Abstract
Cruzain is the major cysteine protease of Trypanosoma cruzi, the infectious agent responsible for Chagas disease, and cruzain inhibitors display considerable antitrypanosomal activity. In the present work we elucidated crystallographic data of fukugetin, a biflavone isolated from Garcinia brasiliensis, and investigated the role of this molecule as cysteine protease inhibitor. The kinetic analyses demonstrated that fukugetin inhibited cruzain and papain by a slow reversible type inhibition with KI of 1.1 and 13.4 µM, respectively. However, cruzain inhibition was about 12 times faster than papain inhibition. Lineweaver–Burk plots demonstrated partial competitive inhibition for cruzain and hyperbolic mixed-type inhibition for papain. Furthermore, the docking results showed that the biflavone binds to ring C′ in the S2 pocket and to ring C in the S3 pocket through hydrophobic interactions and hydrogen bonds. Finally, fukugetin also presented inhibitory activity on proteases of the T. cruzi extract, with IC50 of 7 µM.
| Abbreviations | ||
| IC50, | = | inhibitor concentration to decrease 50% enzymatic activity |
| KI, | = | inhibition constant |
| Vmax | = | maximum velocity |
| Km, | = | Michaelis–Menten constant |
| Kobs, | = | apparent first-order rate constant |
| E, | = | enzyme |
| I, | = | inhibitor |
| kcat, | = | catalytic constant |
| S, | = | substrate |
| E64, | = | L-trans-epoxysuccinyl-leucylamido (4-guanidino)butane |
| MCA, | = | 7-amino-4-methylcoumarin |
| EDTA, | = | ethylenediaminetetraacetic acid |
| DM,F | = | dimethylformamide |
| r.m.s., | = | root mean square. |
Introduction
The major protease of Trypanosoma cruzi is cruzain, a recombinant form of cruzipain or GP57/51, a cysteine protease of the cathepsin L-like papain structure. Cruzain is a member of a large family of closely-related isoforms found in the parasiteCitation1,Citation2. It is involved in intracellular replication and differentiation, and is essential at all stages of the parasite’s life cycle. Inhibitors of cruzainCitation3–6 display considerable antitrypanosomal activityCitation7,Citation8 and some classes have been shown to cure T. cruzi infection in mouse modelsCitation7–10. Since currently available therapeutics is practically ineffective in the acute phase of the disease besides being highly toxic to be safely administered for long periods, efforts have been made with a view to characterizing new therapeutic targets, among which the proteolytic apparatus of T. cruzi is a possible candidateCitation11. Plant flavonoids, considered as nature’s tender drugs, exert several activities in mammalian cellsCitation12. The benzopyranone-ring system, found in flavonoids, is a molecular scaffold of considerable interest, particularly as inhibitors of cysteine proteases, as reported by Zeng and co-authorsCitation13 for biflavones inhibition of cathepsin B and K. In the present study, we evaluated inhibition of papain, cruzain and T. cruzi extracts peptidase activities by fukugetin, a biflavone isolated from Garcinia brasiliensis (). In addition, we elucidated its crystallographic structure and performed flexible molecular docking simulations with papain and cruzain.
Material and methods
Chemicals
Papain (EC 3.4.22.2 from Carica papaya latex) and the substrate carbobenzoxy-phenylalanil-arginyl-7-amido-4-methylcoumarin (Cbz-Phe-Arg-MCA) were commercially obtained from Sigma (St. Louis, USA). Cruzain (EC 3.4.22.51) was obtained from Escherichia coli (strain DH5a containing the expression plasmid) kindly supplied by J. H. McKerrow, University of California, San Francisco, USA, following a previously reported procedureCitation14. The molar concentration of the enzymes’ solution was determined by active site titration with E-6415, and hydrolysis of fluorogenic substrate was followed at λex 380 nm and λem 460 nm (excitation and emission wavelengths for MCA). The spectrofluorometer was calibrated with standard solutions of MCA hydrolyzed in a spectrofluorometer Shimadzu RF-1501 (Shimadzu Corporation, Kyoto, Japan). All other chemicals, solvents and reagents were obtained from commercial sources (Sigma and Merck, Whitehouse Station, NJ). Papain and cruzain assays (previously treated with 5 mM dithiotreitol for 15 min) were performed as previously described by MeloCitation16, using the substrate Cbz-Phe-Arg-MCA prepared by serially diluting a stock solution to 1 mM of H2O:DMF (50:50 v/v). Fukugetin was dissolved in H2O:DMF (50:50 v/v) and diluted in the enzymatic buffer. Enzymes were assayed in 0.1 M sodium phosphate buffer; pH 6.8 containing 1 mM EDTA.
Plant material
G. brasiliensis fruits were collected from trees grown under controlled conditions at the herbarium of the University of Viçosa (latitude 20° 45′ 14″ south and longitude 42° 52′ 55″ west), Minas Gerais, Brazil, where a voucher specimen is deposited (number VIC2604).
