Abstract
A series of dihydro-pyrimidine-5-carbonitrile derivatives (3–16) were synthesized and evaluated for their anticonvulsant activity against MES and scPTZ models. Motor impairment screening was carried out by rotarod test method and CNS depressant effect was determined by Porsolt’s force swim pool method. Compounds 4 and 9 having p-substituted bromo and m-substituted nitro groups, respectively, were found to be most active showing activity both in MES and scPTZ screen at lower doses of 30 mgkg−1 at 0.5 h and 100 mgkg−1 at 4 h. In the rotarod motor impairment screen, compound 4 did not show any motor impairment even at the maximum dose of 300 mgkg−1; however, compound 9 showed motor impairment at 300 mgkg−1 dose after 4.0 h. The compounds were also tested for their CNS depression effect. The compounds 4 and 9 showed 41.38 and 43.44% increase in immobility time with respect to control. The pharmacophore hypothesis also fits best for compounds 4 and 9.
Introduction
Epilepsy is a common neurological disorder characterized by excessive temporary neuronal discharges resulting in unpredictable recurrence of unprovoked seizures. At present, phenytoin is still a drug of choice after its discovery over a period of more than half centuryCitation1. Although several new anticonvulsants are already in clinical use, some types of seizure are still not adequately treated with current therapy. Current drug therapy is accompanied by numerous side effects including drowsiness, ataxia, gastrointestinal disturbances, gingival hyperplasia, hirsutism and megaloblastic anaemiaCitation2,Citation3. These facts give the field of anticonvulsant drug discovery high priority.
These observations place new emphasis on the need as well as search for alternative new and more effective anticonvulsant agents with reduced neurotoxicity and CNS depression effect. Among a wide variety of compounds that have been explored for developing pharmaceutically important anticonvulsant agents, Schiff’s bases have played an important role.
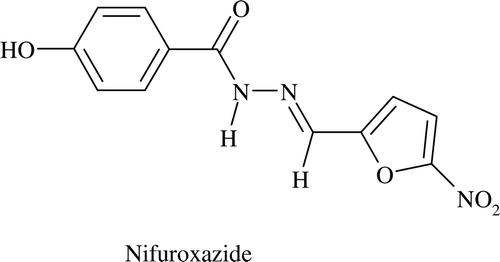
In recent years, Schiff’s bases have gained much attention due to its wide spectrum of biological activities like anticonvulsantCitation4,Citation5, antidepressantCitation6, anti-inflammatoryCitation7, antimalarialCitation8, antimycobacterialCitation9 etc. Nifuroxazide (), an intestinal antisepticCitation10, is a Schiff’s base.
Cakir B. et al.11 have synthesized fifteen Schiff’s bases and out of them N’-(4-fluoro-benzylidene)-2-(2-oxobenzo[d]oxazol-3(2H)-yl) aceto-hydrazide () was found to be a very active anticonvulsant.
According to the literatureCitation12, the compounds with at least one aromatic ring (R), one electron donor (D) and a hydrogen bond acceptor or donor (HBD) combination were found to possess very good anticonvulsant activity. The generated pharmacophore using 3.1 module of Schrodinger-913 showed the presence of all crucial structural components required for a compound to be an anticonvulsant.
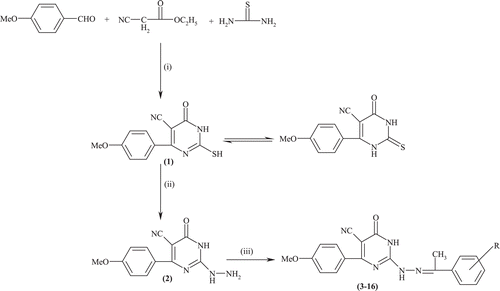
Owing to the versatility of Schiff’s bases and the support of pharmacophore modeling, we have synthesized fourteen new 2-(2-{substituted benzylidene} hydrazinyl)-4-(4-methoxyphenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrileas outlined in . All the final compounds were structurally confirmed on the basis of IR, 1H-NMR and mass spectral data and the final synthesized compounds were evaluated for their anticonvulsant effect in both MES14,15 and scPTZ methodCitation16. They were also tested for their side effects i.e. neurotoxicity effect by rotarod testCitation17 and CNS depression effect by Porsolt’s swim pool testCitation18.
