Abstract
Context: Organoselenium compounds have been described as antioxidant and neuroprotective agents.
Objective: To evaluate the antioxidant action of 2,2′-dithienyl diselenide (DTDS) and its effects in brain monoamine oxidase (MAO) activity in vitro.
Materials and methods: Assays for reactive species (RS), lipid peroxidation, protein oxidation, MAO A and B activities in rat brain homogenate as well as mimetic dehydroascorbate reductase and glutathione S-transferase activities were performed using DTDS (μM range).
Results: DTDS was effective in decreasing the levels of RS as well as lipid peroxidation induced by malonate, sodium nitroprusside or FeCl2/EDTA and protein carbonyl in the rat brain homogenate. DTDS elicited dehydroascorbate reductase-like and glutathione S-transferase-like activities. DTDS was effective in inhibiting both MAO-A and MAO-B activities.
Discussion: The results demonstrated that DTDS is an antioxidant agent with non-selective inhibitory effect on MAO activity.
Conclusion: DTDS is a promising molecule to be evaluated in experimental models of neurological diseases.
Introduction
Oxidative stress, a perturbation of the redox homeostasis, has been implicated to the pathophysiology of several neurological disorders and plays a paramount role in the aging processCitation1,Citation2. In recent years, it has become increasingly clear that mitochondrial dysfunction and oxidative damage are major contributors to neuronal lossCitation3. The brain is especially sensitive to oxidative stress because it utilizes high levels of oxygen, contains large amounts of lipids and exhibits a lower level of antioxidant defenses compared to other tissuesCitation2. Since natural or synthetic antioxidants can protect against oxidative stress, molecules with antioxidant action appear to be an attractive approach for prevention and/or adjuvant treatment of disorders linked to oxidative stress.
Chemicals such as sodium nitroprusside (SNP), malonate and FeCl2/ethylenediamine tetraacetic acid (EDTA) are widely used to induce lipid peroxidation in order to screen novel antioxidant compounds in vitro since. These lipid peroxidation inductors can trigger reactive species (RS) generation by distinct waysCitation4–6. The effects of novel compounds on the increase of RS induced by sodium azide, which causes mitochondrial dysfunction by inhibiting cytochrome oxidase activityCitation7, are also a valuable tool to screen antioxidant activity of compounds. Lastly, it is possible to apply assays for radical scavenging activity to characterize an antioxidant compound.
Monoamine oxidase (MAO) is a flavoprotein located at the outer membranes of mitochondria in neuronal, glial and other cells. MAO is involved in the metabolic cerebral degradation of monoamines (serotonin, noradrenalin and dopamine) and appears to play important roles in many neurological and psychiatric disordersCitation8,Citation9. It is widely speculated that prolonged excessive activity of these enzymes may be conducive to mitochondrial damage and neurodegenerative disturbancesCitation10. The byproducts of MAO reactions include a number of potentially neurotoxic species, such as hydrogen peroxide and ammonia. In particular, hydrogen peroxide can trigger the production of reactive oxygen species (ROS) and induce mitochondrial damage and neuronal apoptosisCitation10. In this sense, compounds having both antioxidant and MAO inhibitory properties could be interesting for treatment of some neurological diseases.
Selenium is an essential trace element and plays a crucial role in several major metabolic pathways, including antioxidant defense systemCitation11. Selenium exerts its antioxidant function mainly in the form of selenocysteine residues as an integral constituent of ROS-detoxifying selenoenzymesCitation12. Besides, the preferential retention of selenium in the brain suggests that it plays important functionsCitation13. In this context, it has been highlighted the importance of the development of compounds containing selenium as preventive or therapeutic agents in neurological conditions.
Generally, organic forms of selenium are more bio-available and less toxic than the inorganic formsCitation14. The interest in the research of organic compounds containing selenium has increased considerably due to the fact that these compounds have been described to possess interesting pharmacological activities, such as neuroprotectionCitation15,Citation16. In fact, the brain appears to be one of the target organs of organoselenides, since they have highly lipophilic natureCitation16. In line with this, we have demonstrated that 2,2′-dithienyl diselenide (DTDS) plays anticonvulsant action in rats exposed to kainic acidCitation17.
