Abstract
The inhibition of two human cytosolic carbonic anhydrase (hCA, EC 4.2.1.1) isozymes I, II and human serum isozyme VI, with a series of tosylited aromatic amine derivatives was investigated. The KI ranges of compounds 1–14 and acetazolamide against hCA I ranged between 1.130 and- 448.2 μM, against hCA II between 0.103 and- 14.3 μM, and against hCA VI ranged between 0.340 and- 42.39 μM. Tosylited aromatic amine derivatives are thus interesting hCA I, II and VI inhibitors, and might be used as leads for generating enzyme inhibitors eventually targeting other isoforms which have not been assayed yet for their interactions with such agents.
Introduction
Carbonic anhydrase (CA, EC 4.2.1.1) enzymes are involved in important physiological and pathological functions, such as pH and CO2 homeostasis, respiration and transport of CO2/HCO3− between metabolizing tissues and the lungs, ion secretion in different tissues/organs and biosynthetic reactions (such as gluconeogenesis, lipogenesis and ureagenesis). Among the sixteen 16 isoenzymes, described up to now, CAI and CAII are present at high concentrations in the cytosol in erythrocytes, and CAII has the highest turnover rate of all the CAsCitation1–8. Carbonic anhydrase VI (CA VI) is a secretory enzyme that was initially described in the ovine parotid gland, saliva and normal human serumCitation8.
The interactions between specific enzymes and various types of inhibitors are of great importance and corresponding investigations have become very popular in the recent years. These substances are sometimes useful in medicinal applications, whereas they have undesirable effects on both human metabolism and other organismsCitation9–21.
Carbonic anhydrase inhibitors or activators have several medical applications, such as in the treatment of glaucoma, as diuretics, in the management of several neurological disorders, including epilepsy, possibly in the treatment of Alzheimer’s disease, whereas several agents are in clinical evaluations as antiobesity or antitumor drugs/diagnostic toolsCitation3–7.
A class of derivatives which showed very promising applications among the various CAIs reported by Supuran’s group in the last years, were the thioureas obtained from isothiocyanato sulfonamides (such as e.g., 4-isothiocyanatobenzenesulfonamide) and amines, hydrazines or amino acidsCitation22–24. Such compounds generally showed potent inhibitory activity against the cytosolic isozyme hCA II as well as the transmembrane, tumor-associated isozyme hCA IX, being thus interesting candidates for developing anti-glaucoma/ anti-tumor therapies based on themCitation22–24.
Many sulfonamide derivatives have been widely used as pro-drugs or drugs. For instance, sulfadiazine is used as an antibiotic, sulfapyridine is mainly used for treatment of bacterial infections, acetazolamide is mainly used anti-glaucoma agent.
Our groups recently investigated the interaction of CA isozymes I, II with salicylic acid derivatives, some antioxidant phenolic compounds, some purine analogue drugs, organic nitrates, and etc.Citation9–13,Citation25,Citation26. We would like to extend these earlier investigations to some benzenesulfonamide derivatives in order to discover novel powerful CAIs which might have implications in medicine.
In this work, toward the discovery of CA inhibitors, we synthesized benzenesulfonamide derivatives. Compounds were evaluated for their ability to inhibit human CA-I, II and VI. Inhibition is reported as KI (μM) and the results are the average of at least three independent experiments.
Materials and methods
Chemicals
All chemicals and solvents are commercially available and were used after distillation or treatment with drying agents. Column chromatography (CC): silica gel (SiO2; 60 mesh, Merck-Darmstadt, Germany). Preparative thick layer chromatography (PLC): 1 mm of SiO2 60 PF (Merck) on glass plates. Mp: cap. melting-point apparatus (BUCHI 530 - Flawil, Switzerland); uncorrected. IR Spectra: solns. in 0.1 mm cells with a Mattson 1000 FT-IR spectrophotometer (Cambrige, England). 1H- and 13C- NMR spectra: 200 (50) and 400 (100)-MHz Varian spectrometer (Danbury, USA); δ in ppm; Me4Si as the internal standard. Elemental analyses: Leco CHNS-932 apparatus (Michigan, USA).
