Abstract
Two series of urea and thiourea derivatives (1a–11a, 1b–11b) have been synthesized; all the 22 compounds were reported for the first time. Their anti-proliferative activities against the melanoma cell line B16-F10 were evaluated. Among the compounds tested, compound 6b exhibited the most potent activity in melanoma cells growth inhibition (IC50 = 0.33 μM). The bioassay tests showed that anti-proliferative activities of these novel compounds were possibly caused by inhibition of ERK1/2 phosphorylation level. Therefore, compound 6b can be a potential anti-melanoma agent and an inhibitor of ERK1/2 phosphorylation deserving further research.
Keywords::
Introduction
Skin cancer is one of the most common human malignancies and its global incidence is rising at an alarming rate, with basal cell carcinoma, squamous cell carcinoma and melanoma being the most common formsCitation1. Due to the rising incidence and lack of effective treatments for advanced disease, melanoma is the most dangerous form, accounting for most skin cancer deaths, so new treatment strategies are urgently neededCitation2,Citation3. The growth of melanoma depends on stimulatory effects of various growth factors, which bind to tyrosine kinase receptors to activate various intracellular signalling pathwaysCitation1,Citation4. Recent studies have provided some new therapeutic approaches to this disease. Extracellular signal-regulated kinase (ERK) is one of the most important members of mitogen-activated protein kinase (MAPK) family, which is activated by tyrosine kinase receptors and other upstream signal molecules, such as RAS (RAS-GTP), RAF-1 and MEK1/2Citation5,Citation6. The pathway offers several junctures for signal-transduction blockade; due to the converging functions of MEK1/2 and ERK1/2, specific inhibition of these proteins is particularly desirableCitation7. The ERK proteins consist of two isoforms (the 44 kDa ERK1 and the 42 kDa ERK2; referred to as ERK1/2)Citation8,Citation9. The activities of ERK1/2 are tightly controlled by dual-phosphorylation at special Thr 183/202 and Tyr 185/204 residuesCitation10,Citation11. The dual-phosphorylated (full-active) forms have determined the structural features required for full-activationCitation11–13. Phosphorylated ERK (pERK, active ERK) 1/2 protein transactivates numerous growth-related genes contribute to tumour cell proliferation, tumour cell survival, migration and metastasisCitation14,Citation15. This pathway has emerged recently as the central growth-stimulatory pathway in melanoma, with ERK being hyperactivated in up to 90% of human melanomasCitation1,Citation16,Citation17.
The urea derivatives such as N-nitrosoureas, benzoylureas, thioureas generally represent one of the most useful classes of anticancer agents, with a wide range of activities against various leukemias and solid tumoursCitation18–21. Our interest in this area is to design and synthesize diverse active urea and thiourea derivatives for anti-tumour agentsCitation22,Citation23. In continuation of our earlier studies, two novel series of N-benzyl-N-(4-hydroxy-3-methoxybenzyl)-N′-phenyl-urea and thiourea (1a–11a, 1b–11b) derivatives were synthesized in this paper, then we found that some of these compounds could inhibit the proliferation of melanoma cell line B16-F10 via block RAS–MEK–ERK signalling pathway.
Experimental procedure Chemistry
All the NMR spectra were recorded on a Bruker DRX 500 or DPX 300 model Spectrometer in CDCl3. Chemical shifts (δ) for 1H NMR spectra were reported in parts per million to residual solvent protons. Melting points were measured on a Boetius micro melting point apparatus. The ESI-MS spectra were recorded on a Mariner System 5304 Mass spectrometer. All chemicals and reagents used in current study were of analytical grade. The TLC was run on the silica gel-coated aluminium sheets (Silica Gel 60 GF254, E. Merck, Germany) and visualized in UV light (254 nm).
