10,11,12,13,14,15,16,-17-tetradecahydro-1H-cyclopenta[a] phenanthren-17-yl)hept-2-enoic acid. Acta Cryst 2011;E67:711.
- Khan MF Rehman FU, Khan I, Khan GM, Khan H. Antibacterial, antimalarial and leishmanicidal activities of Binuclear Ni(II) and Cu(II) Complexes of Diclofenac. Journal of the Pakistan Chemical Society 2010;32:462–466.
- Saeed M, Khan H, Khan MA, Simjee SU, Muhammad N, Khan SA. Phytotoxic, insecticidal and leishmanicidal activities of aerial parts of Polygonatum verticillatum. African Journal of Biotechnology 2010;9:1241–1244.
- Khademvatan S, Gharavi MJ, Rahim F, Saki J. Miltefosine-induced apoptotic cell death on Leishmania major and L. tropica strains. Korean j Parasitol 2011;49:17–23.
- Khan I, Nisar M, Khan N, Saeed M, Nadeem S, Fazal-ur-Rehman et al. Structural insights to investigate Conypododiol as a dual cholinesterase inhibitor from Asparagus adscendens. Fitoterapia 2010;81:1020–1025.
- Rahman AU, Samreen AW, Choudhary MI. Discovery of leishmanicidal agents from medicinal plants. Pure and Applied Chemistry 2008;80:1783–1790.
- Tiwari S, Bala M. Hippophae leaves prevent immunosuppression and inflammation in 60Co-γ-irradiated mice. Phytopharmacology 2011;1:36–48.