Abstract
A new iridoid glycoside, 6′-O-E-caffeoyl-mussaenosidic acidCitation, in addition to one known aglycon, four known triterpenes and one known flavonoid, were isolated from the aerial parts of Scutellaria albida subsp. albida. Furthermore, 12 iridoids with similar structures isolated from Scutellaria sp., were examined for their inhibitory potency on lipoxygenase and lipid peroxidation, as well as their antioxidant activity, in comparison to known antioxidants e.g. caffeic acid, nordihydroguaretic acid (NDGA) and trolox. AAPH, DPPH and soybean lipoxygenase (LOX) assays were used for the tests. This investigation led to interesting observations considering the Structure-Activity Relationship. According to our results, the presence of a p-coumaroyl group optimized and even dramatically changed the biological responses of the investigated iridoids.
Keywords::
Introduction
More than 350 Scutellaria species are of cosmopolitan distribution, many of which are medicinally active. In Eurasia they are found from Syria up to the Altai Mountains and the Himalayas and are also common in Central Africa, Malaysia and Australia. In America 113 species have so far been located from the Arctic Circle to Tierra del Fuego, with the greatest concentration in Central MexicoCitation1.
Previous investigations into Scutellaria albida L. ssp. albidaCitation2,Citation3, collected in Greece (Mount Pelion), showed that the main products of the plant are the iridoids catalpolCitation4,Citation5 and albidosideCitation4, with catalpol being a useful taxonomic marker for the genus ScutellariaCitation6. In continuation of our phytochemical research, we report on the isolation and structure elucidation of one new iridoid glycoside, 6′-O-E-caffeoyl-mussaenosidic acid 1 in addition to 6′-O-E-caffeoylcatalpol, rehmaglutin, the triterpenes stigmasterol, β-sitosterol, betulin, betulinic acid and the flavonoid 4′-hydroxywogonin-7-O-β-D-glucuronic acid glycoside.
Iridoids exhibit a wide range of bioactivityCitation7,Citation8 and are produced in high amounts in many species of Scutellaria. The fact that their anti-inflammatory activity is reported to be most pronounced is quite interesting, since inflammatory diseases include rheumatoid arthritis, allergy, asthma, as well as auto-immune diseases and cancerCitation9. Iridoids seem to exert their anti-inflammatory activity through considerable inhibition on the Arachidonic Acid (AA) cascade. AA is a 20-carbon unsaturated fatty acid produced from membrane phospholipids and its derivatives are potent mediators of inflammation. Therefore, inhibition of the biosynthesis of pro-inflammatory molecules will terminate inflammation and may be achieved at different levels by antioxidants and anti-inflammatory drugsCitation10,Citation11. Oxygen species are known to be key participants in several pathophysiological situations and directly effect lipid peroxidation in membranes and cellular components. Consequently, antioxidants preventing the presumed damaging effects of free radicals in the human body, as well as the deterioration of fats and other constituents in food products are much in demand. Nowadays there is preference for antioxidants from natural rather than from synthetic sourcesCitation12.
Therefore, in this study, we undertook to investigate the above mentioned inhibitory effect of 12 iridoids isolated from two Scutellaria sp., S. albida L. ssp. albida and Scutellaria goulimyi Rech. fCitation13. Their inhibitory potency on lipoxygenase and lipid peroxidation, as well as their antioxidant activity has been examined in comparison to known antioxidants e.g. caffeic acid, nordihydroguaretic acid (NDGA) and trolox (). To our knowledge this is the first report on the bioactivity of the tested compounds. This investigation led to interesting observations considering the Structure-Activity Relationship (SAR), since typically SARs are explored on a case-by-case basis, given a specific target and a series of active compounds.
Methods
Plant material
The aerial parts of S. albida ssp. albida were collected at Mount Pelion (Central Greece) in June 2001. The plant was authenticated by Dr. T. Constantinidis and a voucher specimen was deposited in the Herbarium (ACA-Lazari & Gousiadou 001).