General procedure for N-benzyl-N-(4-hydroxy-3-methoxy-benzyl)-N′-phenyl-urea (1a–11a) and thiourea (1b–11b) preparations
The primary substituted amines (12 mmol) was added to a solution of 3-methoxy-4-hydroxybenzaldehyde (10 mmol), in ethanol (30 mL). The mixture was stirred at room temperature, and the reaction was monitored by TLC. The products were filtered and recrystallised in ethanol. Without further purification, to an ethanolic solution of synthesized Schiff bases, NaBH4 (10 mmol) was slowly added in an ice bath with stirring. The mixture was refluxed for 2 h, then the solvent was evaporated and water (20 mL) was added. The product extraction was carried out with CH2Cl2 (3 × 20 mL). The organic layer was dried over Na2SO4, and after solvent evaporation affording the crude product as yellow oil (1′–11′). To chloroform solution of amines (1′–11′), phenylisocyanate or phenylisothiocyanate (1:1) was slowly added with stirring. The mixture was refluxed overnight. The completion of reaction was checked by TLC. The CHCl3 was removed under reduced pressure and the crude product was purified by column chromatography.
Spectral properties of compounds 1a–11a and 1b–11b 1-(4-Hydroxy-3-methoxybenzyl)-1, 3-diphenylurea (1a)
White powder, yield: 75%. Mp: 124°C–126°C. 1H NMR (500 MHz, CDCl3, δ ppm): 3.83 (s, 3H); 4.80 (s, 2H); 5.57 (s, 1H); 6.61–6.64 (m, 1H); 6.74–7.32 (m, 12H). MS (ESI): 349.1 ([M+H]+). Anal. Calcd for C21H20N2O3: C, 72.40; H, 5.79; N, 8.04. Found: C, 72.51; H, 5.97; N, 8.19.
1-(4-Hydroxy-3-methoxybenzyl)-3-phenyl-1-p-tolylurea (2a)
Needle crystals, yield: 79%. Mp: 146°C–148°C. 1H NMR (300 MHz, CDCl3, δ ppm): 2.37 (s, 3H); 3.84 (s, 3H); 4.81 (s, 2H); 5.54 (s, 1H); 6.61–6.65 (dd, J1 = 1.83, J2 = 8.04 Hz, 1H); 6.76–6.78 (d, J = 8.04 Hz, 1H); 6.88–6.89 (d, J = 1.83 Hz, 1H); 6.96–7.31 (m, 9H). MS (ESI): 363.1 ([M+H]+). Anal. Calcd for C22H22N2O3: C, 72.91; H, 6.12; N, 7.73. Found: C, 72.78; H, 6.05; N, 7.87.
1-(4-Hydroxy-3-methoxybenzyl)-1-(4-methoxyphenyl)-3-phenylurea (3a)
Needle crystals, yield: 60%. Mp: 139°C–142°C. 1H NMR (500 MHz, CDCl3, δ ppm): 3.80 (s, 3H); 3.85 (s, 3H); 4.81 (s, 2H); 5.56 (s, 1H); 6.62–6.65 (dd, 1H); 6.75–6.78 (d, J = 8.0 Hz, 1H); 6.89–7.23 (m, 8H); 7.27–7.32 (m, 2H). MS (ESI): 379.1 ([M+H]+). Anal. Calcd for C22H22N2O4: C, 69.83; H, 5.86; N, 7.40. Found: C, 69.79; H, 5.87; N, 7.23.
1-(4-Fluorophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylurea (4a)
Needle crystals, yield: 74%. Mp: 126°C–128°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.84 (s, 3H); 4.80 (s, 2H); 5.57 (s, 1H); 6.60–6.63(dd, J1 = 2.01, J2 = 8.04 Hz, 1H); 6.77–6.80 (d, J = 8.04 Hz, 1H); 6.86–6.89 (d, J = 1.83 Hz, 1H); 6.98–7.03 (m, 1H); 7.08–7.24 (m, 6H); 7.27–7.30 (m, 2H). MS (ESI): 367.1 ([M+H]+). Anal. Calcd for C21H19FN2O3: C, 68.84; H, 5.23; N, 7.65. Found: C, 68.90; H, 5.43; N, 7.52.
1-(4-Chlorophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylurea (5a)
Needle crystals, yield: 67%. Mp: 143°C–145°C. 1H NMR (500 MHz, CDCl3, δ ppm): 3.84 (s, 3H); 4.81 (s, 2H); 5.58(s, 1H); 6.60–6.62 (dd, 1H); 6.77–6.79 (d, J = 8.0 Hz, 1H); 6.86 (d, J = 1.0 Hz, 1H); 6.98–7.08 (m, 3H); 7.23–7.30 (m, 4H); 7.36–7.38 (d, J = 8.5 Hz, 2H). MS (ESI): 383.1 ([M+H]+). Anal. Calcd for C21H19ClN2O3: C, 65.88; H, 5.00; N, 7.32. Found: C, 65.91; H, 4.97; N, 7.26.