General procedure for the synthesis of N-aryl-ptoluenesulfonamides
To a mixture of aryl amine derivative (10 mmol) and pyridine (10 mL, 120 mmol) in dichloromethane (50 mL) was added slowly p-toluenesulfonyl chloride (2.29 g, 12 mol, 1.2 eq.). The reaction mixture was stirred at room temperature for 16 hCitation27. The reaction mixture was washed with 5% HCl. Then the organic layer was washed with NaHCO3 solution and dried over sodium sulfate, filtered, and evaporated to afford a residue. Purification of the residue by silica gel column chromatography with ethylacetate/hexane provided the corresponding N-aryl-p-toluenesulfonamide 2–11 in 67–100% yields.
Synthesis of 4-methyl-N-p-tolylbenzenesulfonamide (2):
In Quantitative yield, Compound 2 was recrystallized from CH2Cl2-hexane solution. M.P.: 97–99°C. 1H -NMR (400 MHz, CDCl3, δ, ppm): δ = 2.36 (s, 3H in NH-Ts), 7.06 (s, 1H, -NH), 7.10 (d, 2H, J = 8.23 Hz, AA’ part of AA’BB’ system in NH-Ts), 7.22 (m, 5H in Ph), 7.68 (d, 2H, J = 8.23 Hz, BB’ part of AA’BB’ system in NH-Ts). 13C-NMR (100 MHz, CDCl3, ppm): 21.8, 121.7, 125.4, 127.5, 129.5, 129.9, 136.0, 136.8, 144.1. IR (KBr, cm−-1): 3257, 1599, 1496, 1412, 1336, 1303, 1159, 1092. Anal. calcd. for C13H13NO2S (247.31) C, 63.13; H, 5.30; N, 5.66; S, 12.97. Found: C, 62.90; H, 5.40; N, 5.64; S, 12.72.
Synthesis of 4-Methyl-N-(naphthalen-2-yl)benzenesulfonamide (3)
In %98 yield, Compound 3 was recrystallized from CH2Cl2-hexane solution. M.P.: 153–155°C. 1H -NMR (400 MHz, CDCl3, δ, ppm): δ = 2.32 (s, 3H in NH-Ts), 7.14 (d, 2H, J = 8.24 Hz, AA’ part of AA’BB’ system in NH-Ts), 7.25–7.46 (m, 4H in Naph.), 7.64 (d, 2H, J = 8.24 Hz, BB’ part of AA’BB’ system in NH-Ts), 7.70 (d, 1H, J = 6.95 Hz in Naph.), 7.79 (m, 1H in Naph.), 7.85 (d, 1H, J = 8.05 Hz in Naph.). 13C-NMR (100 MHz, CDCl3, ppm): 21.7, 121.7, 122.9, 125.6, 126.5, 126.8, 127.4, 127.6, 128.6, 129.1, 129.7, 131.7, 134.5, 136.7, 144.0. IR (KBr, cm−-1): 3264, 1597, 1454, 1410, 1344, 1314, 1160, 1091. Anal. calcd. for C17H15NO2S (297.37) C, 68.66; H, 5.08; N, 4.71; S, 10.78. Found: C, 68.46; H, 5.20; N, 4.72; S, 10.89.
Synthesis of 4-Methyl-N-(2,4,6-trimethyl-phenyl)-benzenesulfonamide (4)
In %95 yield, Compound 4 was recrystallized from CH2Cl2-hexane solution. M.P.: 166–168°C. 1H -NMR (400 MHz, CDCl3, δ, ppm): δ = 1.98 (s, 6H in Ph-CH3), 2.24 (s, 3H in Ph-CH3) 2.41 (s, 3H in NH-Ts), 5.92 (brs, 1H, -NH), 6.81(s, 2H in Ph-CH3), 7.24 (d, 2H, J = 8.23 Hz, AA’ part of AA’BB’ system in NH-Ts), 7.60 (d, 2H, J = 8.23 Hz, BB’ part of AA’BB’ system in NH-Ts). 13C-NMR (100 MHz, CDCl3, ppm): 18.8, 21.1, 21.7, 127.5, 129.7, 129.8, 130.2, 137.7, 137.8, 138.2, 143.7. IR (KBr, cm−-1): 3282, 1597, 1481, 1400, 1328, 1289, 1161, 1091. Anal. calcd. for C16H19NO2S (289.39) C, 66.41; H, 6.62; N, 4.84; S, 11.08. Found: C, 66.24; H, 6.80; N, 4.85; S, 10.97.