General experimental procedures
Optical rotations were measured on a Perkin–Elmer 341 polarimeter. IR spectra were obtained on a Perkin–Elmer Paragon 500 FT-IR spectrophotometer. UV spectra were recorded on a Shimadzu UV-160A spectrophotometer. IR spectra were obtained on a Perkin–Elmer PARAGON 500 FT-IR spectrophotometer. LCHRESIMS was performed on an AgilentHP 1100 HPLC equipped with a BDS-C18 reversed-phase column running a H2O-MeCN (50 ppm TFA in H2O) gradient. The LC was coupled to a LCT of a TOF MS (Micromass, Manchester, UK) operated in the positive electrospray ion mode using 5-leucineenkephalin as lock mass. 1H and 2D NMR spectra were recorded in CD3OD on Bruker DRX-400 at 295 K. Chemical shifts are given in parts per million (ppm) and were referenced to the solvent signals at δ 3.31 and 49.5 for 1H and 13C NMR, respectively. COSY, HMQC, HSQC, HMBC, and NOESY (mixing time 950 ms) were performed using standard Bruker microprograms. Vacuum liquid chromatography (VLC): silica gel (Merck; 43–63 µm). Column chromatography: silica gel (SDS; 40–63 µm), gradient elution with the solvent mixtures indicated in each case. HPLC support: preparative HPLC was performed using a C18 25 cm × 10 mm Kromasil column on a Jasco system equipped with a PU 980 pump, RI-930 refractive index detector (Jasco Corporation, Tokyo, Japan). Fractionation was always monitored by TLC silica gel 60 F-254, Merck, Art. 5554 with visualization under UV (254 and 365 nm).
Extraction and isolation
The methanolic extract of the aerial parts of S. albida ssp. albida yielded one new iridoid glucoside, 6′-O-E-caffeoyl-mussaenosidic acidCitation1, the iridoid glucoside 6′-O-E-caffeoylcatalpolCitation14, one known iridoid aglycon, rehmaglutinCitation15 and one known flavonoid, 4′-hydroxywogonin-7-O-β-D-glucuronic acid glycosideCitation16. Also, the acetonic extract, offered four known triterpenes, stigmasterolCitation17, β-sitosterolCitation18,Citation19, betulinCitation20, and betulinic acidCitation20,Citation21. The plant material has been extracted as previously describedCitation2. The dried MeOH extract (27.6 g) was subjected to VLC over silica gel (10 × 8 cm) using as eluent CH2Cl2-MeOH mixtures of increasing polarity to yield finally seven fractions (MA′-MR′). Fraction MM’ (8.7 g; eluted with CH2Cl2-MeOH 65:35–60:40) was further applied to VLC over silica gel using EtOAc-MeOH and yielded sixteen fractions (MM′A-MM′P). From fraction MM′C (228.3 mg, eluted with EtOAc-MeOH 92:8) 74.3 mg were further subjected to RP-HPLC (MeOH-H2O 30:70) and yielded 6′-O-caffeoylcatalpol (5.31 mg; tR 23.5 min). Fraction MM′E (370 mg; eluted with EtOAc-MeOH 90:10) was submitted to CC on silica gel (CH2Cl2-MeOH 95:5-50:50) and yielded eight fractions (MM′EA-MM′EH). Fraction MM′EF (33.6 mg, eluted with CH2Cl2-MeOH 85:15) was further subjected to RP-HPLC (MeOH-H2O 35:65) and finally yielded the hitherto unknown compound 1 (2.7 mg; tR 8.0 min). Fraction MO′ (5.6 g, CH2Cl2-MeOH 55:45–40:60) was subjected for further purification to RP-MPLC using a mixture of solvents (H2O-MeOH) of decreasing polarity. The aqueous residue MO′A (1.69 g, H2O 100%) was submitted to CC on silica gel (EtOAc-MeOH-H2O, increasing polarity) and yielded twenty one fractions (MO′AA-MO′AU). Fraction MO′AC (EtOAc-MeOH-H2O, 90:10:1) was rehmaglutin A (2.4 mg). Fraction MO′AR (118 mg, EtOAc-MeOH-H2O, 80:20:2) was further subjected to Sephadex LH-20 (MeOH) and yielded 4′-hydroxywogonin-7-O-β-D-glucuronic acid glycoside (0.4 mg). The dried acetone extract (6.3 g) was subjected to VLC over silica gel (10 × 8 cm) using as eluent cyclohexane-EtOAc mixtures of increasing polarity and subsequently yielded thirteen fractions (AA-AM). Fraction AD (496.6 mg; eluted with cyclohexane-EtOAc 70:30) was further subjected to CC on Sephadex LH-20 (cyclohexane-CH2Cl2-MeOH 70:30:5) and yielded three fractions (ADA′-ADC′). Both fractions ADA′ (355.9 mg) and ADB′ (48.8 mg) were further subjected to HPLC (cyclohexane-CH2Cl2-MeOH 70:30:5) and finally yielded stigmasterol (2.3 mg; tR 42.9 min), β-sitosterol (5.3 mg; tR 21.2 min), betulin (2.0 mg; tR 43.4 min) and betulinic acid (2.7 mg; tR 36.2 min). The known compounds were identified by spectral analysis and direct comparison of their physical properties with those reported previously for these compounds.