Extraction and fukugetin isolation
To obtain the extracts, the fruits were dried and powdered (affording 1 kg), and then were extracted with solvents of growing polarity (hexane, ethyl-acetate and ethanol, in this order, for 24 h with each), at room temperature using the soxhlet equipment. These extracts were concentrated under reduced pressures using a rotary evaporator, and then dried under vacuum, yielding 57.14 g, 105.44 g and 253.06 g, of hexanic, ethyl-acetate and ethanolic extracts, respectively. These dried extracts were used in the assays. Ethyl-acetate extract was chromatographed on silica gel (230–400 mesh) column (8 × 100 cm) eluted with crescent polarity mixtures of n-hexane/ethyl-acetate and ethyl-acetate/ethanol to give 30 fractions. These fractions were then pooled in 10 groups according to their similarities in thin layer chromatography (TLC). The group was rechromatographed with mixtures of n-hexane/ethylacetate and ethyl-acetate/ethanol, and the main fraction was recrystallized several times using methanol solutions, yielding 1.05 g of fukugetin, as described by DerogisCitation17. The structure elucidation was obtained by NMR, MS, UV, and IR techniques of this isolated compound which were appraised by comparison with literature valuesCitation18–20.
Single crystal x-ray diffraction
After the isolation and purification procedures, a well-shaped clear single fukugetin crystal, which was grown by recrystallization from 1:1 dichloromethane and methanol mixture, was selected for the X-ray diffraction experiment. Intensity data were measured at room temperature (293(2) K) and with graphite monochromated MoKα radiation (λ = 0.71073 Å), using the Enraf-Nonius κ-CCD diffractometer. Cell refinements were performed using the software CollectCitation21 and ScalepackCitation22, and the final cell parameters were obtained for all reflections. Data reduction was carried out using the software Denzo-SMN and ScalepackCitation22. Since the absorption coefficient was insignificant (0.116 mm−1), no absorption correction was applied. The structure was solved using the software SHELXS-9723 and refined using the software SHELXL-9724. Non-hydrogen atoms of the molecules were clearly solved and full-matrix least-squares refinement of these atoms with anisotropic thermal parameters was carried on. The C-H hydrogen atoms were positioned stereochemically and were refined with fixed individual displacement parameters [Uiso(H) = 1.2Ueq (Csp2) or 1.5Ueq (Csp3)] using a riding model with aromatic C—H, methyl C—H and methine C—H bond lengths of 0.93 Å, 0.96 Å, and 0.98 Å, respectively. The O-H hydrogen atoms were also positioned stereochemically and were refined with fixed individual displacement parameters [Uiso(H) = 1.5Ueq] using a riding model with O—H bond length of 0.82 Å. The hydrogen of water molecules were modeled using the CALC-OH program. Crystal, collection and structure refinement data are summarized in . Tables were generated by WinGXCitation25 and the structure representations by ORTEP-326 and MERCURYCitation27. Although fukugetin crystallized in a noncentrosymmetric space group, the Flack parameter was not refined during X-ray crystallographic analysis. Since the most electron-rich atom is O, whose anomalous scattering is not large enough (using MoKα radiation) to permit determination of the absolute structure by X-ray diffraction, Friedel pairs were averaged before refinement. Supplementary crystallographic dataset for fukugetin is available in the Cambridge Structural Data Base, deposition number CCDC 813873. Copies of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK (fax: +44123-336-033; e-mail: [email protected] or http:www.ccdc.ac.uk).
Table 1. Crystal data and structure refinement for fukugetin.
Kinetic profile of inhibitions and determination of rate constants
To establish the time-dependent mechanism of inhibition, enzymes and different concentrations of fukugetin were incubated in microtubes. Aliquots of this preincubate were retrieved at different times (0–280 min) and added to assay buffer containing fluorogenic peptide substrate at 25°C. For this trial, papain (8 nM) or cruzain (10 nM) and fukugetin (0.1–7.5 µM) were incubated in 1500 µL assay buffer. Aliquots of 50 µL of this preincubate and the substrate Cbz-Phe-Arg-MCA (10 µM) were added to 950 µL assay buffer in a cuvette. The initial velocity of this incubate was monitored spectrofluorometrically and the progress curves in function of time for each inhibitor concentration were fitted to the rate equation of first-order reaction, according to equation 1Citation28 using the Grafit 5.0 software (Erithacus Software, UK):
where, A is the enzymatic activity, A0 is the enzymatic activity in the absence of inhibitor, t stands for time and kobs represents the apparent first-order rate constant. The values of KI and the apparent association (k7) and dissociation (k−7) constants were calculated from the relation of the first-order rate constant in function of the inhibitor concentration according to equation 2 described by Baici, 2009 Citation29:
where the dissociation and association rate constants are described for fast (k3 and k-3) and slow step (k7 and k−7), S represents of substrate concentration and Km is the Michaelis–Menten constant.