Based on the pharmacological properties presented by synthetic organoselenium compounds, the aim of the present study was to evaluate the antioxidant action of DTDS in vitro. Moreover, since MAO activity is an important drug target for the treatment of neurological disorders, a second objective of this study was to investigate the in vitro effect of DTDS in cerebral MAO-A and MAO-B activities of rats.
Material and methods
Chemicals
DTDS () and ebselen were prepared according to the methods described by Tiecco et al.Citation18 and EngmanCitation19, respectively. Analysis of Citation1HNMR and Citation13CNMR spectra showed that the compounds obtained presented spectroscopic data in full agreement with their assigned structures. The chemical purity of these compounds (99.9%) was determined by gas chromatography–mass spectrometry (GC/MS). Reduced glutathione (GSH), SNP and malonate were purchased from Sigma (St. Louis, MO, USA). 1-Chloro-2, 4-dinitrobenzene (CDNB) was purchased from Aldrich Chemical Co (USA). All other chemicals were of analytical grade and obtained from standard commercial suppliers. DTDS and ebselen were dissolved in dimethylsulfoxide (DMSO). The control group received the vehicle used for dissolving the compounds (DMSO). The DTDS chemical characteristics are given below:
Yield: 0.023 g (40%). H1 NMR (CDCl3, 200 MHz), δ (ppm): 7.47 (dd, J = 1.2, 5.3 Hz, 2H), 7.23 (dq, J = 1.2, 3.5 Hz, 2H), 7.00 (dd, J = 3.5, 5.3 Hz, 2H). C13 NMR (CDCl3, 50 MHz) δ (ppm): 136.9 (2C), 132.9 (2C), 128.0 (2C), 125.5 (2C). MS (EI, 70 eV) m/z (relative intensity): 325 (34), 162 (100), 160 (53), 119 (24), 93 (4), 84 (27), 70 (32), 51 (5).
Animals
Male adult Wistar rats (200–300 g) were obtained from a local breeding colony. Animals were housed in cages with free access to food and water. Animals were kept in a separate animal room, on a 12-h light/12-h dark cycle, with lights on at 7:00 a.m., in an air-conditioned room (22 ± 2°C). The animals were used according to the guidelines of the Committee on Care and Use of Experimental Animal Resources, Federal University of Santa Maria, Brazil.
Antioxidant assays
Tissue preparation
Rats were killed by decapitation and cerebral tissue (whole brain) was rapidly dissected, placed on ice and weighed. Tissues were immediately homogenized in cold 50 mM Tris–HCl buffer, pH 7.4 (1/5, weight/volume). Homogenate freshly prepared was centrifuged at 2400×g for 10 min to yield a pellet that was discarded and a low-speed supernatant (S1). S1 was used to determine the effect of different concentrations of DTDS on lipid peroxidation and reactive species levels. Protein carbonyl was assayed using the fresh brain homogenate (1/10, weight/volume) without centrifugation.
RS measurement
The RS levels were determined by a spectrofluorimetric method, using 2′, 7′-dichlorofluorescein diacetate (DCHF-DA) assayCitation20. To estimate the levels of RS production, 50 μL of S1 was incubated with 2.9 mL of 10 mM Tris–HCl buffer, pH 7.4 and 10 μL of 1 mM DCHF-DA (prepared in ethanol and protected from light and warmth) in the presence or absence of a prooxidant (30 μL of 100 mM sodium azide) and 10 μL of DTDS at different concentrations (i.e., to achieve final concentrations of 1 to 25 μM) resulting in a final volume of 3 mL. The oxidation of DCHF-DA to fluorescent dichlorofluorescein (DCHF) is measured for the detection of RS levels. The DCHF fluorescence intensity emission was recorded at 520 nm (with 480 nm excitation) 15 min after the addition of DCHF-DA to the medium. During the 15 min of incubation at room temperature, tubes were maintained in dark. RS levels were expressed as arbitrary units (AU) of fluorescence.