Methods
Chemistry
Chemicals were purchased from Merck and Sigma-Aldrich as ‘synthesis grade’ and used without further purification. Melting points were determined in open capillary tubes and are uncorrected. Elemental analyses were performed on a Perkin-Elmer model 240 analyzer and found within ±0.4% of theoretical values. The IR spectra were measured as potassium bromide pellets using a Buck Scientific M-500 Infrared spectrophotometer. 1H-NMR spectra were recorded in DMSO as a solvent (using TMS as an internal standard). 13C-NMR of compound 3 and 4 were recorded as a prototype using DMSO as a solvent. The NMR and mass spectra were recorded on Bruker avance-400 MHz and JEOL BX 102/DA-6000 mass spectrometers, respectively. Purity of the compounds was checked by TLC on silica gel G plates using toluene: ethyl acetate: formic acid (5:4:1) as solvent system and the spots were located either under ultra violet light or through exposure to iodine vapors.
The synthesis of title compounds involves three steps and is exemplified by the synthesis of compound 3.
Synthesis of 2-Mercapto-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (1)
Anisaldehyde (1 mmol), ethyl cyanoacetate (1 mmol) and thiourea (1 mmol) were dissolved in absolute alcohol. Potassium carbonate (3 mmol) was added to this reaction mixture and refluxed for 2 h. The solvent was concentrated and poured into ice cold water with stirring. The solution was neutralized with glacial acetic acid, which causes the separation of compound 1 which was filtered, washed with water and recrystallized from methanol. Yield:90%; mp 139–40°C; Rf 0.6;IR (cm−1): 3245 (-NH of amide), 3215 (-NH), 2225 (C≡N), 1674 (C=O), 1165 (C=S);1H-NMR (δ, ppm): 3.83(s, 3H, OCH3), 6.87 (d, 2H, J = 8.4 Hz, H-3,5, Phenyl), 7.86 (d, 2H, J = 8.4 Hz, H-2,6, Phenyl), 9.60 (s, 1H, NH), 12.06 (bs, 1H, NH-C=O).
Synthesis of 2-Hydrazinyl-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (2)
Compound 1 (1 mmol) was dissolved absolute ethanol and to it hydrazine hydrate (99%; 4 mmol) was added and refluxed for 1 h. The reaction mixture was allowed to cool which causes the separation of solid. The precipitate product was filtered and washed with water. It was recrystallized with ethanol. Yield:82%; mp 180°C; Rf 0.2;IR (cm−1): 3285–3218 (2NH+NH2), 2210 (C≡N), 1680 (C=O), 1065 (C-O-C);1H-NMR (δ, ppm): 3.84(s, 3H, OCH3), 3.97 (bs, 2H, NHNH2),6.93 (d, 2H, J = 8.4 Hz, H-3,5, phenyl), 7.77 (d, 2H, J = 8.4 Hz, H-2,6, phenyl), 10.34 (bs, 1H, NH ) 11.71 (bs, 1H, NH-C=O).
Synthesis of 2-(2-{1-phenyl-ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (3)
Compound 2 (1 mmol) was dissolved in a mixture of glacial acetic acid and alcohol (2:8). To this solution, alcoholic solution of acetophenone (1.1 mmol) was added and refluxed for 2–3 h. Solvent was concentrated to half of its volume, poured into ice water. The precipitate obtained was filtered, washed with water and recrystallized from methanol. Mp 203–04°C, Rf-0.66, %Yield-68; IR (cm−1): 3310 (CONH), 3240 (NH), 2218 (C≡N), 1679 (C=O), 1604 (C=N); 1H-NMR (δ, ppm):2.44 (s, 3H, CH3), 3.89 (s, 3H, OCH3), 7.04 (d, 2H, J = 8.8 Hz, H3,5), 7.41–7.43 (m, 3H, H3′,4′,5′), 7.93 (d, 1H, J = 8.4 Hz, H6′), 7.97 (d, 2H, J = 8.8 Hz, H2,6), 8.05 (d, 2H, J = 8.0 Hz, H2′), 11.43 (bs, 1H, NH), 11.71 (bs, 1H, CONH);
13C-NMR (δ, ppm): 15.13, 55.61, 113.73, 127.30, 128.34, 129.88, 130.68, 137.50, 162.05; Mass (m/z) 359(M+). Anal. Calcd. for C20H17N5O2:C, 66.84; H, 4.77; N,19.49. Found: C, 67.02; H, 4.78; N, 19.51.