Lineweaver–Burk plots
To characterize the type and extent inhibition of papain by fukugetin, papain (0.04 nM) was incubated with four concentrations of fukugetin (1.5, 3, 6 and 12 µM). For cruzain, enzyme concentration was 12 nM and inhibitor concentrations varied from 1 µM to 10 µM. Analysis of enzyme kinetic data was carried out using the Lineweaver–Burk plotCitation30 in Grafit 5.0 software. Hydrolytic activity was measured immediately 3 h after, mixing fukugetin and enzymes by addition growing concentrations of Cbz-Phe-Arg-MCA (0.5–22 µM). The Ks was experimentally determined using standard Michaelis–Menten kineticsCitation31 and all experiments were carried out in triplicate. Factor α, β and KI values were initially estimated through inverse of secondary plots obtained from papain inhibition according to equations 3 and 4 described by hyperbolic mixed-type inhibition and confirmed by non-linear regression analysis, as previously describedCitation32:
where, KS is the substrate dissociation constant, KI is the fukugetin dissociation constant, α is the parameter of KS perturbation, I is the inhibitor concentration, and β is the parameter of Vmax (kcat) perturbation. Factors α, β and KI for cruzain inhibition were calculated using equations 5 and 6, which describe a linear partial competitive inhibitionCitation32:
Molecular docking
In order to identify the possible binding sites, we used the program SiteMapCitation33. The program, which has been proven to provide high assertion site identification index, scores the sites using a linear combination of physical descriptor such as: size, the degree of enclosure by the protein, the degree of exposure to solvent and the hydrophobic and hydrophilic character of the site, among other properties. The papain and cruzain structures files were downloaded from the Brookhaven Protein Data Bank (PDB code 1PE6Citation34 and 1F2CCitation35, respectively) and the substrates were removed prior to calculations. The docking procedure is described as follows: (i) the papain and cruzain PDB files were prepared using the Protein Preparation WizardCitation36 implemented in the Schrodinger Suite; (ii) the QM-Polarized Ligand Docking protocolCitation37 was used to obtain the best poses. In this protocol, the partial charges of the ligands are calculated in the field of the receptor using quantum mechanical methods (6-31G*/LACVP* basis set, and B3LYP density functional). These new charges can result in improved docking accuracy.
Inhibition assays with T. cruzi extract
Parasites were kindly provided by Dr. Sergio Schenkman’s lab from Universidade Federal de São Paulo. Soluble protein extraction was carried out as previously describedCitation38. Briefly, 2 × 108 parasites were collected by centrifugation at 4°C, 2000g/10 min and washed in PBS. The parasites were resuspended in lysis buffer (10 mM Tris–HCl pH 6.8, 1%CHAPS). After ice incubation for 40 min, parasites were homogenized and centrifuged at 4°C, 12,000g for 30 min (Beckman OptimaXL-100K). Supernatant was collected and quantified according to the Bradford methodCitation39. Proteolysis was evaluated using 10 µg of soluble protein extracts. Cysteine protease activity was assessed by degradation of Cbz-Phe-Arg-MCA at 10 µM in 0.1 M sodium phosphate buffer; pH 6.8 containing 1 mM EDTA and 10 µg of protein extract.
Results
Single crystal X-ray diffraction
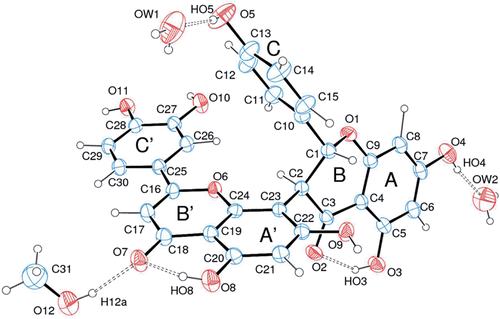
shows an ORTEP-326 view of fukugetin with the atom numbering scheme. Crystal, collection and structure refinement data are summarized in . In the course of the intramolecular analysis, the geometric parameters of fukugetin were analysed using the Mogul softwareCitation27. All geometric values agree with those of other reported similar structures. The rings A, C, A’, B’, and C’ and their respective first neighbours are, as expected, planar. Ring B presents an envelope conformation with atom C1 at the flip point, which deviates 0.645(4) Å from the least-squares plane through the C2, C3, C4 C9 and O1 atoms. The fact that the ring C’ and B’ are almost in the same plane is due to the electronic delocalization at a conjugated system C’ and B’ characterized by keto-enol tautomerism, showing the influence of aromatic hydroxyl groups to the intramolecular crystal structure. Taking this in account, there are three molecular moieties, which individually can be considered almost flat: moiety 1, containing the atoms O2, O3 and O4 and those ones present in rings A and B, except C1; moiety 2, containing ring C and O5; and moiety 3, containing the remaining oxygen atoms and rings A’, B’, and C’. These structural geometries can be explained as a function of either crystal packing forces involving classical intermolecular hydrogen bonds or hindrance effects involving the moieties. Another important piece of information revealed by the X-ray crystallographic analysis is the presence of two crystallization water molecules and one methanol molecule in the crystal structure (). Therefore, the crystal structure reported here can be considered either a pseudo-polymorphic hydrate or solvate form of fukugetin. The water and methanol molecules form intermolecular hydrogen bonds with fukugetin, contributing to the packing stabilization. Hydroxyl oxygen atoms O3 and O8 are hydrogen bond donors to the acceptors O2 and O7, respectively.