Lipid peroxidation induced by malonate and SNP
Malonate and SNP were used as inductors of lipid peroxidation. An aliquot of 100 μL of S1 was added to the reaction mixture containing: 50 μL of 1.5 mM malonate or 0.3 mM SNP, 10 μL of DTDS at different concentrations (i.e., to achieve final concentrations of 1 to 25 μM) and 30 μL of 50 mM Tris–HCl, pH 7.4. Afterward the mixture was pre-incubated at 37°C for 1 h. The reaction product was determined using 500 μL thiobarbituric acid (TBA, 0.8%), 200 μL sodium dodecyl sulfate (SDS, 8.1%) and 500 μL acetic acid (pH 3.4) with subsequent incubation at 95 °C for 2 h. TBA reactive species (TBARS) were spectrophotometrically determined at 532 nm as described by Ohkawa et al.21, using malondialdehyde (MDA, an end product of the peroxidation of lipids) as an external standard. Results were expressed as nmol MDA/g tissue.
Lipid peroxidation induced by FeCl2/EDTA
FeCl2 plus EDTA were used as classical inductors of lipid peroxidation. An aliquot of 200 μL of S1 was added to the reaction mixture containing: 30 µL of 500 μM EDTA solution (in water), 30 µL of 1.44 mM FeCl solution and 10 μL of DTDS at different concentrations (i.e., to achieve final concentrations of 1 to 25 μM) and water to complete a final volume of 300 µL. The FeCl solution was prepared in water, maintained in dark tube on the ice and immediately used. Afterward the mixture was pre-incubated at 37°C for 1 h. The reaction product was determined as described above Results were expressed as nmol MDA/g tissue.
Protein carbonyl determination
Carbonyl content was assayed by a method based on the reaction of protein carbonyls with dinitrophenylhydrazine (DNPH) forming dinitrophenylhydrazone, a yellow compound, measured spectrophotometrically at 370 nmCitation22. Homogenate was diluted with Tris–HCl buffer, pH 7.4 in a proportion of 1:8 (homogenate:Tris–HCl). Aliquots of 940 μL of homogenate dilutions were incubated at 37°C for 2 h in the presence of 10 μL of DTDS at different concentrations (i.e., to achieve final concentrations of 1 to 25 μM) and 50 μL of 20 mM SNP. SNP was used to stimulate the protein carbonyl production and was prepared in water, maintained in dark tube on the ice and immediately used. In two tubes, it was added 200 μL of 10 mM DNPH in 2.0 M HCl. In the third tube, only 200 μL of 2.0 M HCl solution (blank) was added. All tubes were incubated for 1 h at room temperature, in dark and shaken using a vortex mixer every 15 min. After that, 0.5 mL of denaturizing buffer (sodium phosphate buffer, pH 6.8, containing 3% SDS), 1.5 mL of ethanol and 1.5 mL of hexane were added to all tubes. The tubes were shaken with a vortex mixer for 40 s and centrifuged for 15 min at 2400×g. The pellet obtained was separated, washed two times with 1 ml of ethanol: ethyl acetate (1:1, volume/volume), and dried at room temperature for 2 min. The pellet was immediately dissolved in 1 mL of denaturizing buffer solution with mixing. Absorbance was measured at 370 nm. Results were expressed as carbonyl content (nmol carbonyl content/mg protein).
Dehydroascorbate (DHA) reductase-like assay
The DHA reductase-like activity of DTDS was assayed as described previouslyCitation23,Citation24 with minor modifications. In brief, 10 μL of DTDS at different concentrations (i.e., to achieve final concentrations of 1 to 25 μM) were incubated (2 min) with 955 μL of 100 mM sodium phosphate buffer, pH 6.9, at 25°C in the presence of 10 μL of 100–300 mM GSH (final concentrations of 1–3 mM, diluted in water) in a final volume of 1 mL. The mixture was incubated at 25°C for 2 min. The DHA reductase assay was initiated by adding 25 μL of 20 mM DHA to a final volume of 1.0 mL. DHA solution was prepared on the day of experiments. For this end, ascorbic acid was added to a solution containing 10 mM sodium phosphate dibasic and 0.5 mM EDTA to achieve a final concentration of 20 mM ascorbic acid. The pH of mixture was adjusted to 5.5 with NaOH. After that, 10 μL of bromine to each 2 ml of ascorbic acid solution pH 5.5 were added and mixed at room temperature for 30 s. Afterwards, the solution was bubbled in argon for 10 min. The DHA solution obtained was stored protected from light in ice for up to 4 h. Ascorbic acid regeneration from DHA was recorded at 265 nm. A blank without DTDS was run, and the difference gave the DTDS DHA reductase activity in nmol/min using the molar extinction coefficient of ascorbic acid of 14,700 cm−1M−1. Ebselen (1–25 μM) was used as a positive controlCitation25.