The remaining compounds were synthesized with analogous procedure.
2-(2-{1-[4-Bromo-phenyl]ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile(4) mp 255–56°C, Rf-0.65, %Yield-66; IR (cm−1): 3346 (CONH), 3311 (NH), 2213 (C≡N), 1675 (C=O), 1608 (C=N); 1H-NMR (δ, ppm):2.40 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 6.94 (d, 2H, J = 8.8 Hz, H3,5), 7.39 (d, 2H, J = 8.0 Hz, H3′,5′), 7.62 (d, 2H, J = 8.0 Hz, H2′,6′), 7.91 (d, 2H, J = 8.8 Hz, H2,6), 11.08 (bs, 1H, NH), 11.68 (bs, 1H, CONH);
13C-NMR (δ, ppm): 14.87, 55.56, 113.66, 123.68, 129.08, 130.66, 131.28, 136.54, 162.04; Mass (m/z) 43 8(M+), 440 (M+2). Anal. Calcd. for C20H16BrN5O2:C, 54.81; H, 3.68; N, 15.98. Found: C, 54.96; H, 3.67; N, 15.97.
2-(2-{1-[3-Bromo-phenyl]ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (5) mp 265–66°C, Rf-0.65, %Yield-72; IR (cm−1): 3308 (CONH), 3234 (NH), 2225 (C≡N), 1676 (C=O), 1604 (C=N); 1H-NMR (δ, ppm):2.42 (s, 3H, CH3), 3.84 (s, 3H, OCH3), 6.99 (d, 2H, J = 8.8 Hz, H3,5), 7.37 (dd, 1H, J = 8.0, 7.6 Hz, H5′), 7.51 (d, 1H, J = 8.0 Hz, H6′), 7.57 (d, 1H, J = 7.6 Hz, H4′), 7.79 (s, 1H, H2′), 7.95 (d, 2H, J = 8.4 Hz, H2,6), 11.26 (bs, 1H, NH), 11.96 (bs, 1H, CONH); Mass (m/z) 438(M+), 440 (M+2). Anal. Calcd. for C20H18BrN5O2:C, 54.56; H, 4.12; N, 15.98. Found: C, 54.72; H, 4.11; N, 15.98.
2-(2-{1-[4-Fluoro-phenyl]ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (6) mp 271–72°C, Rf−0.64, %Yield-60; IR (cm−1): 3319 (CONH), 3284 (NH), 2220 (C≡N), 1672 (C=O), 1605 (C=N); 1H-NMR (δ, ppm):2.43 (s, 3H, CH3), 3.90 (s, 3H, OCH3), 7.03 (d, 2H, J = 8.4 Hz, H3,5), 7.16 (t, 2H, J = 8.4 Hz, H3′,5′), 7.93 (d, 2H, J = 8.4 Hz, H2,6), 8.07 (d, 2H, J = 8.4 Hz, H2′,6′), 10.49 (bs, 1H, NH), 11.78 (bs, 1H, CONH); Mass (m/z) 377(M+). Anal. Calcd. for C20H16FN5O2:C, 63.66; H, 4.27; N, 18.56. Found: C, 63.75; H, 4.28; N, 18.57.
2-(2-{1-[4-Chloro-phenyl]ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (7) mp 235–36°C, Rf-0.64, %Yield-75; IR (cm−1): 3316 (CONH), 3281 (NH), 2219 (C≡N), 1678 (C=O), 1607 (C=N); 1H-NMR (δ, ppm):2.42 (s, 3H, CH3), 3.79 (s, 3H, OCH3), 6.95 (d, 2H, J = 8.4 Hz, H3,5), 7.38 (d, 2H, J = 8.0 Hz, H2′,6′), 7.69 (d, 2H, J = 8.0 Hz, H3′,5′), 7.83 (d, 2H, J = 8.4 Hz, H2,6), 10.52 (bs, 1H, NH), 11.46 (bs, 1H, CONH); Mass (m/z) 394(M+), 396(M+2). Anal. Calcd. for C20H16ClN5O2:C, 61.00; H, 4.09; N, 17.78. Found: C, 61.13; H, 4.08; N, 17.77.