Synthesis of 4-Methyl-N-o-tolyl-benzenesulfonamide (5)
In %96 yield, Compound 5 was recrystallized from CH2Cl2-hexane solution. M.P.: 102–104°C. 1H -NMR (400 MHz, CDCl3, δ, ppm): δ = 2.01 (s, 3H in Ph-CH3),2.38 (s, 3H in NH-Ts), 6.60 (brs, 1H, -NH), 7.06 (m, 2H (ortho, para) in Ph-CH3), 7.12 (m, 2H (meta) in Ph-CH3), 7.21 (d, 2H, J = 8.26 Hz, AA’ part of AA’BB’ system in NH-Ts), 7.31 (d, 1H, J = 8.06 Hz (meta) in Ph-CH3), 7.62 (d, 2H, J = 8.26 Hz, BB’ part of AA’BB’ system in NH-Ts). 13C-NMR (100 MHz, CDCl3, ppm): 17.8, 21.7, 124.6, 126.4, 127.1, 127.4, 129.8, 131.0, 131.6, 134.8, 137.0, 144.0. IR (KBr, cm−-1): 3278, 1598, 1495, 1403, 1330, 1290, 1162, 1092. Anal. calcd. for C14H15NO2S (261.34) C, 64.34; H, 5.79; N, 5.36; S, 12.27. Found: C, 64.18; H, 5.84; N, 5.34; S, 12.25.
Synthesis of N-(2-cyanophenyl)-4-methylbenzenesulfonamide (6)
In %67 yield, Compound 6 was recrystallized from CH2Cl2-hexane solution. M.P.: 129–131°C. 1H -NMR (400 MHz, CDCl3, δ, ppm): δ = 2.38 (s, 3H in NH-Ts), 7.17 (m, 1H (meta) in Ph-CN), 7.25 (d, 2H, J = 8.42 Hz, AA’ part of AA’BB’ system in NH-Ts), 7.46 (dd, 1H (meta) in Ph-CN, J = 7.7, 1.5 Hz), 7.52 (brdt, 1H(para) in Ph-CN, J = 8.8, 1.5 Hz), 7.70 (d, 2H, J = 8.42 Hz, BB’ part of AA’BB’ system in NH-Ts), 7.72 (d, 1H(ortho) in Ph-CN, J = 8.80 Hz). 13C-NMR (100 MHz, CDCl3, ppm): 21.8, 104.5, 115.9, 121.9, 125.4, 127.6, 130.2, 133.0, 134.4, 135.7, 139.6, 145.0. IR (KBr, cm−-1): 3241, 2230, 1599, 1493, 1455, 1413, 1342, 1163, 1091. Anal. calcd. for C14H12N2O2S (272.32) C, 61.75; H, 4.44; N, 10.29; S, 11.77. Found: C, 61.64; H, 4.54; N, 10.36; S, 11.61.
Synthesis of 4-Methyl-N-(2-nitrophenyl)benzenesulfonamide (7)
In %75 yield, Compound 7 was recrystallized from CH2Cl2-hexane solution. M.P.: 108–110°C. 1H -NMR (400 MHz, CDCl3, δ, ppm): δ = 2.37 (s, 3H in NH-Ts), 7.14 (ddd, 1H (meta) in Ph-NO2, J = 8.4, 7.3, 1.5 Hz), 7.25 (d, 2H, J = 8.2 Hz, AA’ part of AA’BB’ system in NH-Ts), 7.57 (ddd, 1H (para) in Ph-NO2, J = 8.4, 7.3, 1.5 Hz), 7.72 (d, 2H, J = 8.2 Hz, BB’ part of AA’BB’ system in NH-Ts), 7.83 (dd, 1H (meta) in Ph-CN, J = 8.4, 1.5 Hz), 8.09 (dd, 1H (ortho) in Ph-CN, J = 8.4, 1.5 Hz), 9.84 (brs, 1H, -NH). 13C-NMR (100 MHz, CDCl3, ppm): 21.8, 121.2, 124.0, 126.4, 127.5 (2C), 130.2, 134.2, 135.9, 136.1, 145.0. IR (KBr, cm−-1): 3286, 1610, 1529, 1487, 1393, 1348, 1275, 1169, 1090. Anal. calcd. for C13H12N2O4S (292.31) C, 53.42; H, 4.14; N, 9.58; S, 10.97. Found: C, 53.31; H, 4.17; N, 9.56; S, 10.90.