1-(4-Bromophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylurea (6a)
Crystals, yield: 80%. Mp: 150°C–152°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.84 (s, 3H); 4.81 (s, 2H); 5.58 (s, 1H); 6.60–6.64 (dd, J1 = 1.83, J2 = 8.03 Hz, 1H); 6.77–6.80 (d, J = 7.8 Hz, 1H); 6.85–6.86 (d, J = 1.83 Hz, 1H); 6.98–7.03 (m, 3H); 7.22–7.31 (m, 4H); 7.51–7.54 (m, 2H). MS (ESI): 427.0 ([M+H]+). Anal. Calcd for C21H19BrN2O3: C, 59.03; H, 4.48; N, 6.56. Found: C, 59.16; H, 4.45; N, 6.62.
1-(2-Fluorophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylurea (7a)
White powder, yield: 43%. Mp: 126°C–129°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.85 (s, 3H); 4.76 (s, 2H); 5.65 (s, 1H); 6.57–7.19 (m, 8H); 7.27–7.43 (m, 4H). MS (ESI): 367.1 ([M+H]+). Anal. Calcd for C21H19FN2O3: C, 68.84; H, 5.23; N, 7.65. Found: C, 68.63; H, 5.09; N, 7.75.
1-(2-Chlorophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylurea (8a)
Needle crystals, yield: 56%. Mp: 117°C–119°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.84 (s, 3H); 4.79 (s, 2H); 5.57 (s, 1H); 6.58–7.03 (m, 5H); 7.17–7.34 (m, 7H). MS (ESI): 383.1 ([M+H]+). Anal. Calcd for C21H19ClN2O3: C, 65.88; H, 5.00; N, 7.32. Found: C, 65.71; H, 5.07; N, 7.51.
1-(2-Bromophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylurea (9a)
White powder, yield: 80%. Mp: 134°C–137°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.84 (s, 3H); 4.81 (s, 2H); 5.69 (s, 1H); 6.59–7.51 (m, 12H). MS (ESI): 427.0 ([M+H]+). Anal. Calcd for C21H19BrN2O3: C, 59.03; H, 4.48; N, 6.56. Found: C, 60.07; H, 4.59; N, 6.46.
1-(2, 4-Difluorophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylurea (10a)
White powder, yield: 53%. Mp: 142°C–145°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.83 (s, 3H); 4.76 (d, 2H); 5.62 (s, 1H); 6.60–6.64 (m, 1H); 6.73–7.30 (m, 10H). MS (ESI): 385.1 ([M+H]+). Anal. Calcd for C21H18F2N2O3: C, 65.62; H, 4.72; N, 7.29. Found: C, 65.81; H, 4.75; N, 7.40.
1-(2, 4-Dichlorophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylurea (11a)
White powder, yield: 46%. Mp: 144°C–145°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.83 (s, 3H); 4.82 (d, 2H); 5.61 (s, 1H); 6.59–6.62 (dd, 1H); 6.77–7.11 (m, 5H); 7.23–7.36 (m, 5H). MS (ESI): 417.1 ([M+H] +). Anal. Calcd for C21H18Cl2N2O3: C, 60.44; H, 4.35; N, 6.71. Found: C, 60.52; H, 4.33; N, 6.58.
1-(4-Hydroxy-3-methoxybenzyl)-1, 3-diphenylthiourea (1b)
Needle crystals, yield: 71%. Mp: 136°C–138°C. 1H NMR (500 MHz, CDCl3, δ ppm): 3.84 (s, 3H); 5.48 (s, 2H); 5.57(s, 1H); 6.64–6.66 (dd, 1H); 6.75–6.77 (d, J = 8.0 Hz, 1H); 6.93 (s, 1H); 7.09–7.10 (m, 2H); 7.16–7.19 (m, 1H); 7.30–7.43 (m, 6H). MS (ESI): 365.4([M+H]+). Anal. Calcd for C21H20N2O2S: C, 69.20; H, 5.53; N, 7.69. Found: C, 69.13; H, 5.46; N, 7.56.