6’-O-E-caffeoyl-mussaenosidic acid 1
Yellowish powder; [a]D20 = −13.04 (c 0.25, MeOH); UV (MeOH) λ maximum (log ϵ):300.5 sh, 312; IR (CaF2): νmax cm-1: 3352 (O-H), 2914 (C-H), 1644 (C = O), 1607 (C=C); for 1H and 13C NMR spectra, see ; HR-ESI-MS m/z 539.1684 [M+H]+ (calcd for C25H30O13: 539.1686).
Table 1. Spectral data of compound 1 in CD3OD Hz (400 MHz).
In vitro assays
In are given the concentrations of the stock and final solutions of the tested compounds in ethanol or DMSO. A Perkin–Elmer Lambda 20 UV-Vis spectrophotometer has been used for the radical scavenging activity experiments. Each in vitro experiment was performed at least in triplicate and the standard deviation of absorbance was less than 10% of the mean. All the chemicals used were of analytical grade and commercially available by Merck. 1,1-Diphenyl-2-picrylhydrazyl (DPPH), trolox and nordihydroguairetic acid (NDGA) were purchased from the Aldrich Chemical Co. Milwaukee, WI, (USA). Soybean Lipoxygenase, linoleic acid sodium salt and 2, 2′-Azobis (2-amidinopropane) dihydrochloride (AAPH) were obtained from Sigma Chemical, Co. (St. Louis, MO, USA).
Soybean lipoxygenase inhibition activity
The soybean lipoxygenase (LOX) assay was used as an indication of anti-inflammatory activityCitation22. LOX is a key enzyme in the inflammatory cascade, whose inhibition is correlated to the ability of the inhibitors to reduce Fe+3 at the active site to the catalytically inactive Fe+2. LOXs contain a “non-heme” iron per molecule in the enzyme active site as high-spin Fe+2 in the native state and the high-spin Fe+3 in the activated state. Several LOX inhibitors, such as phenolic derivatives, are excellent ligands for Fe+3.
Ethanolic solution of the tested compound was incubated with sodium linoleate (0.1 mM) and 0.2 mL of soybean lipoxygenase solution (1/9 × 10–4 w/v in saline) at room temperature. The conversion of sodium linoleate to 13-hydroperoxylinoleic acid was recorded at 234 nm and compared with the standard inhibitor caffeic acid according to the procedure previously reportedCitation23.
Interaction with 1,1-diphenyl-picrylhydrazyl (DPPH) activity
Several methods are used for the estimation of efficiency of synthetic/natural antioxidants, like the 2, 2′-azobis(2-amidinopropane) dihydrochloride (AAPH)/linoleic acid assayCitation24, 1,1-diphenyl-1-picrylhydrazyl (DPPH) assayCitation25–29. The antioxidant activity of the 12 iridoids has been evaluated in both in vitro tests. In view of the differences among the test systems available, the results of a single assay can give only a suggestion on the protective potential of phytochemicals.