Synthesis of 4-Methyl-N-p-tolyl-benzenesulfonamide (8)
In Quantitative yield, Compound 8 was recrystallized from CH2Cl2-hexane solution. M.P.: 115–117°C. 1H -NMR (400 MHz, CDCl3, δ, ppm): δ = 2.24 (s, 3H in Ph-CH3), 2.35 (s, 3H in NH-Ts), 6.89 (brs, 1H, -NH), 7.00 (d, 2H, J = 4.21 Hz, AA’ part of AA’BB’ system (ortho) in Ph-CH3), 7.18 (d, 2H, J = 8.23 Hz, AA’ part of AA’BB’ system in NH-Ts), 7.21 (d, 2H, J = 4.21 Hz, BB’ part of AA’BB’ system (meta) in Ph-CH3), 7.67 (d, 2H, J = 8.23 Hz, BB’ part of AA’BB’ system in NH-Ts). 13C-NMR (100 MHz, CDCl3, ppm): 21.0, 21.7, 122.4, 127.5, 129.8, 130.0, 134.1, 135.4, 136.4, 143.9. IR (KBr, cm−-1): 3258, 1598, 1511, 1450, 1393, 1331, 1160, 1092. Anal. calcd. for C14H15NO2S (261.34) C, 64.34; H, 5.79; N, 5.36; S, 12.27. Found: C, 64.12; H, 5.86; N, 5.38; S, 12.35.
Synthesis of N-(4-ethylphenyl)-4-methylbenzenesulfonamide (9)
In %98 yield, Compound 9 was recrystallized from CH2Cl2-hexane solution. M.P.: 90–92°C. 1H -NMR (400 MHz, CDCl3, δ, ppm): δ = 1.17 (t, 3H, J = 7.7 Hz in Ph-C2H5), 2.37 (s, 3H in NH-Ts), 2.55 (q, 2H, J = 7.7 Hz in Ph-C2H5), 6.76 (brs, 1H, -NH), 6.98 (d, 2H, J = 8.6 Hz, AA’ part of AA’BB’ system (ortho) in Ph-C2H5), 7.05 (d, 2H, J = 8.60 Hz, BB’ part of AA’BB’ system (meta) in Ph-C2H5), 7.21 (d, 2H, J = 8.05 Hz, AA’ part of AA’BB’ system in NH-Ts), 7.64 (d, 2H, J = 8.05 Hz, BB’ part of AA’BB’ system in NH-Ts). 13C-NMR (100 MHz, CDCl3, ppm): 15.6, 21.7, 28.4, 122.5, 127.5, 128.8, 129.8, 134.2, 136.5, 141.9, 143.9. IR (KBr, cm−-1): 3259, 1598, 1512, 1457, 1397, 1333, 1160, 1092. Anal. calcd. for C15H17NO2S (275.37) C, 65.43; H, 6.22; N, 5.09; S, 11.64. Found: C, 65.29; H, 6.20; N, 5.12; S, 11.65.
Synthesis of N-(4-cyanophenyl)-4-methylbenzenesulfonamide (10)
In %72 yield, Compound 10 was recrystallized from CH2Cl2-hexane solution. M.P.: 177–179°C. 1H -NMR (400 MHz, CDCl3, δ, ppm): δ = 2.39 (s, 3H in NH-Ts), 7.18 (d, 2H, J = 9.0 Hz, AA’ part of AA’BB’ system (meta) in Ph-CN), 7.27 (d, 2H, J = 8.2 Hz, AA’ part of AA’BB’ system in NH-Ts), 7.51 (d, 2H, J = 9.0 Hz, BB’ part of AA’BB’ system (ortho) in Ph-CN), 7.76 (d, 2H, J = 8.2 Hz, BB’ part of AA’BB’ system in NH-Ts). 13C-NMR (100 MHz, CDCl3, ppm): 21.8, 107.9, 118.7, 119.5, 127.5, 130.2, 133.8, 135.8, 141.3, 145.0. IR (KBr, cm−-1): 3237, 2226, 1607, 1508, 1462, 1402, 1342, 1159, 1090. Anal. calcd. for C14H12N2O2S (272.32) C, 61.75; H, 4.44; N, 10.29; S, 11.77. Found: C, 61.63; H, 4.46; N, 10.28; S, 11.70.