2-(2-{1-[4-Nitro-phenyl]ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (8) mp 241–42°C, Rf-0.65, %Yield-65; IR (cm−1): 3353 (CONH), 3273 (NH), 2224 (C≡N), 1668 (C=O), 1613 (C=N); 1H-NMR (δ, ppm):2.36 (s, 3H, CH3), 3.85 (s, 3H, OCH3), 6.96 (d, 2H, J = 8.4 Hz, H3,5), 7.68 (d, 2H, J = 8.8 Hz, H2′,6′), 7.94 (d, 2H, J = 8.4 Hz, H2,6), 8.26 (d, 2H, J = 8.8 Hz, H3′,5′), 11.39 (bs, 1H, NH), 11.92 (bs, 1H, CONH); Mass (m/z) 404(M+). Anal. Calcd. for C20H16N6O4:C, 59.40; H, 3.99; N, 20.78. Found: C, 59.27; H, 3.98; N, 20.77.
2-(2-{1-[3-Nitrophenyl]ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (9) mp 245–46°C, Rf-0.64, %Yield-75; IR (cm−1): 3326 (CONH), 3276 (NH), 2227 (C≡N), 1683 (C=O), 1607 (C=N); 1H-NMR (δ, ppm):2.39 (s, 3H, CH3), 3.86 (s, 3H, OCH3), 7.04 (d, 2H, J = 8.8 Hz, H3,5), 7.62 (dd, 1H, J = 8.8, 8.4 Hz, H5′), 7.93 (d, 2H, J = 8.8 Hz, H2,6), 8.09 (d, 1H, J = 8.8 Hz, H6′), 8.24 (d, 1H, J = 8.4 Hz, H4′), 8.37 (s, 1H, H2′), 10.36 (bs, 1H, NH), 12.21 (bs, 1H, CONH); Mass (m/z) 404(M+). Anal. Calcd. for C20H16N6O4:C, 59.40; H, 3.99; N, 20.78. Found: C, 59.25; H, 3.98; N, 20.79.
2-(2-{1-[2-Nitro-phenyl]ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (10) mp 241–42°C, Rf-0.64, %Yield-80; IR (cm−1): 3342 (CONH), 3251 (NH), 2222 (C≡N), 1689 (C=O), 1602 (C=N); 1H-NMR (δ, ppm):2.36 (s, 3H, CH3), 3.90 (s, 3H, OCH3), 6.97 (d, 2H, J = 8.4 Hz, H3,5), 7.58 (t, 1H, J = 7.6 Hz, H4′), 7.73 (t, 1H, J = 8.0 Hz, H5′), 7.82 (d, 1H, J = 8.4 Hz, H6′), 7.89 (d, 2H, J = 8.44 Hz, 44 Hz, H2,6), 8.13 (d, 1H, J = 8.0 Hz, H3′), 1110.21 (bs, 1H, NH), 11.64 (bs, 1H, CONH); Mass (m/z) 404(M+). Anal. Calcd. for C20H16N6O4:C, 59.40; H, 3.99; N, 20.78. Found: C, 59.31; H, 4.00; N, 20.77.
2-(2-{1-[4-Hydroxy-phenyl]ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (11) mp 251–52°C, Rf-0.66, %Yield-76; IR (cm−1): 3418 (OH), 3345 (CONH), 3246 (NH), 2215 (C≡N), 1674 (C=O), 1606 (C=N); 1H-NMR (δ, ppm):2.26 (s, 3H, CH3), 3.78 (s, 3H, OCH3), 6.71 (d, 2H, J = 8.4 Hz, H3′,5′), 7.00 (d, 2H, J = 8.4 Hz, H3,5), 7.80 (d, 2H, J = 8.4 Hz, H2′,6′), 7.85 (d, 2H, J = 8.4 Hz, H2,6), 9.96 (s, 1H, OH), 11.25 (bs, 1H, NH), 11.73 (bs, 1H, CONH); Mass (m/z) 375(M+). Anal. Calcd. for C20H17N5O3:C, 63.99; H, 4.56; N, 18.66. Found: C, 63.79; H, 4.57; N, 18.67.