Among the plethora of methods used for the evaluation of the antioxidant activity, the DPPH test is very useful in the micromolar range, demanding minutes to hours for both lipophilic and hydrophilic samples. In literature, many studies concerning the antioxidant activity of natural substances tested with DPPH have been reportedCitation25–29. In the presence of an antioxidant, which can donate an electron to DPPH, the purple color is typical of the free DPPH radical decays, a change which can be followed spectrophotometrically (517 nm). This interaction indicates its radical scavenging ability in an iron-free system.
To a solution of DPPH (0.05 mM) in absolute ethanol, an equal volume of 0.1 mM or 20 μL (at the final 2 mL volume) ethanolic solution of the tested compound was added. After 20 and 60 min the absorbance was recorded at 517 nm and compared with the appropriate standard NDGA. Ethanol was used as a controlCitation23.
Inhibition of linoleic acid lipid peroxidation
This assay can be used to follow oxidative changes and to understand the contribution of each tested compound. Azo compounds generating free radicals through spontaneous thermal decomposition are useful for in vitro studies of free radical production. The water soluble azo compound AAPH has been extensively used as a clean and controllable source of thermally produced alkylperoxyl free radicals. Oxidation of linoleic acid to conjugated diene hydroperoxide in an aqueous dispersion is monitored at 234 nm. AAPH was used as a free radical initiator. Ten microliters of the 16 mM linoleic acid dispersion was added to the UV cuvette containing 0.93 mL of 0.05 M phosphate buffer, pH 7.4.
The oxidation reaction was initiated under air by the addition of 50 μL of 40 mM AAPH solution. Oxidation was carried out in the presence of compounds (10 μL). In the assay no antioxidant lipid oxidation was measured in the presence of the same level of ethanol. The rate of oxidation at 37°C was monitored by recording the increase of absorption at 234 nm caused by conjugated diene hydroperoxides. The results were compared to the standard inhibitor troloxCitation24,Citation29.
Results
Extraction and isolation
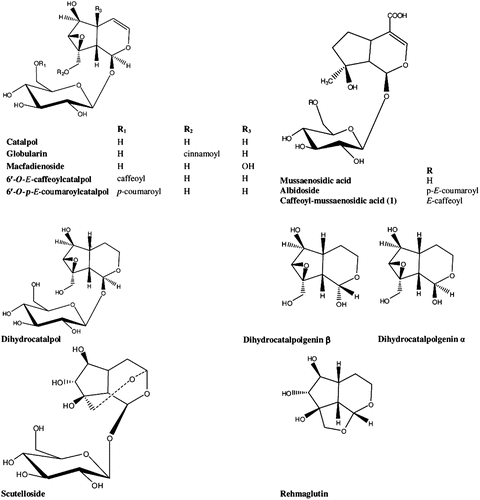
Compound 1 was obtained as yellowish powder with a molecular formula C25H30O13. 1D and 2D NMR spectral data () indicated that 1 consisted of a 4-substituted iridoid structure containing a β-glucopyranosyl moiety esterified with an aromatic acid at C-6′. Detailed analysis revealed the presence of a mussaenosidic acid moietyCitation30, esterified to a caffeoyl group. The 1H NMR spectrum of 1 exhibited signals of a substituted aromatic ring (ABX system) and the vinylic proton signals of a trans double bond, which together with HSQC and HMBC data led to the identification of a trans-caffeoyl moiety. Furthermore, downfield shifts from normal of the sugar protons H-6a′ and H-6b′ were observed, which indicated esterification at C-6′ of the β-glucopyranosyl moiety. In the 13C spectrum, the C-6′ resonance of the β-D-glucopyranose was typically deshielded (α-effect) while the C-5′ resonance was shifted upfield (β-effect) due to the acylation of the primary hydroxyl function. HMBC confirmed the position of the caffeoyl residue by showing a clear long-range correlation peak between the carbonyl carbon and H-6′a of the glucopyranosyl unit. Therefore 1 was assigned as 6′-O-E-caffeoylcatalpolcaffeoyl-mussaenosidic acid ().
The presence of 1 bears biosynthetic significanceCitation2, since it is in concert with data indicating that biosynthetically, all iridoids in S. albida ssp. albida are likely to arise from 8-epi-deoxy-loganic acidCitation30.