1-(4-Hydroxy-3-methoxybenzyl)-3-phenyl-1-p-tolylthiourea (2b)
Needle crystals, yield: 65%. Mp: 130°C–132°C. 1H NMR (300 MHz, CDCl3, δ ppm):2.36 (s, 3H); 3.85 (s, 3H); 5.46(s, 2H); 5.56 (s, 1H); 6.63–6.66(dd, J1 = 1.83, J2 = 8.07 Hz, 1H); 6.75–6.78 (d, J = 8.07 Hz, 1H); 6.95–6.98 (m, 3H); 7.13–7.34 (m, 7H). MS (ESI): 379.1 ([M+H]+). Anal. Calcd for C22H22N2O2S: C, 69.81; H, 5.86; N, 7.40. Found: C, 69.85; H, 6.00; N, 7.47.
1-(4-Hydroxy-3-methoxybenzyl)-1-(4-methoxyphenyl)-3-phenylthiourea (3b)
Needle crystals, yield: 67%. Mp: 146°C–148°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.81 (s, 3H); 3.85 (s, 3H); 5.44 (s, 2H); 5.55 (s, 1H); 6.63–6.66 (dd, J1 = 1.83 Hz, J1 = 8.04 Hz, 1H); 6.75–6.78 (d, J = 8.04 Hz, 1H); 6.89–7.00 (m, 5H); 7.12–7.20 (m, 1H); 7.27–7.35 (m, 4H). MS (ESI): 395.1 ([M+H]+). Anal. Calcd for C22H22N2O3S: C, 66.98; H, 5.62; N, 7.10. Found: C, 66.85; H, 5.70; N, 7.26.
1-(4-Fluorophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylthiourea (4b)
Needle crystals, yield: 59%. Mp: 136°C–139°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.85 (s, 3H); 5.45 (s, 2H); 5.57 (s, 1H); 6.62–6.65 (dd, 1H); 6.76–6.78 (d, J = 8.04 Hz, 1H); 6.89 (s, 1H); 7.06–7.32 (m, 9H). MS (ESI): 383.1 ([M+H]+). Anal. Calcd for C21H19FN2O2S: C, 65.95; H, 5.01; N, 7.32. Found: C, 66.02; H, 5.03; N, 7.51.
1-(4-Chlorophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylthiourea (5b)
Needle crystals, yield: 65%. Mp: 138°C–140°C. 1H NMR (500 MHz, CDCl3, δ ppm): 3.85 (s, 3H); 5.45 (s, 2H); 5.58(s, 1H); 6.63–6.65 (dd, J1 = 1.5 Hz, J2 = 8.0 Hz, 1H); 6.77–6.78 (d, J = 8.0 Hz, 1H); 6.88 (s, 1H); 7.02–7.04 (d, J = 8.5 Hz, 2H); 7.10–7.11 (d, J = 1.5 Hz, 1H); 7.18–7.21 (m, 1H); 7.31–7.40(m, 5H). MS (ESI): 399.1 ([M+H]+). Anal. Calcd for C21H19ClN2O2S: C, 63.23; H, 4.80; N, 7.02. Found: C, 63.14; H, 4.86; N, 6.88.
1-(4-Bromophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylthiourea (6b)
Crystals, yield: 73%. Mp: 150°C–152°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.85 (s, 3H); 5.44 (s, 2H); 5.58 (s, 1H); 6.63–6.66 (dd, 1H); 6.76–6.79 (d, J = 7.86 Hz, 1H); 6.88 (s, 1H); 6.96–6.99 (m, 2H); 7.10–7.11 (d, J = 1.83 Hz, 1H); 7.17–7.21 (m, 1H); 7.30–7.56 (m, 5H). MS (ESI): 443.0 ([M+H]+). Anal. Calcd for C21H19BrN2O2S: C, 56.89; H, 4.32; N, 6.32. Found: C, 56.81; H, 4.29; N, 6.24.
1-(2-Fluorophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylthiourea (7b)
White powder, yield: 38%. Mp: 133°C–135°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.85 (s, 3H); 5.39 (s, 2H); 5.64 (s, 1H); 6.65–7.05 (m, 4H); 7.16–7.26 (m, 5H); 7.30–7.37 (m, 3H). MS (ESI): 383.1 ([M+H]+). Anal. Calcd for C21H19FN2O2S: C, 65.95; H, 5.01; N, 7.32. Found: C, 65.91; H, 5.06; N, 7.26.