2-(2-{1-[2-Hydroxy-phenyl]ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (12) mp 213–14°C, Rf-0.67, %Yield-70; IR (cm−1): 3426 (OH), 3334 (CONH), 3264 (NH), 2215 (C≡N), 1683 (C=O), 1606 (C=N); 1H-NMR (δ, ppm):2.28 (s, 3H, CH3), 3.85 (s, 3H, OCH3), 6.95–7.02 (m, 4H, H3,5,3′,5′), 7.42 (t, 1H, J = 7.6 Hz, H4′), 7.73 (d, 1H, J = 8.0 Hz, H6′), 7.87 (d, 2H, J = 8.4 Hz, H2,6), 10.334 (s, 1H, OH), 11.13 (bs, 1H, NH), 12.01 (bs, 1H, CONH); Mass (m/z) 375(M+). Anal. Calcd. for C20H17N5O3:C, 63.99; H, 4.56; N, 18.66. Found: C, 63.74; H, 4.55; N, 18.67.
2-(2-{1-[4-Methoxy-phenyl]ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (13) mp 223–24°C, Rf-0.67, %Yield-68; IR (cm−1): 3287 (CONH), 3229 (NH), 2216 (C≡N), 1671 (C=O), 1603 (C=N); 1H-NMR (δ, ppm):2.39 (s, 3H, CH3), 3.81 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 6.90 (d, 2H, J = 7.6 Hz, H3′,5′), 6.97 (d, 2H, J = 8.4 Hz, H3,5), 7.76 (d, 2H, J = 7.6 Hz, H2′,6′), 7.84 (d, 2H, J = 8.4 Hz, H2,6), 11.13 (bs, 1H, NH), 11.84 (bs, 1H, CONH); Mass (m/z) 389(M+). Anal. Calcd. for C21H19N5O3:C, 64.77; H, 4.92; N, 17.98. Found: C, 64.93; H, 4.91; N, 17.97.
2-(2-{1-[3,4-Dimethoxy-phenyl]ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (14) mp 167–68°C, Rf-0.67, %Yield-78; IR (cm−1): 3318 (CONH), 3265 (NH), 2221 (C≡N), 1677 (C=O), 1610 (C=N); 1H-NMR (δ, ppm):2.41 (s, 3H, CH3), 3.88 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 6.93 (d, 1H, J = 8.4 Hz, H5′), 6.99 (d, 2H, J = 8.8 Hz, H3,5), 7.47 (s, 1H, H2′), 7.58 (d, 1H, J = 8.4 Hz, H6′), 7.93 (d, 2H, J = 8.8 Hz, H2,6), 11.29 (bs, 1H, NH), 12.13 (bs, 1H, CONH); Mass (m/z) 419(M+). Anal. Calcd. for C22H21N5O4:C, 63.00; H, 5.05; N, 16.70. Found: C, 63.17; H, 5.04; N, 16.71.
2-(2-{1-[2,4-Dimethoxy-phenyl]ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (15) mp 161–62°C, Rf-0.66, %Yield-82; IR (cm−1): 3319 (CONH), 3236 (NH), 2218 (C≡N), 1674 (C=O), 1609 (C=N); 1H-NMR (δ, ppm):2.40 (s, 3H, CH3), 3.88 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 6.81 (s, 1H, H3′), 6.91 (d, 1H, J = 7.6 Hz, H5′), 6.99 (d, 2H, J = 8.4 Hz, H3,5), 7.32 (d, 1H, J = 7.6 Hz, H6′), 7.91 (d, 2H, J = 8.4 Hz, H2,6), 11.11 (bs, 1H, NH), 11.72 (bs, 1H, CONH); Mass (m/z) 419(M+). Anal. Calcd. for C22H21N5O4:C, 63.00; H, 5.05; N, 16.70. Found: C, 63.12; H, 5.04; N, 16.69.
2-(2-{1-[4-Methyl-phenyl]ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile(16) mp 215–16°C, Rf-0.64, %Yield-74; IR (cm−1): 3320 (CONH), 3274 (NH), 2223 (C≡N), 1681 (C=O), 1601 (C=N); 1H-NMR (δ, ppm):2.31 (s, 3H, CH3), 2.39 (s, 3H, CH3), 3.81 (s, 3H, OCH3), 6.98 (d, 2H, J = 8.4 Hz, H3,5), 7.21 (d, 2H, J = 7.6 Hz, H3′,5′), 7.47 (d, 2H, J = 7.6 Hz, H2′,6′), 7.89 (d, 2H, J = 8.4 Hz, H2,6), 11.27 (bs, 1H, NH), 11.81 (bs, 1H, CONH); Mass (m/z) 373(M+). Anal. Calcd. for C21H19N5O2:C, 67.55; H, 5.13; N, 18.75. Found: C, 67.45; H, 5.14; N, 18.76.