Synthesis of 4-Methyl-N-(4-nitrophenyl)benzenesulfonamide (11)
In %71 yield, Compound 11 was recrystallized from CH2Cl2-hexane solution. M.P.: 181–183°C. 1H -NMR (400 MHz, CDCl3, δ, ppm): δ = 2.40 (s, 3H in NH-Ts), 7.20 (d, 2H, J = 9.2 Hz, AA’ part of AA’BB’ system (meta) in Ph-NO2), 7.29 (d, 2H, J = 8.3 Hz, AA’ part of AA’BB’ system in NH-Ts), 7.77 (d, 2H, J = 8.3 Hz, BB’ part of AA’BB’ system in NH-Ts), 8.12 (d, 2H, J = 9.2 Hz, BB’ part of AA’BB’ system (meta) in Ph-CN). 13C-NMR (100 MHz, CDCl3, ppm): 21.8, 118.9, 125.6, 127.5, 130.3, 135.8, 142.9, 145. IR (KBr, cm−-1): 3254, 1596, 1520, 1497, 1342, 1296, 1161, 1090. Anal. calcd. for C13H12N2O4S (292.31) C, 53.42; H, 4.14; N, 9.58; S, 10.97. Found: C, 53.27; H, 4.16; N, 9.63; S, 11.03.
Purification of carbonic anhydrase isozymes from human blood by affinity chromatography
Purification of hCA I and hCA II were previously described: hCA I and hCA II in Citation18. Serum were purified from fresh human blood obtained from the Blood Center of the Research Hospital at Atatürk University. The blood samples were centrifuged at 5000 rpm for 15 min and precipitant were removed. The serum was isolated. The pH of the hemolysate was adjusted to 8.7 with solid TrisCitation9–11. Sepharose-4B-aniline-sulfanylamide affinity column equilibrated with 25 mM Tris-HCl/0.1M Na2SO4 (pH 8.7). The affinity gel was washed with 25 mM Tris-HCl/22 mM Na2SO4 (pH 8.7). The human carbonic anhydrase (hCA-VI) isozyme were eluted with 0,.25 M H2NSO3H / 25 mM Na2HPO4 (pH = 6,.7). All procedures were performed at 4°CCitation9–12.
Esterase activity assay
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a period of 3 min at 25°C using a spectrophotometer (CHEBIOS UV-VIS) according to the method described by Verpoorte et al.Citation28. The inhibitory effects of compounds 1–14 and AZA were examined. All compounds were tested in triplicate at each concentration used. Control cuvette activity in the absence of inhibitor was taken as 100%. For each inhibitor an Activity%- [Inhibitor] graph was drawn. To determine KI values, three different inhibitor concentrations were tested;. In these experiments, 4-nitrophenylacetate was used as substrate at five different concentrations (0.15–0.75 mM). The Lineweaver-Burk curves were drawnCitation29. Regression analysis graphs were drawn for IC50 using inhibition % values by a statistical package (SPSS-for windows; version 10.0) on a computer (Student’s t-test; n: 3).
Protein determination
Protein during the purification steps was determined spectrophotometrically at 595 nm according to the Bradford method, using bovine serum albumin as the StandardCitation30.
SDS polyacrylamide gel electrophoresis
SDS polyacrylamide gel electrophoresis was performed after purification of the enzymes. It was carried out in 10% and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to LaemmliCitation31.
Results and discussion
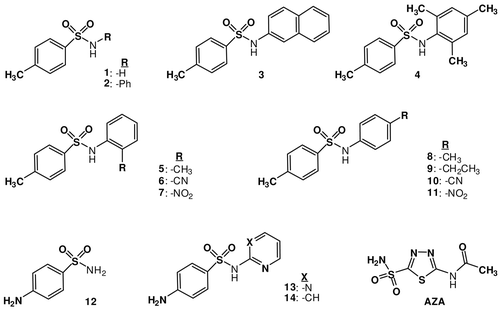
Benzenesulfonamide derivatives (1–14) investigated for the inhibition of CA I, II and CA VI isozymes. Compounds 1, 12–14 and AZA are clinically used compounds (). We (commercially available) have also been assayed compound (1, 12–14), since no such data are available in the literature.
Recently, Supuran’s group investigated the interactions of some methoxy-benzenesulfonamide derivatives and some of its substituted derivatives with isozymes, CA I, II and IXCitation32, evidencing some low micromolar/submicromolar inhibitors as well as the possibility to design isozyme selective CAIs. Indeed, the inhibition profile of various isozymes with this class of agents is very variable, with inhibition constants ranging from the millimolar to the submicromolar range for some methoxy-benzenesulfonamide or acid hydroxamide derivativesCitation32. It appeared thus of interest to extend the previous studiesCitation7,Citation9–14, including in this research some benzenesulfonamides with clinical applications, sulfapyridine 13, sulfadiazine 14, as well as some of its substituted derivatives incorporating amino, cyano, methyl and nitro moieties as substituents at the aromatic ring in different positions.