1-(2-Chlorophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylthiourea (8b)
Needle crystals, yield: 34%. Mp: 146°C–149°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.85 (s, 3H); 5.43 (s, 2H); 5.58(s, 1H); 6.59–7.38 (m, 12H). MS (ESI): 399.1 ([M+H]+). Anal. Calcd for C21H19ClN2O2S: C, 63.23; H, 4.80; N, 7.02. Found: C, 63.30; H, 4.72; N, 7.16.
1-(2-Bromophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylthiourea (9b)
White powder, yield: 63%. Mp: 143°C–145°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.85 (s, 3H); 5.31 (s, 2H); 5.49 (s, 1H); 6.57–7.17 (m, 9H); 7.19–7.23 (m, 1H); 7.29–7.32 (m, 2H). MS (ESI): 443.0 ([M+H]+). Anal. Calcd for C21H19BrN2O2S: C, 56.89; H, 4.32; N, 6.32. Found: C, 56.94; H, 4.22; N, 6.39.
1-(2, 4-Difluorophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylthiourea (10b)
White powder, yield: 45%. Mp: 135°C–137°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.85 (s, 3H); 5.44 (s, 2H); 5.57 (s, 1H); 6.59–6.63 (dd, J1 = 1.8 Hz, J2 = 7.86 Hz, 1H); 6.74–6.77 (d, J = 7.86 Hz, 1H); 6.86–7.05 (m, 5H); 7.18–7.23 (m, 1H); 7.29–7.36 (m, 3H). MS (ESI): 401.1 ([M+H]+). Anal. Calcd for C21H18F2N2O2S: C, 62.99; H, 4.53; N, 7.00. Found: C, 62.95; H, 4.48; N, 6.85.
1-(2, 4-Dichlorophenyl)-1-(4-hydroxy-3-methoxybenzyl)-3-phenylthiourea (11b)
White needle crystals yield: 38%. Mp: 125°C–127°C. 1H NMR (300 MHz, CDCl3, δ ppm): 3.83 (s, 3H); 5.39 (d, 2H); 5.60 (s, 1H); 6.60–6.62 (dd, 1H); 6.77–7.21 (m, 7H); 7.32–7.41 (m, 3H). MS (ESI): 433.1 ([M+H]+). Anal. Calcd for C21H18Cl2N2O2S: C, 58.20; H, 4.19; N, 6.46. Found: C, 58.07; H, 4.23; N, 6.53.
Cell culture
B16-F10 cells obtained from American Type Culture Collection (ATCC) were cultured in RPMI-1640 medium (GibcoBRL) with 10% (v/v) fetal bovine serum (FBS), 100 U/mL penicillin G and 100 μg/mL streptomycin. Cells were incubated at 37°C in a humidified atmosphere of air/CO2 (95%: 5%).
Cell proliferation assay
The anti-proliferative activity was determined using a standard (MTT)-based colorimetric assay (Sigma). Briefly, cell lines were seeded at a density of 7 × 103 cells/well in 96-well microtiter plates (Costar). After 24 h, exponentially growing cells were exposed to the indicated compounds at final concentrations ranging from 0.1 to 100 μg/mL. After 48 h, cell survival was determined by the addition of an MTT solution (10 μL of 5 mg/mL MTT in PBS). After 4 h, 100 μL of 10% SDS (Sigma) in 0.01 N HCl was added, and the plates were incubated at 37°C for a further 18 h; optical absorbance was measured at 570 nm on an LX300 Epson Diagnostic microplate reader. Survival ratios are expressed in percentages with respect to untreated cells. The IC50 values were determined from replicates of 6 wells from at least two independent experiments.
Cell-based enzyme inhibition measurement by ELISA
Detection of the effect of compounds on p-MEK1/2, t-ERK1/2, pERK1/2, p-Rsk1 and p-Elk-1 activity in B16-F10 cells was performed using ELISA kits (Invitrogen) and strictly according to the manufacturer’s instructions. Cells were pre-treated with 30 nM PMA (Sigma) in the absence or presence of these compounds.