Pharmacology
Swiss albino mice used in the present study were housed and kept in accordance with the Hamdard University Animal Care Unit, which applies the guidelines and rules laid down by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India. Albino mice of either sex weighing 22–25 g were used. The animals were housed in groups of six and acclimatized to room conditions for at least 2 days before the experiments. Food and water were freely available up to the time of experiments except during the short time they were removed from the cages for testing.
Anticonvulsant activity
The synthesized compounds were evaluated for their anticonvulsant activity using maximal electroshock (MES14,15) and scPTZ methodCitation16. The convulsion was measured using Convulsiometer.
Maximal electroshock (MES) test
Maximal electroshock seizureCitation14,Citation15 was elicited with a 60 cycle altering current of 50 mA intensity delivered for 0.25 s via ear clip electrodes. Animals were previously given the test drug i.p. Abolition of the hind limb tonic extension spasm was recorded as the anticonvulsant activity. In preliminary screening, each compound was administered through an i.p. injection at three dose levels (30, 100 and 300 mgkg−1 body mass) and the anticonvulsant activity was assessed after 0.5 and 4 h intervals of administration.
Subcutaneous pentylenetetrazole (scPTZ) seizure threshold test
scPTZCitation16 was conducted by administering PTZ dissolved in 0.9% sodium chloride solution in the posterior midline of the animals. A minimal time of 30 min subsequent to s.c. administration of PTZ was used for seizure detection. Protection was referred to as the failure to observe an episode of clonic spasms of at least 5 s during this time period.
Neurotoxicity
The synthesized drugs interference with motor coordination was checked by the rotarod test at a dose level of 30, 100 and 300 mgkg−1 body mass. In that test, mice were trained to stay on the knurled plastic rod having diameter of 3.2 cm. Normal mice could maintain equilibrium on the rotating rod for longer period of time. The neurotoxicityCitation17 was indicated by inability of mice to maintain equilibrium on the rod for at least 1 min in each of the three trials. The dose which impairs the ability of 50% of the animals to remain on the revolving rod was considered the end point.
CNS depressant study
The forced swim pool method (Porsolt’s swim pool test) was followed to study CNS depressionCitation18. Male albino mice were placed in a chamber (diameter 45 cm, height: 20 cm) containing water up to a height of 15 cm at 25 ± 2°C. Two swim sessions were conducted, an initial 15 min pre-test, followed by a 5-min test session 24 h later. The animals were administered an i.p. injection (100 mgkg−1) of the test compounds 30 min before the test session. The period of immobility (passive floating without struggling, making only those movements which are necessary to keep its head above the surface of water) during the 5-min test period was measured.
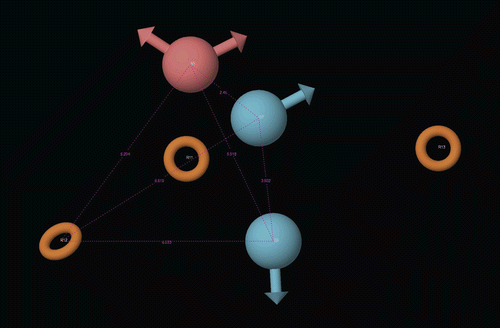
Pharmacophore modeling
Various 3D pharmacophoreCitation13 hypotheses have been generated using Phase 3.1 module of Schrodinger-9 and pharmacophore distance was compared with average distance for existing anticonvulsant drugsCitation12. The data related to this are presented in .
Result and discussion
A series of 2-(2-{1-phenyl-ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile derivatives (3–16) were synthesized by refluxing 2-hydrazino-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile (2) with different substituted acetophenones in glacial acetic acid and alcohol mixture 2:8).