The purification of human erythrocyte CA I and II isoenzymes were performed using simple single-step method by Sepharose-4B-anilin-sulfanilamide affinity gel chromatographyCitation18. We report here the first study on the inhibitory effects of benzenesulfonamides (1–14) and AZA on the CA esterase activity. Data of show the following regarding inhibition of hCA-I, II and VI with compounds 1–15:
Table 1. hCA I, II and VI inhibition data with new sythetizedsynthesized sulfanilamine derivatives 1-12, sulfapyridine (13), sulfadiazine (14) and acetazolamide (15), by an esterase assay with 4-nitrophenylacetate as substrate.
(i) Against the slow cytosolic isozyme hCA I, sulfonamides 2–5 and 9 behave as weak inhibitors, with KI values in the range of 148.27–448.2 μM. Isoform hCA I was moderately inhibited by sulfonamides 1, 6, 8, 12–15 reported here. Thus, several derivatives, such as 1, 6 and 8, showed medium inhibitory activity, with inhibition constants in the range of 18.24–50.02 μM, in the same range as the clinically used compounds sulfapyridine (13), sulfadiazine (14) and acetazolamide (15) (KI of 26.19–36.2 μM for both compounds against this isoform, ). The remaining sulfonamides were more effective hCA I inhibitors as compared to derivatives discussed above, with KI-s in the range of 1.130–5.380 μM. Obviously, both the aromatic sulfonamide head as well as the aryl/hetaryl-acetyl moiety influence the biological activity of these hCA I inhibitorsCitation6. It may be observed that efficient inhibitors incorporate both sulfanilamide, 4-Methyl-N-(4-nitrophenyl) benzenesulfonamide and N-(4-cyanophenyl)-4-methylbenzenesulfonamide. Except for the five less active compounds mentioned above (2-5 and 9) which incorporate methylbenzenesulfonamide (1 or 8), cyanophenyl (6), 4-amino-pyridin (13) or amino-pyrimidin (14) moiety, the remaining compounds showed a quite compact behavior of moderately-efficient hCA I inhibitors. Thus, benzenesulfonamides lead to significant hCA I inhibition, but all these compounds possess KI-s > 0.100 μM, being thus only moderately active. Kinetic investigations (Lineweaver Burk plots, data not shown) indicate that similarly to sulfonamides and inorganic anionsCitation33–38, all the investigated compounds act as noncompetitive inhibitors with 4-NPA as substrate, i.e., they bind in different regions of the active site cavity as compared to the substrate. However, the binding site of 4-NPA itself is unknown, but it is presumed to be in the same region as that of CO2, the physiological substrate of this enzymeCitation39.
(ii) A rather strong activity of these compounds has been observed also for the inhibition of the rapid cytosolic isozyme hCA-II (). Indeed, again the same one compound (2) showed weaker hCA II inhibitory activity, with KI in the range of 14.3 μM, whereas compounscompounds 7, 8 behave as moderate inhibitors (KI-s in the range of 0.977–1.015 μM). It should be noted that the three clinically used compounds (13, 14 and AZA) and 1, 3–6, 9–12 show much more potent hCA II inhibitory activity, with KI-s in the range of 0.103–0.715 μM (). Theses finding clearly illustrate that a very big variation in the structure of a CAI (such as the presence of an additional CN, NO2 and CH3 moiety, in this case) may have drastic consequences for the enzyme inhibitory activity and selectivity profile against various isozymes of such derivatives. Thus, except for the one less active compound 2, all derivatives showed moderate or powerful hCA-II inhibitory activity ().
(iii) Sulfapyridine 13 and some of its congeners such as 1–9, and 14 are also weak inhibitors of the secreted isozyme hCA VI, with KI-s of 4.710–42.39 μM. However, again the compounds 10–12 are medium potency inhibitors (KI of 1.112–1.230 μM), and AZA (15) show a higher affinity for this isozyme, with inhibition constant in the range of 0.340 μM ().