Inhibition of papain and cruzain activities by fukugetin
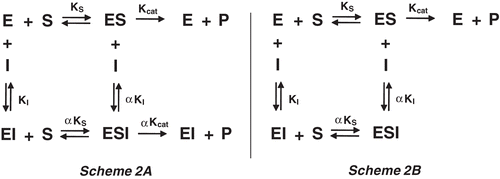
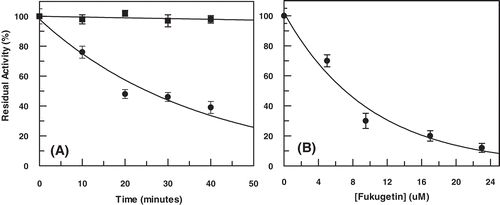
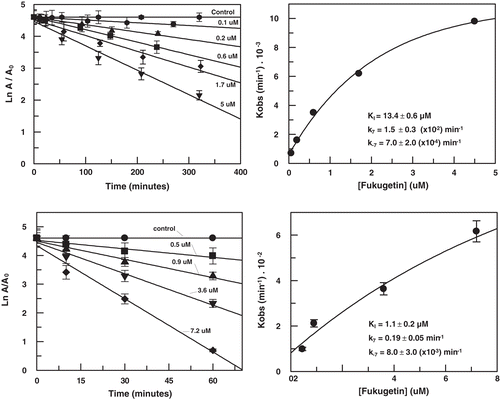
shows that fukugetin inhibited both papain and cruzain endopeptidase activity in a time- and concentration-dependent manner, and the kinetic data treatment was conducted as previously reportedCitation29. The inhibition of fukugetin upon papain and cruzain endopeptidase activities can be described as a slow reversible type inhibition, depicted in . The association and dissociation constants of a slow-binding fukugetin were determined by plotting the apparent first-order rate constant (kobs) against fukugetin concentration. In principle, there are two ways in which a slow-binding inhibitor gains existence: the direct binding of inhibitor or the slow production of an enzyme-inhibitor complex (EI) preceding a rapid equilibration between E and I to form an adsorptive complex (EI28,29). Depending on the inhibition mechanism, the plot is a straight line in the direct model or a hyperbole, in the isomerization model. In , the statistical analysis from non-linear regression of the first-order rate constant plot versus [I] suggests a hyperbole as best fit, which indicates that the binding of fukugetin to the enzymes is a reversible slow-binding inhibition. After overnight incubation of fukugetin with papain and cruzain, the substrate addition was able to return the enzymatic activity, proving the reversible inhibition mechanism (data not shown). The results obtained for papain were KI = 13.4 ± 0.6 µM, k7 = 0.015 ± 0.001 min−1 and k-7 = 0.0007 ± 0.0002 min−1, while for cruzain the constants were KI = 1.1 ± 0.2 µM, k7 = 0.19 ± 0.05 min−1 and k-7 = 0.008 ± 0.003 min−1.
Figure 3. Slow-binding inhibition of papain (upper) and cruzain (lower) by fukugetin. Left− time course of inactivation. Right− first-order constant (Kobs) as function of inhibitor concentration.
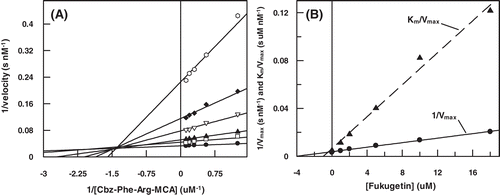
In order to better characterize the molecular mechanism of fukugetin papain and cruzain inhibition, the initial velocity of enzymes was measured at various substrate and inhibitor concentrations. The double reciprocal plotCitation30 () showed convergent lines crossing at the same point in the second quadrant, for both enzymes. shows that the presence of fukugetin in the papain kinetic assays resulted in a decrease in kcat values for the hydrolysis of substrate Cbz-Phe-Arg-MCA, and also markedly decreases the affinity of the proteases for this substrate. In the inhibition papain, both replots of slope versus fukugetin concentration and intercept (1/vmaxapp) versus fukugetin concentration are hyperbolic (). These data show that fukugetin inhibits papain activity upon the substrate Cbz-Phe-Arg-MCA by a hyperbolic mixed-type inhibition fashionCitation32 depicted in . The estimated Vmax and Ks values for papain inhibition by fukugetin are summarized in . Compared with the uninhibited reaction (control), addition of fukugetin resulted in significant changes of Vmax and Km that ranged between 8.8 ± 0.4 and 1.6 ± 0.1 µMs−1 and 10.1 ± 0.5 and 12.5 ± 0.9 µM, respectively. Papain inhibition by fukugetin shows a situation system in which papain (E) and papain-fukugetin (EI) bind to substrate (S), but with different affinities. Both ES and ESI form product, but at different rates. This mixed type system is considered to be a mixture of partial competitive and partial noncompetitive inhibitionCitation32. The data were fitted to the Equation described in the section “Material and methods” and the values for the constants were determined. Factor α, the factor by which Ks changes when fukugetin occupied papain, was 1.8 ± 0.3, while factor β, the factor by which kcat is altered when the inhibitor occupies the enzyme, was 0.12 ± 0.02. The results shows that fukugetin binds free papain (E) with a KI = 13.4 ± 0.2 µM, and the complex enzyme-substrate (ES) with a dissociation constant of αKI = 24 ± 4 µM. Also, fukugetin induced a 1.8-fold decrease in the affinity of papain for the substrate Cbz-Phe-Arg-MCA, Ks value increased from 10.0 ± 0.5 µM to 18.0 ± 4 µM in the presence of fukugetin (α = 1.8 ± 0.3), whereas kcat decreased eight times in the presence of fukugetin (β = 0.12 ± 0.02 µM). Thus, the catalytic efficiency for the substrate Cbz-Phe-Arg-MCA in the presence of fukugetin decreased (β/α = 0.07 ± 0.02). Curiously, in the cruzain inhibition assays, the reciprocal plot shows a pattern of linear mixed inhibition, the replot of Vmax axis intercept versus fukugetin concentration and the replot of slope versus fukugetin concentration is linear (differently from papain), indicating that one fukugetin molecule binds to cruzain (). The effect of fukugetin on the hydrolysis of substrate Cbz-Phe-Arg-MCA by cruzain is described by the kinetic model in the section “Material and methods”, and the equilibrium describing this system is shown in . The results show that fukugetin binds cruzain with a KI of 1.0 ± 0.2 µM. The simplest mixed system is one in which EI has a lower affinity than E for S, and the ESI complex is nonproductive. The system may be considered a mixture of partial competitive inhibition and pure noncompetitive inhibition. As long as fukugetin is present, some of the enzyme is nonproductive ESI form, even at an infinitely high S. Consequently, Vmaxapp is lower than Vmax, as shown in . Also, at any fukugetin concentration, a portion of the enzyme available for combination with S exists in the lower affinity EI form. Therefore, Ksapp increased about 4.5 times (factor α) in the presence of fukugetin, in relation to control. At an infinitely high fukugetin concentration, the enzyme is driven to the EI and ESI forms. Because ESI is nonproductive, the velocity is driven to zero by increasing of fukugetin and the factor β is numerically equal to zero. Since β is equal to zero, the slope versus [I] and the 1/v axis intercept versus [I] replots are linear. Because the replots are linear, this type of mixed system found for cruzain is called partial competitive inhibition.