The required intermediate compound 2 was synthesized from 2-mercapto-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile 1 upon nucleophilic attack by the hydrazine hydrate. The compound 1 was synthesized by modified Biginelle condensationCitation19 method using anisaldehyde, ethyl cyanoacetate and thiourea in presence of potassium carbonate.
In general, the IR spectral data of all the compounds showed characteristic peaks around 3346 cm−1 for -NH of amide group, 2224 cm−1 for C≡N and 1669 cm−1 for C=O indicatingthe formation of cyanopyrimidinone. Similarly, peak around 1617 cm−1 indicates formation of Schiff’s base. In 1H-NMR spectral data, all the compounds showed characteristic peak at appropriate δ-values.
The synthesized compounds were screened for their anticonvulsant activity against two standard models MES and scPTZ for their ability to reduce seizure spread and to evaluate seizure threshold, respectively. Motor impairment screening was carried out by rotarod test method and CNS depressant effect of the compounds was determined by Porsolt’s force swim pool method. The anticonvulsant activity was reported after 0.5 and 4.0 h time intervals at dose levels of 30, 100 and 300 mgkg−1 body weight. The CNS depressant was studied at a dose level of 100 mgkg−1 body weight. Phenytoin and carbamazepine were used as standard drugs.
In this series, compounds 4 and 9 having p-substituted bromo and m-substituted nitro groups, respectively, were found to be most active of the series showing activity both in MES and scPTZ screen at lower doses of 30 mgkg−1 at 0.5 h and 100 mgkg−1 at 4 h. In the rotarod motor impairment screen, compound 4 did not show any motor impairment even at the maximum dose of 300 mgkg−1; however, compound 9 showed motor impairment at 300 mgkg−1 dose after 4.0 h.
Some selected compounds having significant anticonvulsant activity were also tested for their CNS depressant effect. The compounds 4 and 9 showed 41.38 and 43.44% increase in immobility time with respect to control where as standard drug carbamazepine showed 45.18% increase in the immobility time (). Rest of the tested compounds also showed a good increase in immobility time.
Table 1. Anticonvulsant, neurotoxicity screening and CNS depression study of compounds 3–16.
Table 2. Comparison of distance between the essential structural elements of existing drugs and synthesized compounds.
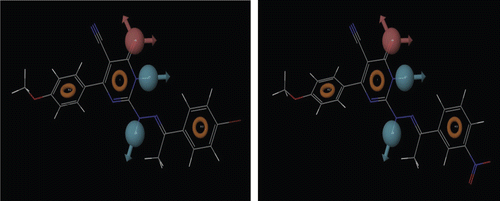
The generated pharmacophore using Phase 3.1 module of Schrodinger-9 also showed the presence of all crucial structural components i.e. the aryl ring centre or the lipophilic group (R12), an electron donor atom (D6), a hydrogen bond acceptor (HA) and a hydrogen bond donor (HD7) required for a compound to be an anticonvulsant. On the basis of fitness of the highest active ligands i.e. compound 4 and 9, ADDRRR-24 pharmacophore hypothesis was selected () and the selected pharmacophore hypothesis was found to fit best for most active ligands which can be visualized by .
The result indicates that compounds 4 and 9 are the most active compounds of this study as it is effective in both MES and scPTZ screen showing no motor impairment effect. The compound 4 and 9 also showed reduced CNS depressant effect in comparison to standard drug carbamazepine.
Conclusion
Fourteen new 2-(2-{1-phenyl-ethylidene}hydrazinyl)-4-(4-methoxy-phenyl)-6-oxo-1,6-dihydro-pyrimidine-5-carbonitrile derivatives were successfully synthesized. Biological evaluation results showed that the compounds are promising anti-convulsant agents with no motor impairment and good decrease in immobility time indicating that the synthesized molecules have lesser CNS depressant effect.
Among the newer derivatives, two compounds 4 and 9 emerged as lead compounds. The synthesized compounds were also supported by the pharmocophore model.
Acknowledgements
The authors are thankful to Jamia Hamdard (New Delhi) for providing research facilities to pursue this work.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Meritt HH, Putnam JJ. Experimental determination of the anticonvulsant properties of some phenyl derivatives. Science 1937;85:525–526.
- Brodie MJ. Established anticonvulsants and treatment of refractory epilepsy. Lancet 1990;336:350–354.