3. Conclusions
Benzenesulfonamide derivatives 1–14 used in this study affect the activity of CA isozymes due to the presence of the different functional groups (CH3, CH2CH3, CN, NO2 and NH2) moieties present in their aromatic scaffold. It has been determined in our study that compounds 1–14 are effective inhibitors of hCA-II compared to AZA, which is used as reference inhibitor for carbonic anhydrase. Our findings here indicate thus the well-known class of possible CAIs of interest, sulfonamides/sulfamates/sulfamides. Indeed, some benzenesulfonamide derivatives investigated here showed effective CA I, II and VI inhibitory activity, in the low micromolar range, by the esterase method which usually gives KI-s an order of magnitude higher as compared to the CO2 hydrase assay.Citation13 Although benzenesulfonamide derivatives used in this study has previously synthesized, there was not such detailed spectroscopic data about these substances in previous works. Also, most of these derivatives could not be obtained in one-step with high yields. These findings point out that substituted benzenesulfonamide derivatives may be used as leads for generating potent CAIs eventually targeting other isoforms which have not been assayed yet for their interactions with such agents.
Declaration of interest
This study was financed by Turkish Republic Prime Ministry State Planning Organization (DPT), (project no: 2010K120440) and Agri Ibrahim Cecen University Scientific Research Council, (project no: Agri BAP-2010/K-10) for (MS).
References
- Ozensoy O, Arslan O, Sinan SO. A new method for purification of carbonic anhydrase isozymes by affinity chromatography. Biochemistry Mosc 2004;69:216–219.
- Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Hilvo M, Baranauskiene L, Salzano AM, Scaloni A, Matulis D, Innocenti A et al. Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J Biol Chem 2008;283:27799–27809.
- Supuran CT, Scozzafava A, Conway J. Carbonic Anhydrase-Its Inhibitors and Activators. CRC Press: Boca Raton, New York, London, 2004. pp. 1–363.
- Winum JY, Rami M, Scozzafava A, Montero JL, Supuran C. Carbonic anhydrase IX: A new druggable target for the design of antitumor agents. Med Res Rev 2008;28:445–463.
- Güzel O, Innocenti A, Scozzafava A, Salman A, Parkkila S, Hilvo M et al. Carbonic anhydrase inhibitors: Synthesis and inhibition studies against mammalian isoforms I-XV with a series of 2-(hydrazinocarbonyl)-3-substituted-phenyl-1H-indole-5-sulfonamides. Bioorg Med Chem 2008;16:9113–9120.
- Durdagi S, Sentürk M, Ekinci D, Balaydin HT, Göksu S, Küfrevioglu ÖI et al. Kinetic and docking studies of phenol-based inhibitors of carbonic anhydrase isoforms I, II, IX and XII evidence a new binding mode within the enzyme active site. Bioorg Med Chem 2011;19:1381–1389.
- Kivelä J, Parkkila S, Waheed A, Parkkila AK, Sly WS, Rajaniemi H. Secretory carbonic anhydrase isoenzyme (CA VI) in human serum. Clin Chem 1997;43:2318–2322.
- Sentürk M, Ekinci D, Göksu S, Supuran CT. Effects of dopaminergic compounds on carbonic anhydrase isozymes I, II, and VI. J Enzyme Inhib Med Chem 2011. (doi: 10.3109/14756366.2011.591290).
- Ekinci D, Cavdar H, Talaz O, Sentürk M, Supuran CT. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms I and II. Bioorg Med Chem 2010;18:3559–3563.
- Ekinci D, Ceyhun SB, Sentürk M, Erdem D, Küfrevioglu OI, Supuran CT. Characterization and anions inhibition studies of an a-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–748.
- Alp C, Ekinci D, Gültekin MS, Sentürk M, Sahin E, Küfrevioglu OI. A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis of carbonic anhydrase inhibitory potencies. Bioorg Med Chem 2010;18:4468–4474.
- Ekinci D, Ceyhun SB, Aksakal E, Erdogan O. IGF and GH mRNA levels are suppressed upon exposure to micromolar concentrations of cobalt and zinc in rainbow trout white muscle. Comp Biochem Physiol C Toxicol Pharmacol 2011;153:336–341.
- Ceyhun SB, Sentürk M, Ekinci D, Erdogan O, Ciltas A, Kocaman EM. δmethrin attenuates antioxidant defense system and induces the expression of heat shock protein 70 in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:215–223.
- Erdogan O, Ceyhun SB, Ekinci D, Aksakal E. Impact of δmethrin exposure on mRNA expression levels of metallothionein A, B and cytochrome P450 1A in rainbow trout muscles. Gene 2011;484:13–17.
- Ekinci D, Sentürk M, Beydemir S, Küfrevioglu OI, Supuran CT. An alternative purification method for human serum paraoxonase 1 and its interactions with sulfonamides. Chem Biol Drug Des 2010;76:552–558.