Immunofluorescence
The B16-F10 cells were seeded in 6-well plates at a seeding density of 105 cells/mL. When all the cells were adhered, various concentrations of compound 6b added. For immunofluorescence analysis, B16-F10 cells were treated with PMA (30 nM) and two different concentrations (0 and 0.25 μM) of compound 6b. After 24 h incubation, cells washed with PBS, fixed with 2% paraformaldehyde for 2h and permeabilized with 0.2% Triton X-100. After blocking with 1% BSA, the cells were incubated for 3h at room temperature with primary anti-pERK1/2 (Boster, 1:100). The cells were incubated with secondary antibodies: FITC-labelled goat anti-rabbit IgG (Boster) at dilution 1:100 for 1h at 37°C in the dark. Slides were analysed under Nikon E800 (Nikon, Japan) microscope.Results and discussion
Chemistry
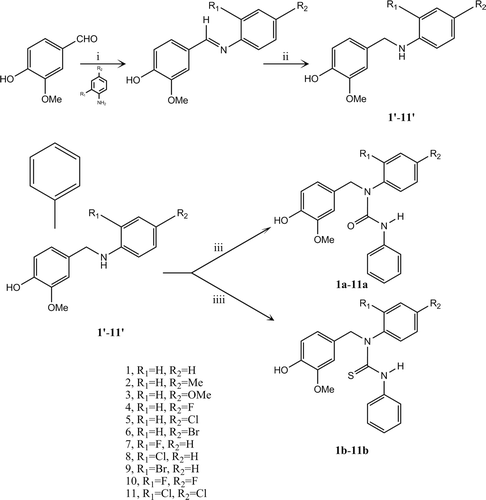
The synthesis of compounds 1a–11a and 1b–11b followed the general pathway outlined in . The two series of urea and thiourea derivatives were synthesized in three steps. First, we chose 11 different substituted anilines to prepare the corresponding Schiff bases. The secondary amines (1′-11′) were obtained from the corresponding Schiff bases after the reduction reactions with sodium borohydride. In the next step, the secondary amines were condensed with phenylisocyanate or phenylisothiocyanate in chloroform as the solvent, affording the target compounds 1a-11a and 1b-11b. The reactions were monitored by thin layer chromatography (TLC) and the products were purified by column chromatography. All the urea and thiourea 1a–11a and 1b–11b were synthesized for the first time and all the compounds were fully characterized by 1H NMR, ESI MS and elemental analysis. Furthermore, the crystal data, data collection and refinement parameter for compound 6b are listed in supplementary information. The structure was solved by direct methods and refined on F2 by full-matrix least-squares methods using SHELX-97Citation24.Biological activity study
Scheme 1. Synthesis route of compounds 1a–11a and 1b–11b. Reagents and conditions: (i) ethanol, rt, 4 h; (ii) ethanol, NaBH4, reflux, 2 h; (iii) phenylisocyanate, chloroform, reflux, overnight; (iiii) phenylisothiocyanate, chloroform, reflux, overnight.
In vitro cytotoxic activities of these N-benzyl-N-(4-hydroxy-3-methoxybenzyl)-N′-phenylurea and thiourea derivatives were studied in B16-F10 melanoma cell line. A number of synthesized compounds displayed potent cytotoxic activities against B16-F10. The cytotoxic activity of the 22 compounds in B16-F10 was closely associated with their structures, as shown in and expressed as the half maximal inhibitory concentration (IC50). All 22 compounds exhibited good activity with IC50 values of < 10.0 μM, among which 7 compounds demonstrated IC50 values of 5.0–10.0 μM, 15 compounds showed IC50 less than 5.0 μM, compound 6b exhibited the most potent inhibitory activity in melanoma cells growth inhibition (IC50 = 0.33 μM), which was more potent than positive control cisplatin (IC50 = 4.24 μM) and carboplatin (IC50 = 7.35 μM).
Table 1. Cytotoxicity of compounds 1a–11a and 1b–11b against B16-F10 cells.