- Eadia MJ. Anticonvulsant drugs: An update. Drugs 1984;27:328–363.
- Ragavendran JV, Sriram D, Patel SK, Reddy IV, Bharathwajan N, Stables J et al. Design and synthesis of anticonvulsants from a combined phthalimide-GABA-anilide and hydrazone pharmacophore. Eur J Med Chem 2007;42:146–151.
- Arzu K, Meral O, Şamil I, James PS, Sevim D. Synthesis, anticonvulsant and antimicrobial activities of some new 2-acetylnaphthalene derivatives. Bioorg Med Chem2010;18:2902–2911.
- Ergenc N, Gunay NS. Synthesis and antidepressant evaluation of new 3-phenyl-5-sulfonamidoindole derivatives. Eur J Med Chem 1998;33:143–148.
- Duarte CD, Tributino JL, Lacerda DI, Martins MV, Alexandre-Moreira MS, Dutra F et al. Synthesis, pharmacological evaluation and electrochemical studies of novel 6-nitro-3,4-methylenedioxyphenyl-N-acylhydrazone derivatives: Discovery of LASSBio-881, a new ligand of cannabinoid receptors. Bioorg Med Chem 2007;15:2421–2433.
- Bernardino AM, Gomes AO, Charret KS, Freitas AC, Machado GM, Canto-Cavalheiro MM et al. Synthesis and leishmanicidal activities of 1-(4-X-phenyl)-N’-[(4-Y-phenyl)methylene]-1H-pyrazole-4-carbohydrazides. Eur J Med Chem 2006;41:80–87.
- Imramovský A, Polanc S, Vinsová J, Kocevar M, Jampílek J, Recková Z et al. A new modification of anti-tubercular active molecules. Bioorg Med Chem 2007;15:2551–2559.
- Rollas S, Gulerman N, Erdeniz H. Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines. Farmaco 2002;57:171–174.
- Cakir B, Dag O, Yildirim E, Erol K, Sahin MF. Synthesis and anticonvulsant activity of some hydrazones of 2-[(3H)-oxobenzoxazolin-3-yl-aceto]hydrazide. J Fac Pharm Gazi 2001;18:99–106.
- Yogeeswari P, Sriram D, Thirumurugan R, Raghavendran JV, Sudhan K, Pavana RK et al. Discovery of N-(2,6-dimethylphenyl)-substituted semicarbazones as anticonvulsants: hybrid pharmacophore-based design. J Med Chem 2005;48:6202–6211.
- Phase, version 3.1, Schrodinger, LLC, New York, NY, 2009.
- Krall RL, Penry JK, White BG, Kupferberg HJ, Swinyard EA. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia 1978;19:409–428.
- Löscher W, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. II. Maximal electroshock seizure models. Epilepsy Res 1991;8:79–94.
- Porter RJ, Cereghino JJ, Gladding GD, Hessie BJ, Kupferberg HJ, Scoville B et al. Antiepileptic Drug Development Program. Cleve Clin Q 1984;51:293–305.
- Yogeeswari P, Sriram D, Saraswat V, Ragavendran JV, Kumar MM, Murugesan S et al. Synthesis and anticonvulsant and neurotoxicity evaluation of N4-phthalimido phenyl (thio) semicarbazides. Eur J Pharm Sci 2003;20:341–346.
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 1978;47:379–391.
- Kappe CO. 100 years of the biginelli dihydropyrimidine synthesis. Tetrahedron 1993;49:6937.
- Arpana R, Nadeem S, Suroor AK, Haque SE, Bhat MA. N-{[(6-Substituted-1,3-benzothiazole-2-yl)amino]carbonothioyl}-2/4-substituted benzamides: Synthesis and pharmacological evaluation. Eur J Med Chem 2008;43:1114–1122.
- Flaherty PT, Greenwood TD, Manheim AL, Wolfe JF. Synthesis and evaluation of N-(phenylacetyl)trifluoromethanesulfonamides as anticonvulsant agents. J Med Chem 1996;39:1509–1513.
![Figure 2. N’-(4-fluoro-benzylidene)-2-(2-oxobenzo[d]oxazol-3(2H)-yl) aceto-hydrazide.](/cms/asset/5968e069-e358-451d-9dc3-4d3cdd7fc2ba/ienz_a_618129_f0002_b.gif)