Glutathione S-transferase (GST)-like assay
The reaction of GSH with CDNB is typically the preferred system used to measure the catalysis imparted by naturally occurring GSTsCitation26. An aliquot of 10 μL of DTDS at different concentrations (i.e., to achieve final concentrations of 1 to 25 μM) was incubated with 20 μL of 50 mM GSH and 950 μL of 100 mM sodium phosphate buffer, pH 6.9 at 25°C for 3 min. The reaction was initiated by adding 20 μL of 25 mM CDNB to achieve a final volume of 1.0 mL and recorded for 3 min at 340 nm. CDNB was used as substrate. A blank without DTDS was included and the difference was expressed as ΔAbs (delta absorbance)/min. Ebselen (1–25 μM) was used as a positive controlCitation25.
Scavenging activity of 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical
The determination of the ABTS•+ radical scavenging activity was performed according to the method described by Re et al.Citation27, with some modifications. Initially, the ABTS radical was generated by reacting 7 mM ABTS solution in water with 140 mM potassium persulfate in the dark for 12–16 h. In the day of the assay, the pre-formed ABTS radical solution was diluted in potassium phosphate buffer in a proportion of 1:88 (1 mL ABTS radical + 87 mL 10 mM potassium phosphate buffer, pH 7.0). Briefly, 1 mL of ABTS radical solution was added to tubes containing 10 μL of DTDS at different concentrations (i.e., to achieve final concentrations of 1 to 25 μM). The mixture was incubated at 25°C for 30 min in dark. The decrease in absorbance was measured at 734 nm. Ascorbic acid (1–25 μM) was used as a positive control. Results were expressed as percentage of the control.
Scavenging activity of 2,2′-diphenyl-1-picrylhydrazyl (DPPH) radical
Radical-scavenging activity was determined by the reaction of the stable DPPH radical with the compound in accordance the method described by Choi et al.Citation28. An aliquot of 10 μL of DTDS at different concentrations (i.e., to achieve final concentrations of 1 to 25 μM) was mixed with 1 mL of methanolic solution containing DPPH radical, resulting in a final concentration of 85 μM DPPH. The mixture was left to stand for 30 min at room temperature in dark and the absorbance was measured at 517 nm. Ascorbic acid (1–25 μM) was used as a positive control. Results are expressed as percentage of the control.
MAO activity
Preparation of cerebral mitochondria
A preparation of cerebral mitochondria was carried out as described by Soto-Otero et al.Citation29. Rat whole brain was removed and washed in ice-cold isolation medium (pH 7.4, Na2PO4/KH2PO4 isotonized with sucrose). Cerebral mitochondria were then obtained by differential centrifugation. Briefly, after removing blood vessels and pial membranes, brain were manually homogenized with four volumes (weight/volume) of the isolation medium. Then, the homogenate was centrifuged at 900×g at 4°C for 5 min. The supernatant was centrifuged at 12,500×g for 15 min. The mitochondria pellet was then washed once with isolation medium and centrifuged again under the same conditions. Finally, the mitochondrial pellet was reconstituted in a buffer solution (Na2PO4/KH2PO4 isotonized with KCl, pH 7.4). MAO activity was performed immediately after mitochondria isolation.
Enzymatic assay
MAO activity was determined as described by KrajlCitation30 with some modifications of Matsumoto et al.Citation31. An aliquot of 100 μL of samples (100 μg of protein) was incubated at 37°C for 10 min in a medium containing buffer solution (Na2PO4/KH2PO4 isotonized with KCl, pH 7.4), specific inhibitors [selegiline (a MAO-B inhibitor, 250 nM) or clorgiline (a MAO-A inhibitor, 250 nM)] and DTDS (1–100 μM) at a final volume of 600 μL. Then 20 μL of kynuramine dihydrobromide was added to the reaction mixture (final concentration of 90 μM for MAO-A and 60 μM for MAO-B) as substrate. Samples were then incubated at 37 °C for 30 min. After incubation, the reaction was terminated by adding 300 μL of 10% trichloroacetic acid (TCA). After cooling and centrifugation at 3,000×g for 15 min, an aliquot of 1 mL of the supernatant was added to 1 mL of 1 M NaOH. The fluorescence intensity was detected spectrofluorimetrically with excitation at 315 nm and emission at 380 nm. The concentration of 4-hydroxyquinoline was estimated from a corresponding standard fluorescence curve of 4-hydroxyquinoline. MAO activity was expressed as nmol 4-OH quinoline/mg protein/min.