Table 2. Kinetic parameters from papain and cruzain inhibition by fukugetin (means ± standard deviation).
Figure 4. (A) Lineweaver–Burk plot for hydrolysis of Cbz-Phe-Arg-MCA by papain in the presence and absence of fukugetin and (B) the secondary plots of showing the hyperbolic inhibition type mixed on fukugetin concentration. The reaction was carried out in the absence (• ) and in the presence of fukugetin; Δ, 1.5µM; ▪, 3µM; ◊, 6µM and ◆, 12µM.
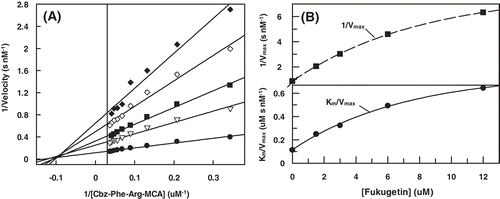
Figure 5. (A) Lineweaver–Burk plot for hydrolysis of Cbz-Phe-Arg-MCA by cruzain in the presence and absence of fukugetin and (B) the secondary plots showing the dependence of the axis 1/Vmax and the Km/Vmax on the fukugetin concentration. The reaction was carried out in the absence (• ) and in the presence of fukugetin; □ 1µM; ▴, 2µM; ◊, 5µM; ◆, 10µM and ◊, 18µM.
Molecular docking
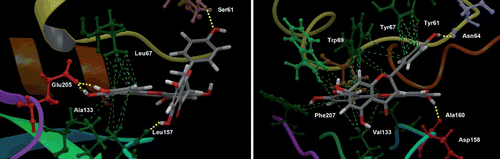
The docking simulations of fukugetin/papain and fukugetin/cruzain, respectively, showed very similar modes of binding (). The fukugetin molecule is shown in stick representation, whereas the proteins are represented through its electrostatic potential surface (EPS). When the structures of fukugetin bound to papain or bound to cruzain are aligned and superimposed, they show a high degree of similarity to one another (data not shown). In both simulations, the distance between the active site cysteine and the ketone group at C18 (7.104 Å− papain and 6.335 Å− cruzain) does not favor the establishment of a covalent bond. In fact, the ketone moiety and Cys are oriented in conformations that are incompatible with covalent bond formation. As found in crystal structure, an interaction mediated through hydrogen bond between the hydroxyl group at C20 and the ketone group of fukugetin stabilizes this group, reducing its nucleophilicity. Due to the similarities in the papain and cruzain active sites, the inhibitor binds in a similar fashion with the ring C’ in the S2 pocket and the ring C in the S3 pocket. The S1 and S’1 subsites of proteases remain vacant. shows details of interaction of fukugetin with papain and cruzain. In papain, ring C establishes hydrophobic interactions with Tyr61and Tyr67 present in the S3 pocket. There are also hydrophobic interactions between ring C’ and Val133, Phe207, Trp69 and Tyr67 residues. Hydrogen bonds involve the amino acid residue Asp158 with the hydroxyl group at C20 and the amino acid residue Asn64 with the hydroxyl group at C13. In cruzain, hydrophobic interactions in S2 pocket is mediated between amino acid residues Leu67, Ala133, Leu157 and ring C’. Moreover, hydrogen bonds are established through (i) the Glu205 present at the bottom S2 subsite and the hydroxyl groups at C27 and C28, (ii) the amino acid residue Ser61 and hydroxyl group at C13, and (iii) carbonyl group backbone of Leu157 and hydroxyl group at C5. In both enzymes, the interactions (hydrophobic and hydrogen bond) are at different sides of the fukugetin C2-C23 flexible bridge, giving to this pose a better stability. The docking GlideScore and the Emodel score (a combination of GlideScore, the nonbonded interaction energy, and, for flexible docking, the excess internal energy of the generated ligand conformation) were −7.4 and −54.9 for papain; −6.0 and −91.9 for cruzain, respectively. Both results were obtained for the best poses and indicate a correlation between the binding energy and the experimental inhibitory potency.
Figure 6. Molecular docking of papain (left) and cruzain (right) fukugetin inhibitor. The proteins are represented based on electrostatic potential surface (EPS); inhibitor carbons are pink, hydrogens are white and oxygens are red. The papain and cruzain structure files were downloaded from the Protein Data Bank (PDB code 1PE6 and 1F2C, respectively).