Structure-activity relationships (SARs) of these urea and thiourea derivatives demonstrated that phenyl thiourea derivatives have less IC50 values than those corresponding phenyl urea derivatives. The result also indicated that compounds with substitution at the para (1a-6a, 1b-6b) position showed more potent activities than those with substitution on the ortho position (7a-9a, 7b-9b). A comparison of the para position substitution on benzene ring demonstrated that a para halogen group (4a-6c, 4b-6b) may have slightly more improved anti-proliferative activity than a methyl or a methoxy group, and it showed the most potent inhibitory activity when the para position was substituted by bromine. A significant loss of activity was observed when the halogen substituent was moved to the ortho (7a-9a, 7b-9b) position. This trend was observed in all compounds whether they were urea or thiourea. The compounds (10a, 11a, 10b, 11b) substituted by halogen groups both on para position and ortho position showed more potent activities than compounds with ortho halogen substituents, but less than compounds with para halogen substituents. So we deduced that substitution at the para position of the N-phenyl ring plays an important role in the anti-proliferative activity.
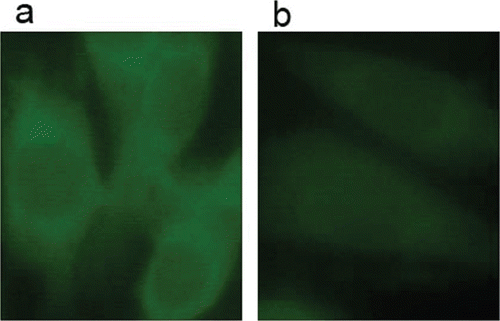
As described above, the ERK signalling pathway plays a central role in several steps of melanoma development, including cancer cell proliferation and the development of resistance to apoptosis. The ERK1/2 is therefore considered a prominent therapeutic target for the development of chemotherapeutic drugs. The synthesized urea and thiourea were evaluated for their ability to inhibit phosphorylation (activation) of ERK in B16-F10 cells. As illustrated in , all the compounds displayed potent activities to induce the reduction in the amounts of pERK1/2. These results suggested that compounds with substitution at the para (1a-6a, 1b-6b) position showed more potent activities than those with substitution at the ortho position, and compound 6b, which displayed the most potent activity in tumour growth inhibition, also showed the lowest IC50 value (IC50 = 1.27 μM). It is indicated that there is a reasonable correlation between the pERK1/2 inhibitory activities and the cytotoxicities against B16-F10 cells of these compounds. Next, compound 6b was evaluated for its ability to inhibit pERK1/2 level by using an immunofluorescence analysisCitation25. Samples were immunocytochemically labelled with fluorescein isothiocyanate (FITC) for phosphorylated ERK1/2 in green. The results were summarized in . Compared to controls, the cells treated with compound 6b displayed a significant reduction of fluorescence, which verified that this thiourea derivative could inhibit pERK1/2 level in B16-F10 cell line.
Table 2. Inhibition (IC50) of pERK1/2 in B16-F10.
Figure 1. Immunofluorescence staining of pERK1/2 in B16-F10 cells after 24 h incubation. Cells were treated in the absence or presence of 6b. Green staining indicates the immunofluorescence of human pERK1/2 (FITC-labelled) in B16-F10 cells (a) untreated (b) or treated with 0.25 μM compound 6b.
As shown in , we tested the inhibitory activities of selected compounds (3b, 4b, 5b, 6a, 6b) against phosphorylated MEK1/2 and total ERK1/2 (upstream factors of pERK), phosphorylated Rsk1 and phosphorylated Elk-1 (downstream factors of pERK). These compounds decreased p-Rsk1 and p-Elk-1 amounts (the downstream substrates of ERK1/2) in B16-F10 cells with good effects. However, these compounds did not show obvious inhibitory ability against p-MEK1/2, t-ERK1/2. Combining these enzyme assay results, we deduced that these compounds could specially inhibit the phosphorylation of ERK1/2 and then block the RAS–MEK–ERK signalling pathway in B16-F10 cells.
Table 3. Inhibition of p-MEK1/2, t-ERK1/2, p-Rsk1 and p-Elk1 in B16-F10 cell line by tested compounds.
Conclusion
In summary, two series of novel N-benzyl-N-(4-hydroxy-3-methoxy-benzyl)-N′-phenylurea and thiourea derivatives (1a–11a, 1b–11b) were synthesized. Their anti-proliferative activities against the melanoma cell line B16-F10 were evaluated. Some compounds displayed good inhibitory activities and the SARs have also been studied. The consequences demonstrated that substitution at the para position of the N-phenyl ring played an important role in the anticancer activity. Among the compounds tested, we found compound 6b had demonstrated significant ERK phosphorylation inhibitory activity (IC50 = 1.27 μM against pERK1/2) and anti-proliferative activity in melanoma cells growth inhibition (IC50 = 0.33 μM). In enzyme assays, 6b did not show obvious inhibitory ability against upstream factors of pERK1/2. Above all, compound 6b would be a potential anti-melanoma agent through specially inhibit phosphorylation of ERK1/2.