Protein quantification
Protein concentration was measured by the method of BradfordCitation32, using bovine serum albumin (1mg/mL) as the standard. For this, S1 was diluted in 50 mM Tris–HCl buffer (pH 7.4) in a proportion of 1:10. Then, 50 μL of S1 dilution was added to 2.5 mL of Coomassie (Bradford reagent) and mixed. After 10 min, the color product was measured at 595 nm.
Statistical analysis
Data were statistically analyzed by one-way analysis of variance (ANOVA), followed by the Newman-Keuls test when appropriate. The IC50 values were determined by linear regression from individual experiments using “GraphPad Software” (GraphPad software, San Diego, CA, USA). The IC50 values were calculated considering responses between 20 and 80% and reported as geometric means accompanied by their 95% confidence limits. The maximal inhibition (Imax) values were calculated at the most effective concentration used using “GraphPad Software”.
Results
Effect of DTDS on RS levels induced by sodium azide
The increase in RS levels induced by sodium azide was protected by DTDS at concentrations equal or greater than 10 μM (). The values for IC50 and Imax were 23.89 μM (19.46–29.34) and 47 ± 30%, respectively ().
Table 1. In vitro action of DTDS in RS, TBARS and protein carbonyl levels in the rat brain homogenate.
Table 2. Calculated IC50 (μM) and Imax (%) values for RS, lipid peroxidation (TBARS), protein carbonyl levels and MAO activity for DTDS.
Effect of DTDS on lipid peroxidation levels induced by malonate, SNP or FeCl2/EDTA
In the present study, DTDS was effective against the increase in lipid peroxidation levels induced by malonate at concentrations equal or greater than 1 μM (). The values for IC50 and Imax were 4.72 μM (2.15–10.33) and 82 ± 20%, respectively ().
In addition, DTDS showed antioxidant potential against lipid peroxidation induced by SNP at concentrations equal or greater than 10 μM (). The calculated IC50 value was 5.94 μM (4.36–8.10) while the Imax value was 92 ± 20% ().
The lipid peroxidation induced by FeCl2/EDTA in rat brain was significantly decreased by DTDS at the concentration of 25 μM (). The values for IC50 and Imax were 15.33 μM (14.31–16.4) and 61 ± 12%, respectively ().
Effect of DTDS on protein carbonyl formation induced by SNP
Statistical analysis demonstrated that DTDS, at concentrations equal or greater than 2.5 μM, was effective against protein oxidation induced by SNP () in the rat brain homogenate. The calculated IC50 value was 11.89 μM (6.31–22.40) while the Imax value was 73 ± 60% ().
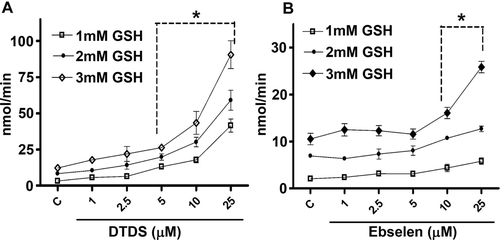
DHA reductase-like activity
The data show that DTDS acted as a GSH-dependent DHA reductase, and the rate of reduction was closely proportional to the concentration of GSH and DTDS. DTDS, at concentrations equal or greater than 5 μM, was effective in reducing DHA to ascorbic acid (. Ebselen, the positive control, at concentrations of 10 μM and greater elicited DHA reductase-like activity, which was dependent on the GSH concentration (. The effect of DTDS was superior to that of ebselen DHA reductase-like activity.
GST-like activity
In the presence of GSH, DTDS at concentrations equal or greater than 2.5 μM demonstrated GST-like activity. Ebselen (positive control) at concentrations equal or greater than 10 μM also presented GST-like activity. The results demonstrated that GST-like activity of DTDS was more effective than that of ebselen, the positive control ().
Table 3. GST-like, ABTS and DPPH radical scavenging assays for DTDS and positive controls.