Also, to our knowledge, this is the first report concerning the presence of rehmaglutin A in the Scutellaria genus.
The chemical profile of plants belonging to the genus Scutellaria varies widely. Some are characterized by the presence of iridoids, especially catalpol and its esters, while in others different metabolic routes are engagedCitation31. In this investigation we have been able to trace in small amounts a wogonin derivative, 4′-hydroxywogonin-7-O-β-D-glucuronic acid glycoside. Wogonin is a major constituent of S.baicalensisCitation32, a non-iridoid producing member of the genus.
Bioactivity
Iridoids with similar structures () after being submitted to three biological assays exhibited different levels of bioactivity. Most of them exhibited none or negligible inhibitory activity on lipoxygenase, except for albidoside, which inhibited with IC50 value of 62.5 μM (). This value was ten times lower than that of the known lipoxygenase inhibitor, caffeic acid ().
Table 2. Interaction% with DPPH reducing ability RA%; in vitro inhibition of soybean lipoxygenase at 0.1 mM LO)/ (IC50); %Inhibition of lipid peroxidation (AAPH%) at 0.1 mM.
The tested iridoids interacted with DPPH variably. The results are time independent. Macfadienoside, catalpol, rheumaglutin and mussaenosidic acid showed no activity. Scutelloside, albidoside, dihydrocatalpol, caffeoyl-mussaenosidic acid and the mixture of dihydrocataipolgenins A and B interacted weakly. Globularin, 6′-O-p-E-coumaroylcatalpol and 6′-O-E-caffeoylcatalpol exhibited strong interaction, though less than that of the appropriate standard NDGA ().
Most of the tested iridoids exhibited weak or negligible LP inhibition, with the exception of albidoside, dihydrocatalpolgenins A and B and caffeoyl-mussaenosidic acid, which showed a very strong inhibition activity, much stronger than the standard inhibitor trolox ().
Discussion and conclusions
Structure activity relationship
Our objective has been to evaluate closely related structures and provide data for consequent SAR establishment, if possible, since the exploration of SARs is an important task in medicinal chemistry and drug design. SARs can display very different features. We observed that small chemical differences between active molecules altered, often dramatically, biological responses. The magnitude of the response of active compounds to chemical variations distinguishes many SARs. For example, small chemical differences can render active molecules completely or nearly inactive or, alternatively, increase their potency. Large-magnitude biological responses to minor chemical variations are characteristic of SARs that are discontinuous in nature. In our investigation, we have encountered such cases as will be analytically described further onCitation33,Citation34. However, it is well-recognized that SAR characteristics often depend on the types of molecules under study. For instance, it has been reportedCitation35 that the antioxidant activity exhibited in the case of phenylethanoid glucosides, may be mainly related to the number of aromatic methoxy and hydroxyl groups and the structure of the acyl moiety, while its position is without significance. Deacylation of phenylethanoids led to a 30-fold decrease of activity and resulted in compounds with moderate antioxidative effects. We have found this to be partly true for iridoids. In a series of four molecules with identical main structure, namely catalpol, globularin, 6′-O-E-caffeoylcatalpol and 6′-O-p-E-coumaroylcatalpol, the presence of the acyl moiety was decisive for the appearance of activity while its position made no difference. But we also observed that the activity did not increase with the number of hydroxyl groups. On the contrary, while the presence of the p-coumaroyl moiety made 6′-O-p-E-coumaroylcatalpol the strongest antioxidant, a weak LOX inhibitor and a strong LP inhibitor, followed closely by globularin bearing a cinnamoyl substitution, the least active with a considerable difference was 6′-O-E-caffeoylcatalpol, substituted with a caffeoyl group. Catalpol was found to be almost inactive, exhibiting only a weak LP inhibition. Macfadienoside, bearing an extra hydroxyl group on C5 compared to catalpol, was inactive.