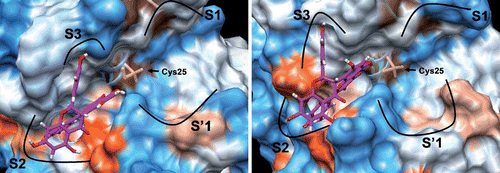
Figure 7. Best docking poses for fukugetin inside the subsites of cruzain (left) and papain (right). The hydrogen bonds that contribute for inhibitor anchoring are shown as yellow lines and the hydrophobics interactions as green lines. Some amino acids were highlighted due to their key roles in the binding of inhibitor. H atoms in C-H bonds are omitted.
Inhibition assays in T. cruzi extract
Trypanosoma parasite extract was initially treated with fukugetin at 10 µM, and the enzymatic activity was measured. As observed in , fukugetin inhibited approximately 20% the parasite peptidase activity after 10 min and about 60% after 40 min of incubation, showing clear time dependence. Furthermore, 1 h of parasite extract incubation in the presence of increased fukugetin concentrations showed IC50 at 7.0 ± 0.6 µM ().
Discussion
The amino acid sequences of papain and cruzain are 38% homologous, and their active sites are nearly identical. Structural studies showed that the active site region of recombinant cruzain is very similar to that of other papain-like members, extending over seven substrate-binding sites, four of which (S4–S1) are located on the acyl side of the cleaved bond and three (S’1-S’3) on the amino sideCitation40,Citation41. Papain was selected because it is commercially available in large quantities and its structure is known. Our kinetic analyses demonstrated that fukugetin was a potent papain and cruzain inhibitor with slow-binding and reversibility kinetics. The time dependence inhibition was also observed with parasite extract. The kinetic data analysis indicates that the inhibition occurs by formation of a slow enzyme-inhibitor complex (EI) preceding a rapid equilibration between E and I to form an adsorptive complex (EI*). Fukugetin is a dimer of flavones linked at C2 and C23, and the most interesting structural feature of this molecule, as well as others biflavones, is the rigidity of benzopyranone moiety as well as flexibility between phenyl ring and benzopyranone because the torsional angles at bound C2-C23, C16-C25 and C1-C10 can twist from 0 to 360°Citation48. In the X-ray structure, fukugetin is crystallized with two water molecules and one methanol molecule, indicating that this molecule may be solvated in solution. Docking results showed ring C’ in the S2 subsite and ring C in the S3 subsite, which are hydrophobic, with fukugetin in a conformational structure completely different from the crystal. Thus, the molecule flexibility and the change from an aqueous to a hydrophobic environment explain the time dependence inhibition. One of the rings (C’ or C) interacts with the S2 or S3 pocket, respectively, resulting in an enzyme-inhibitor complex. Then the other ring establishes a new interaction in one of the subsites, forming a complex more stable, which is also reversible. Moreover, intramolecular aromatic stacking interactions stabilize fukugetin into the enzymatic active site. Aromatic stacking, according to Burley and PetskoCitation42, consists of pairs of aromatic residues with phenyl ring centroid separations between 4.5 and 7.0 Å and dihedral angles between aromatic planes within 30–90°. These separations and angles between the inhibitor’s phenyl rings A-C’and A’-C are 5.019 Å and 38.7°, and 4.545 Å and 35.5°, respectively, and between phenyl ring C-Tyr67 of papain the values are 5.570 Å and 31.4°.
In papain-family cysteine proteases, the P2 position can be a key determinant of specificity. The S2 pocket of cruzain is lined with hydrophobic residues such as Leu67, Met68, Ala133, and Leu157, which readily accommodate the aromatic side chain of the substrates and inhibitors as shown for ring C’ of fukugetin. Besides the hydrophobic interactions, the residue Glu-205 at the bottom of the S2 subsite increases the interaction through hydrogen bonds with hydroxyl groups at C27 and C28. Studies have shown the importance of this residue Glu205 as a different target, when compared to others proteins of peptidase C1 family membersCitation43. Although papain and cruzain to be isostructural in the region of catalytic sites, different conformations was found for the inhibitor Cbz-Phe-Ala-FMK where there is a 60° rotation of Phe side chain in cruzain relative to papainCitation44 and too in the region of the loops and turns. Despite the majority of prior inhibitor development for cruzain has focused on the S1′, S1, and S2 pockets, inhibitors with changes focusing S3 pockets showed a potential inhibition. Because the S3 pocket of cruzain to be large and open-ended, unlike others cysteine proteases as cathepsin S45 wich normally displays a small and well-defined S3 subsite, studies also showed heterocycles were chosen with potential for hydrophobic interactions with the hydrophobic side of the pocket and with potential for hydrogen-bonding interactions with the serine residue in the S3 pocketCitation46 and the ring C of fukugetin performs this role mentioned above. Cruzain also substitutes Tyr61 and Tyr67 for the smaller side chains of Ser and Leu, respectively, changing the contacts with the P3 side chain. The different details between these proteases show because cruzain was inhibited about 12 times faster than papain in the kinetics mechanism inhibition besides we found partial competitive inhibition for cruzain and hyperbolic mixed-type inhibition for papain. Thus, although these proteases are very similar, cruzain was inhibited more effectively than papain by fukugetin in the realized experiments.