ABTS and DPPH radical-scavenging activity
DTDS, at all concentrations tested, had neither ABTS nor DPPH radical-scavenging activity (p > 0.05). Ascorbic acid (positive control) showed ABTS and DPPH radical-scavenging activity at concentrations equal or greater than 5 μM and 2.5 μM, respectively ().
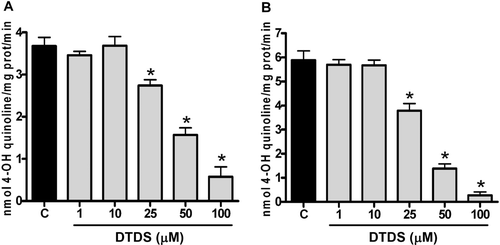
Effect of DTDS on MAO activity
Statistical analysis revealed that DTDS, at concentrations equal or greater than 25 µM, significantly inhibited both MAO-A ( and MAO-B ( activities when compared to the control tube. The IC50 values were 44.56 μM (38.26–51.91) for MAO-A and 33.28 μM (29.37–37.71) for MAO-B. The Imax values for MAO-A and MAO-B by DTDS were 84 ± 60% and 95 ± 20%, respectively ().
Discussion
Results of this study demonstrate that DTDS exhibited antioxidant action in vitro by a mechanism that involves DHA reductase- and GST-like activities. In addition, DTDS revealed to be a non-selective MAO inhibitor since it was effective in inhibiting MAO-A and MAO-B activities in vitro.
High RS levels can be critical for cells since RS can attack various biomolecules including lipids and proteins. Emerging data from a number of neurological diseases suggest that there may be common features of toxicity that are related to oxidative damageCitation3. On the other hand, selenium has been described as a potent protective agent for neurons through redox regulationCitation13. Here, DTDS, an organic compound containing selenium in its structure, showed antioxidant action by decreasing the augment of RS levels induced by sodium azide. Although selenium is probably involved in the antioxidant effect, it is interesting to consider the contribution of tiophene portion of molecule in this effect since it is known that several thiophene compounds have antioxidant propertyCitation33.
In the central nervous system, lipids are among the main targets of RS because their membranes are rich in polyunsaturated fatty acids that are highly susceptible to lipid peroxidationCitation2. The results clearly indicate that the compound DTDS protected against brain lipid peroxidation induced by SNP, malonate and FeCl2/EDTA exerting its antioxidant effect irrespective of the chemical. These results suggest that DTDS is a promising antioxidant agent and is effective in protecting lipid biomolecules by preventing the generation of toxic products resulting from several chemical inductors of lipoperoxidation.
The protein carbonyl formation can occur as a result of oxidative stress and has been shown to play an important role in a number of human diseasesCitation34. ROS are known to convert amino groups of proteins and thereby alter protein structure or functionCitation34. Interestingly, the protein carbonyl levels were reduced by DTDS in the rat brain homogenate. This finding substantiates the antioxidant action of DTDS and demonstrates that it is effective in protecting different biomolecules against the RS action.
The present data show that DTDS, at low concentrations (5 μM and greater), acted as a GSH-dependent DHA reductase, an enzyme that catalyzes the reduction of DHA to ascorbic acidCitation24. Moreover, DHA reductase-like activity of DTDS was superior to that of ebselen, a well-recognized antioxidantCitation25. Ascorbic acid is able to protecting against lipoperoxidation by acting as a scavenger of ROS and by one-electron reduction of lipid hydroperoxyl radicals via the vitamin E redox cycleCitation35. Thus, the antioxidant activity of DTDS can be attributed, at least in part, to its DHA reductase-like activity, since this recycling leads to the accumulation of ascorbic acid in tissue increasing their antioxidant capacity.
GST-like activity can also contribute to the antioxidant effect of DTDS, since this compound showed GST-like activity starting at the concentration of 2.5 μM. It is known that GSTs play an important role in cellular protection against oxidative stressCitation36 besides its detoxification function of xenobioticsCitation37. GSTs can reduce lipid hydroperoxides through their selenium-independent glutathione peroxidase activity and also detoxify lipid peroxidation end products, such as 4-hydroxynonenalCitation36. The findings presented here demonstrated that the GST-like activity of DTDS was more effective than that of ebselen, the positive control used in this test.