- Aksakal E, Ceyhun SB, Erdogan O, Ekinci D. Acute and long-term genotoxicity of δmethrin to insulin-like growth factors and growth hormone in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:451–455.
- Ceyhun SB, Aksakal E, Ekinci D, Erdogan O, Beydemir S. Influence of cobalt and zinc exposure on mRNA expression profiles of metallothionein and cytocrome P450 in rainbow trout. Biol Trace Elem Res. Doi: 10.1007/s12011-011-9068-z.
- Cakmak R, Durdagi S, Ekinci D, Sentürk M, Topal G. Design, synthesis and biological evaluation of novel nitroaromatic compounds as potent glutathione reductase inhibitors. Bioorg Med Chem Lett 2011;21:5398–5402.
- Aksakal E, Ekinci D, Erdogan O, Beydemir S, Alim Z, Ceyhun SB. Increasing stocking density causes inhibition of metabolic-antioxidant enzymes and elevates mRNA levels of heat shock protein 70 in rainbow trout. Livest Sci 2011. Doi:10.1016/j.livsci.2011.07.006.
- Siktar E, Ekinci D, Siktar E, Beydemir S, Gulcin I, Gunay M. Protective role of L-carnitine supplementation against exhaustive exercise-induced oxidative stress in rats. Eur J Pharmacol 2011. Doi: 10.1016/j.ejphar.2011.07.032.
- Casini A, Scozzafava A, Mincione F, Menabuoni L, Ilies MA, Supuran CT. Carbonic anhydrase inhibitors: Water-soluble 4-sulfamoylphenylthioureas as topical intraocular pressure-lowering agents with long-lasting effects. J Med Chem 2000;43:4884–4892.
- Cecchi A, Winum JY, Innocenti A, Vullo D, Montero JL, Scozzafava A et al. Carbonic anhydrase inhibitors: Synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with sulfonamides derived from 4-isothiocyanato-benzolamide. Bioorg Med Chem Lett 2004;14:5775–5780.
- Innocenti A, Casini A, Alcaro MC, Papini AM, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: The first on-resin screening of a 4-sulfamoylphenylthiourea library. J Med Chem 2004;47:5224–5229.
- Balaydin HT, Durdagi S, Ekinci D, Senturk M, Goksu S, Menzek A. Inhibition of human carbonic anhydrase isozymes I, II and VI with a series of bisphenol, metoxy and bromophenol compounds. J Enzym Inhib Med Ch 2011. Doi:10.3109/14756366.2011.596836.
- Senturk M, Kufrevioglu OI. In vitro effects of some nucleoside analogue drugs on enzyme activities of carbonic anhydrase isozymes I and II from human erythrocytes. Hacettepe J Biol Chem 2009;37:289–294.
- Park KK, Lee JJ, Ryu J. Photo-Fries rearrangement of N-arylsulfonamides to aminoaryl sulfone derivatives. Tetrahedron 2003;59:7651–7659.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–4229.
- Lineweaver H, Burk D. The determination of enzyme dissocation constants. J Am Chem Soc 1934;56:658–666.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685.
- Nuti E, Orlandini E, Nencetti S, Rossello A, Innocenti A, Scozzafava A et al. Carbonic anhydrase and matrix metalloproteinase inhibitors. Inhibition of human tumor-associated isozymes IX and cytosolic isozyme I and II with sulfonylated hydroxamates. Bioorg Med Chem 2007;15:2298–2311.
- Briganti F, Pierattelli R, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Part 37. Novel classes of isozymes I and II inhibitors and their mechanism of action. Kinetic and spectroscopic investigations on the native and cobalt-substituted enzymes. Eur J Med Chem 1996;31:1001–1010.
- Sentürk M, Gülçin I, Dastan A, Küfrevioglu OI, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–3211.
- Balaydin HT, Soyut H, Ekinci D, Goksu S, Beydemir S, Menzek A, Sahin E. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols including natural products. J Enzym Inhib Med Ch 2011. Doi: 10.3109/14756366.2011.574131.
- Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 1995;64:375–401.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: Current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Ceyhun SB, Sentürk M, Yerlikaya E, Erdogan O, Küfrevioglu OI, Ekinci D. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 2011;32:69–74.
- Nair SK, Ludwig PA, Christianson DW. Two-site binding of phenol in the active site of human carbonic anhydrase II: Structural implications for substrate association. J Am Chem Soc 1994;116:3659–3660.