Acknowledgement
Qing-Shan Li and Peng-Cheng Lv have contributed equally to the work.
Declaration of interest
This work was supported by Jiangsu National Science Foundation (No. BK2009239).
References
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature 2007;445:851–857.
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H et al. Distinct sets of genetic alterations in melanoma. n Engl j Med 2005;353:2135–2147.
- Marks R. Epidemiology of melanoma. Clin Exp Dermatol 2000;25:459–463.
- Gao L, Feng Y, Bowers R, Becker-Hapak M, Gardner J, Council L et al. Ras-associated protein-1 regulates extracellular signal-regulated kinase activation and migration in melanoma cells: two processes important to melanoma tumorigenesis and metastasis. Cancer Res 2006;66:7880–7888.
- Calvo F, Agudo-Ibáñez L, Crespo P. The Ras-ERK pathway: understanding site-specific signaling provides hope of new anti-tumor therapies. Bioessays 2010;32:412–421.
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 2001;410:37–40.
- Yap JL, Worlikar S, MacKerell AD Jr, Shapiro P, Fletcher S. Small-molecule inhibitors of the ERK signaling pathway: Towards novel anticancer therapeutics. Chemmedchem 2011;6:38–48.
- Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta 2007;1773:1213–1226.
- Kohno M, Pouyssegur J. Pharmacological inhibitors of the ERK signaling pathway: application as anticancer drugs. Prog Cell Cycle Res 2003;5:219–224.
- Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell 1997;90:859–869.
- Kinoshita T, Yoshida I, Nakae S, Okita K, Gouda M, Matsubara M et al. Crystal structure of human mono-phosphorylated ERK1 at Tyr204. Biochem Biophys Res Commun 2008;377:1123–1127.
- Seger R, Seger D, Lozeman FJ, Ahn NG, Graves LM, Campbell JS et al. Human T-cell mitogen-activated protein kinase kinases are related to yeast signal transduction kinases. j Biol Chem 1992;267:25628–25631.
- Robbins DJ, Zhen E, Owaki H, Vanderbilt CA, Ebert D, Geppert TD et al. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. j Biol Chem 1993;268:5097–5106.
- Marshall C. How do small GTPase signal transduction pathways regulate cell cycle entry? Curr Opin Cell Biol 1999;11:732–736.
- Frau M, Biasi F, Feo F, Pascale RM. Prognostic markers and putative therapeutic targets for hepatocellular carcinoma. Mol Aspects Med 2010;31:179–193.
- Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol 2004;5:875–885.
- Cohen C, Zavala-Pompa A, Sequeira JH, Shoji M, Sexton DG, Cotsonis G et al. Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin Cancer Res 2002;8:3728–3733.
- Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell 1990;61:203–212.
- Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem 2000;69:373–398.
- Dai Y, Guo Y, Frey RR, Ji Z, Curtin ML, Ahmed AA et al. Thienopyrimidine ureas as novel and potent multitargeted receptor tyrosine kinase inhibitors. j Med Chem 2005;48:6066–6083.
- Li HQ, Yan T, Yang Y, Shi L, Zhou CF, Zhu HL. Synthesis and structure-activity relationships of N-benzyl-N-(X-2-hydroxybenzyl)-N’-phenylureas and thioureas as antitumor agents. Bioorg Med Chem 2010;18:305–313.
- Li HQ, Lv PC, Yan T, Zhu HL. Urea derivatives as anticancer agents. Anticancer Agents Med Chem 2009;9:471–480.
- Li HQ, Zhu TT, Yan T, Luo Y, Zhu HL. Design, synthesis and structure-activity relationships of antiproliferative 1,3-disubstituted urea derivatives. Eur j Med Chem 2009;44:453–459.
- Sheldrick GM. SHELX-97. Program for X-ray Crystal Structure Solution and Refinement. 1997.
- Abagyan R, Orry A, Raush E, Budagyan L and Totrov M. ICM Manual Version 3.0. 2007.