It was demonstrated in this study that DTDS had neither ABTS nor DPPH radical-scavenging activity, radicals widely used as antioxidant activity screening assaysCitation38. Therefore, the antioxidant activity of DTDS probably is not related to its ability in stabilizing non-natural radicals.
In addition to the antioxidant action, the findings demonstrated that DTDS acted as nonselective MAO inhibitor, since this compound was effective in inhibiting the activity of the isoforms -A and -B of MAO in the mitochondrial preparation of rat brain. Thus, there is no evidence that MAO inhibitors need to be selective for therapeutic effect since each MAO isoform can assume the function of the other when one is inhibitedCitation39. This suggests that nonselective MAO inhibitors might have broader therapeutic effects than selective MAO-A or MAO-B inhibitorsCitation40.
Oxidative deamination of monoamines by MAO is accompanied by the reduction of molecular oxygen to hydrogen peroxideCitation41, a major contributor to oxidative stress. As there are suggestions that oxidative stress plays a role in neurological diseases, we proposed that DTDS, by eliciting both antioxidant and nonselective MAO inhibitory properties, could represent an interesting neuroprotective strategy. These effects confer to DTDS a potential to be tested in experimental models of brain diseases.
Conclusions
The present study revealed that DTDS, at low concentrations (μM range), exhibited antioxidant action in vitro evidenced by the reduction of RS, lipid peroxidation and protein oxidation levels in the rat brain homogenate. The ability to mimic DHA-reductase and GST enzymes may significantly contribute to the antioxidant potential of this organoselenium compound. In addition, the results showed that DTDS is effective in inhibiting MAO-A and MAO-B activities in vitro, characterizing it as a nonselective MAO inhibitor. Therefore, DTDS might be a good candidate for future drug development for prevention or treatment of neurological diseases linked to oxidative stress.
Acknowledgments
The financial support by UFSM, CAPES, CNPq, FAPERGS/CNPq (PRONEX) research grant # 10/0005-1 and FAPERGS research grant # 10/0711-6 is gratefully acknowledged.
Declaration of interest
The authors declare that there are no conflicts of interest.
References
- Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O. Antioxidant enzyme activities and oxidative stress in affective disorders. Int Clin Psychopharmacol 2004;19:89–95.
- Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci 2005;827:65–75.
- Trushina E, McMurray CT. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience 2007;145:1233–1248.
- Dedeoglu A, Ferrante RJ, Andreassen OA, Dillmann WH, Beal MF. Mice overexpressing 70-kDa heat shock protein show increased resistance to malonate and 3-nitropropionic acid. Exp Neurol 2002;176:262–265.
- Rauhala P, Khaldi A, Mohanakumar KP, Chiueh CC. Apparent role of hydroxyl radicals in oxidative brain injury induced by sodium nitroprusside. Free Radic Biol Med 1998;24:1065–1073.
- Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett 1995;82-83:969–974.
- Chen JJ, Swope DM, Dashtipour K. Comprehensive review of rasagiline, a second-generation monoamine oxidase inhibitor, for the treatment of Parkinson’s disease. Clin Ther 2007;29:1825–1849.
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci 1999;22:197–217.
- Shih JC, Thompson RF. Monoamine oxidase in neuropsychiatry and behavior. Am J Hum Genet 1999;65:593–598.
- Bortolato M, Chen K, Shih JC. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Deliv Rev 2008;60:1527–1533.
- Stazi AV, Trinti B. [Selenium deficiency in celiac disease: risk of autoimmune thyroid diseases]. Minerva Med 2008;99:643–653.
- Steinbrenner H, Sies H. Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta 2009;1790:1478–1485.
- Ozdemir E. Physiological role of selenium and selenoprotein in neuropsychiatric disease. J Med Sci 2011;11:11–18.
- Doucha J, Lívanský K, Kotrbácek V, Zachleder V. Production of Chlorella biomass enriched by selenium and its use in animal nutrition: a review. Appl Microbiol Biotechnol 2009;83:1001–1008.
- Nogueira CW, Zeni G, Rocha JB. Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem Rev 2004;104:6255–6285.
- Nogueira CW, Rocha JBT. Diphenyl Diselenide a Janus-Faced Molecule. J Braz Chem Soc 2010;21:2055–2071.