Compounds isolated from Garcinia subelliptica (Guttiferae) displayed trypanocidal activity against trypomastigotes and epimastigotes of T. cruzi47. Although in this mentioned study fukugetin showed no activity in vivo, our results demonstrated fukugetin was a potent inhibitor of cruzain and parasites extracts with concentrations in nonetheless in the low micromolar range. It is not uncommon that potent inhibitors from in vitro screening prove to be ineffective against intracellular proteases due to poor membrane permeability. To enhance membrane permeability, further optimization of the inhibitors is being made by our group utilizing fukugetin as a prototype. This optimization has focused on the preparation of more neutral, hydrophobic compounds balanced with aqueous solubility to achieve a potential therapeutic agent.
Conclusion
Fukugetin is a nonpeptidic small molecule inhibitor exhibiting selectivity across two similar cysteine proteases and activity against T. cruzi extract. The structural characteristic and the possibility of hydroxyl group substitutions, either increasing potency or activity in vivo, makes this compound a good candidate for development of protease inhibitors involved the parasite cycle life with applications in infections by Trypanosoma cruzi.
Acknowledgments
This work was supported in Brazil by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). M. B. was supported by Grant 08/54894-4. IC was supported by Grant APQ-01420-08.
Declaration of interest
References
- Campetella O, Henriksson J, Aslund L, Frasch AC, Pettersson U, Cazzulo JJ. The major cysteine proteinase (cruzipain) from Trypanosoma cruzi is encoded by multiple polymorphic tandemly organized genes located on different chromosomes. Mol Biochem Parasitol 1992;50:225–234.
- Lima AP, Tessier DC, Thomas DY, Scharfstein J, Storer AC, Vernet T. Identification of new cysteine protease gene isoforms in Trypanosoma cruzi. Mol Biochem Parasitol 1994;67:333–338.
- Caffrey CR, Hansell E, Lucas KD, Brinen LS, Alvarez Hernandez A, Cheng J et al. Active site mapping, biochemical properties and subcellular localization of rhodesain, the major cysteine protease of Trypanosoma brucei rhodesiense. Mol Biochem Parasitol 2001;118:61–73.
- Eakin AE, Mills AA, Harth G, McKerrow JH, Craik CS. The sequence, organization, and expression of the major cysteine protease (cruzain) from Trypanosoma cruzi. J Biol Chem 1992;267:7411–7420.
- Nkemgu NJ, Grande R, Hansell E, McKerrow JH, Caffrey CR, Steverding D. Improved trypanocidal activities of cathepsin L inhibitors. Int J Antimicrob Agents 2003;22:155–159.
- Steverding D, Caffrey CR, Sajid M. Cysteine proteinase inhibitors as therapy for parasitic diseases: advances in inhibitor design. Mini Rev Med Chem 2006;6:1025–1032.
- Engel JC, Doyle PS, McKerrow JH. [Trypanocidal effect of cysteine protease inhibitors in vitro and in vivo in experimental Chagas disease]. Medicina (B Aires) 1999;59 Suppl 2:171–175.
- Vicik R, Hoerr V, Glaser M, Schultheis M, Hansell E, McKerrow JH et al. Aziridine-2,3-dicarboxylate inhibitors targeting the major cysteine protease of Trypanosoma brucei as lead trypanocidal agents. Bioorg Med Chem Lett 2006;16:2753–2757.
- Engel JC, Doyle PS, Hsieh I, McKerrow JH. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. J Exp Med 1998;188:725–734.
- Engel JC, Doyle PS, Palmer J, Hsieh I, Bainton DF, McKerrow JH. Cysteine protease inhibitors alter Golgi complex ultrastructure and function in Trypanosoma cruzi. J Cell Sci 1998;111 (Pt 5):597–606.
- McKerrow JH, Doyle PS, Engel JC, Podust LM, Robertson SA, Ferreira R et al. Two approaches to discovering and developing new drugs for Chagas disease. Mem Inst Oswaldo Cruz 2009;104 Suppl 1:263–269.
- Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 2000;52:673–751.
- Zeng GZ, Pan XL, Tan NH, Xiong J, Zhang YM. Natural biflavones as novel inhibitors of cathepsin B and K. Eur J Med Chem 2006;41:1247–1252.
- Eakin AE, Mills AA, Harth G, McKerrow JH, Craik CS. The sequence, organization, and expression of the major cysteine protease (cruzain) from Trypanosoma cruzi. J Biol Chem. 1992, 267: 7411–7420.
- Barrett AJ, Kirshke H. Cathepsin B, cathepsin H, cathepsin L. Methods Enzymol. 1991, 80: 535–561.
- Melo RL, Alves LC, Del Nery E, Juliano L, Juliano MA. Synthesis and hydrolysis by cysteine and serine proteases of short internally quenched fluorogenic peptides. Anal Biochem 2001;293:71–77.
- Derogis PB, Martins FT, de Souza TC, de C Moreira ME, Souza Filho JD, Doriguetto AC et al. Complete assignment of the 1H and 13C NMR spectra of garciniaphenone and keto-enol equilibrium statements for prenylated benzophenones. Magn Reson Chem 2008;46:278–282.
- Konoshima M, Ikeshiro Y, Nishinaga A, et al. The constitution of flavonoids from hook. Tetr Lett 1969;10:121–124.
- Botta B, Mac-Quhae MM, Monache GM, Monache FM, De Mello JF. Chemical Investigation of the Genus Rheedia, V. Biflavonoids and Xanthochymol. J Nat Prod. 1984, 47:1053.
- Li X, Joshi AS, Tan B, Elsohly HN, Walker LA, Zjawiony JK, Ferreira D. Absolute configuration, conformation, and chiral properties of flavanone-(3→8″)-flavone biflavonoids from Rheedia acuminate. Tetrahedron. 2002, 58:8709–8717.