- Bortolatto CF, Jesse CR, Wilhelm EA, Ribeiro LR, Rambo LM, Royes LF et al. Protective effect of 2,2′-dithienyl diselenide on kainic acid-induced neurotoxicity in rat hippocampus. Neuroscience 2011;193:300–309.
- Tiecco M, Testaferri L, Bagnoli L, Marini F, Temperini A, Tomassini C, Santi C. Electrophilic 2-Thienylselenenylation of thiophene. Preparation of oligo (seleno-2,5-thienylenes). Tetrahedron 2000;56:3255–3260.
- Engman L. Expedient synthesis of ebselen and related compounds. J Org Chem 1989;54:2964–2966.
- Loetchutinat C, Kothan S, Dechsupa S, Meesungnoen J, Jay-Gerin J, Mankhetkorn S. Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2′, 7′-dichlorofluorescein diacetate assay. Radiat Phy Chem 2005;72:323–331.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–358.
- Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Meth Enzymol 1994;233:357–363.
- Washburn MP, Wells WW. Identification of the dehydroascorbic acid reductase and thioltransferase (Glutaredoxin) activities of bovine erythrocyte glutathione peroxidase. Biochem Biophys Res Commun 1999;257:567–571.
- Wells WW, Xu DP, Washburn MP. Glutathione: dehydroascorbate oxidoreductases. Meth Enzymol 1995;252:30–38.
- Jung CH, Washburn MP, Wells WW. Ebselen has dehydroascorbate reductase and thioltransferase-like activities. Biochem Biophys Res Commun 2002;291:550–553.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–7139.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26:1231–1237.
- Choi CW, Kim SC, Hwang SS, Choi BK, Ahn HJ, Lee MY, Park SH, Kim SK. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci 2002;163:1161–1168.
- Soto-Otero R, Méndez-Alvarez E, Hermida-Ameijeiras A, Sánchez-Sellero I, Cruz-Landeira A, Lamas ML. Inhibition of brain monoamine oxidase activity by the generation of hydroxyl radicals: potential implications in relation to oxidative stress. Life Sci 2001;69:879–889.
- Krajl M. A rapid microfluorimetric determination of monoamine oxidase. Biochem Pharmacol 1965;14:1684–1686.
- Matsumoto T, Furuta T, Nimura Y, Suzuki O. 3-(p-hydroxyphenyl)propionic acid as a new fluorogenic reagent for amine oxidase assays. Anal Biochem 1984;138:133–136.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Abu-Hashem AA, El-Shehry MF, Badria FA. Design and synthesis of novel thiophenecarbohydrazide, thienopyrazole and thienopyrimidine derivatives as antioxidant and antitumor agents. Acta Pharm 2010;60:311–323.
- Almroth BC, Sturve J, Berglund A, Förlin L. Oxidative damage in eelpout (Zoarces viviparus), measured as protein carbonyls and TBARS, as biomarkers. Aquat Toxicol 2005;73:171–180.
- Halliwell B, Gutteridge JM. Free radicals in biology and medicine. New York: Oxford University Press, 1999.
- Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal 2004;6:289–300.
- Dourado DF, Fernandes PA, Mannervik B, Ramos MJ. Glutathione transferase A1-1: catalytic importance of arginine 15. J Phys Chem B 2010;114:1690–1697.
- Baltrušaitytė V, Venskutonis PR, Čeksterytė V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem 2007;101:502–514.
- Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW. Effects of selective monoamine oxidase inhibitors on the in vivo release and metabolism of dopamine in the rat striatum. J Neurochem 1990;55:981–988.
- Aubin N, Barneoud P, Carter C, Caille D, Sontag N, Marc C et al. SL25.1131 [3(S),3a(S)-3-methoxymethyl-7-[4,4,4-trifluorobutoxy]-3,3a,4,5-tetrahydro-1,3-oxazolo[3,4-a]quinolin-1-one], a new, reversible, and mixed inhibitor of monoamine oxidase-A and monoamine oxidase-B: biochemical and behavioral profile. J Pharmacol Exp Ther 2004;310:1171–1182.
- Rigby SE, Basran J, Combe JP, Mohsen AW, Toogood H, van Thiel A et al. Flavoenzyme catalysed oxidation of amines: roles for flavin and protein-based radicals. Biochem Soc Trans 2005;33:754–757.