- Enraf-Nonius COLLECT.;Nonius BV. Delft, The Netherlands, 1997–2000.
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 1997, 276: 307–326.
- Sheldrick GM. SHELXS-97 Program for Crystal Structure Resolution. Germany:University of Göttingen, 1997.
- Sheldrick GM. SHELXL-97 Program for Crystal Structure Refinement. Germany: University of Göttingen, 1997.
- Farrugia LJ. WinGX suite for small-molecule single-crystal crystallography. J Appl Crystallogr. 1999, 32:837.
- Farrugia LJ. ORTEP-3 for Windows - a version of ORTEP-III with a Graphical User Interface (GUI). J Appl Crystallogr 1997, 30:565.
- Bruno IJ, Cole JC, Edgington PR, Kessler M, Macrae CF, McCabe P et al. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr, B 2002;58:389–397.
- Morrison JF, Walsh CT. The behavior and significance of slow-binding enzyme inhibitors. Adv Enzymol Relat Areas Mol Biol 1988;61:201–301.
- Baici A, Schenker P, Wächter M, Rüedi P. 3-Fluoro-2,4-dioxa-3-phosphadecalins as inhibitors of acetylcholinesterase. A reappraisal of kinetic mechanisms and diagnostic methods. Chem Biodivers 2009;6:261–282.
- Lineweaver H, Burk D. The Determination of Enzyme Dissociation Constants. J Am Chem Soc 1934 56:658–666.
- Michaelis L, Menten ML. Die Kinetik der Inwertin Wirkung. Biochem Z 1913, 49:333–339.
- Segel IH. Enzyme kinetics behavior and analysis of rapid equilibrium and steady-state enzyme systems, pp. 470–473, New York:John Wiley & Sons.
- SiteMap, version 2.3, Schrödinger, LLC, New York, NY, 2009.
- Hof P, Mayr I, Huber R, Korzus E, Potempa J, Travis J et al. The 1.8 A crystal structure of human cathepsin G in complex with Suc-Val-Pro-PheP-(OPh)2: a Janus-faced proteinase with two opposite specificities. EMBO J 1996;15:5481–5491.
- Brinen LS, Hansell E, Cheng J, Roush WR, McKerrow JH, Fletterick RJ. A target within the target: probing cruzain’s P1′ site to define structural determinants for the Chagas’ disease protease. Structure 2000;8:831–840.
- Schrödinger Suite 2009 Protein Preparation Wizard; Epik version 2.0, Schrödinger, LLC, New York, NY, 2009; Impact version 5.5, Schrödinger, LLC, New York, NY, 2009; Prime version 2.1, Schrödinger, LLC, New York, NY, 2009.
- Schrödinger Suite 2009 QM-Polarized Ligand Docking protocol; Glide version 5.5, Schrödinger, LLC, New York, NY, 2009; Jaguar version 7.6, Schrödinger, LLC, New York, NY, 2009; QSite version 5.5, Schrödinger, LLC, New York, NY, 2009.
- Alves CR, Corte-Real S, Bourguignon SC, Chaves CS, Saraiva EM. Leishmania amazonensis: early proteinase activities during promastigote-amastigote differentiation in vitro. Exp Parasitol 2005;109:38–48.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72:248–254.
- McGrath ME, Eakin AE, Engel JC, McKerrow JH, Craik CS, Fletterick RJ. The crystal structure of cruzain: a therapeutic target for Chagas’ disease. J Mol Biol 1995;247:251–259.
- Gillmor SA, Craik CS, Fletterick RJ. Structural determinants of specificity in the cysteine protease cruzain. Protein Sci 1997;6:1603–1611.
- Burley SK, Petsko GA. Aromatic-aromatic interaction: a mechanism of protein structure stabilization. Science 1985;229:23–28.
- Durrant JD, Keranen H, Wilson BA, McCammon JA. Computational identification of uncharacterized cruzain binding sites. PLoS Negl Trop Dis 2010, 4:676.
- Gilles AM, Keil B. Evidence for an active-center cysteine in the SH-proteinase α-clostripain through use of N-tosyl-L-lysine chloromethyl ketone. FEBS Lett 1984;173:58–62.
- Brak K, Doyle PS, McKerrow JH, Ellman JA. Identification of a new class of nonpeptidic inhibitors of cruzain. J Am Chem Soc 2008;130:6404–6410.
- Brak K, Kerr ID, Barrett KT, Fuchi N, Debnath M, Ang K et al. Nonpeptidic tetrafluorophenoxymethyl ketone cruzain inhibitors as promising new leads for Chagas disease chemotherapy. J Med Chem 2010;53:1763–1773.
- Abe F, Nagafuji S, Okabe H, Higo H, Akahane H. Trypanocidal constituents in plants 2.xanthones from the stem bark of Garcinia subelliptica. Biol Pharm Bull 2003;26:1730–1733.
- Pan X, Tan N, Zeng G, Zhang Y, Jia R. Amentoflavone and its derivatives as novel natural inhibitors of human Cathepsin B. Bioorg Med Chem 2005;13:5819–5825.

![Figure 1. Structure of fukugetin. IUPAC name: 8-[(2S,3R)-5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-2, 3-dihydrochromen-3-yl]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one.](/cms/asset/62426d34-6703-4ffa-a757-a68c5d6e6dd5/ienz_a_668539_f0001_b.gif)