Most interesting was the case of mussaenosidic acid, albidoside and caffeoyl-mussaenosidic acid, respectively, another series of molecules with identical main structure, differently substituted. The nonsubstituted mussaenosidic acid had no antioxidant activity and was a weak LOX and LP inhibitor. A caffeoyl moiety as a substitute increased slightly the antioxidant activity and dramatically the LP inhibition of caffeoyl-mussaenosidic acid. But it was the presence of a p-coumaroyl group that changed completely the biological response of albidoside, making this compound a strong LOX and LP inhibitor, with a performance far beyond that of the standard inhibitors caffeic acid and trolox respectively. Furthermore, these results were not in concert with previous papers reporting that only the iridoid aglycons could possibly exert such activityCitation36,Citation37. In conclusion, according to our results, the presence of a p-coumaroyl group optimized and even changed dramatically the biological responses of the investigated iridoids. This seems to be a case of discontinuous SAR.
The dihydro-derivative of catalpol, dihydrocatalpol, exhibited a moderate interaction with DPPH, while the 1:1 mixture of its aglycons dihydrocatalpolgenin A and B were weak antioxidants but excellent LP inhibitors. The aglycons alone did not inhibit LOX, which according to literature should be the caseCitation37.
Finally, scutelloside and the aglycon rehmaglutin were nearly or completely inactive, despite the increased number of free hydroxyl groups on their main structure. This could be attributed to the presence of an ether linkage in these compounds, resulting to the formation of a rigid three ring skeleton which renders them inactive. Rehmaglutin is probably a degradation product from catalpol.
Acknowledgments
We thank Dr. K. F. Nielsen, BioCentrum, DTU, DK, for providing high-resolution ESIMS data, as well as to Dr. Theophanis Constantinidis (Assistant Professor, Faculty of Biology, University of Athens) for the identification of the plant material.
Declaration of interest
The authors report no declarations of interest.
References
- Cole I, Saxena P, Murch S. Medicinal biotechnology in the genus Scutellaria. In Vitro Cell Dev Biol Plant 2007; 43: 318–327.
- Gousiadou C, Karioti A, Heilmann J, Skaltsa H. Iridoids from Scutellaria albida ssp. albida. Phytochemistry 2007;68:1799–1804.
- Skaltsa H, Lazaris D, Loukis A, Harvala C. Flavonoids from Scutellaria albida L. ssp. albida. Scientia Pharmaceutica 1996; 64: 103–106.
- Çalis I, Ersoz T, Saracoglou I, Sticher O. Scalbidoside and albidoside, two iridoid glycosides from Scutellaria albida subsp. colchica. Phytochemistry 1993; 32: 1213–1217.
- Chaudhuri RK, Sticher O. New iridoid glucosides and a lignan diglucoside from Globularia alypum. Helv Chim Acta 1981; 64:3–15.
- Cole MD, Paton AJ, Harley RM, Fellows LA. The significance of the iridoid glycoside, catalpol, in Scutellaria. Biochem Syst Ecol 1991;19: 333–335.
- Villaseñor MI. Bioactivities of Iridoids. Anti-Inflamm Anti-Aller Age Medi Chem 2007; 6: 307–314.
- Ghisalberti EL. Biological and pharmacological activity of naturally occurring iridoids and secoiridoids. Phytomedicine 1998; 5:147–163.
- Inflammation, Wikipedia, the free encyclopedia. Available at: http://en.wikipedia.org/wiki/Inflammation
- Lévy-Toledano S. Platelet signal transduction pathways: could we organize them into a ‘hierarchy’? Haemostasis 1999;29:4–15.
- Grantström E. The arachidonic acid cascade: the prostaglandins, thromboxanes and leukotrienes. Inflammation 1984; 8 Supplement: 15–25.
- Abdalla A, Roozen J. Effect of plant extracts on the oxidative stability of sunflower oil and emulsion. Food Chemistry 1999; 64: 323–329.
- Gousiadou C, Gotfredsen CH, Jensen SR, Tsoukalas M. Iridoids from Scutellaria goulimyi Rech. f., Lamiaceae. Morphological and chemical relations with Scutellaria albida L. ssp albida. Biochemical Systematics and Ecology, Accepted
- Taskova RM, Kokubun T, Ryan KG, Garnock-Jones PJ, Jensen SR. Iridoid and phenylethanoid glucosides from Veronica lavaudiana. J Nat Prod 2011;74:1477–1483.
- Morota T, Nishimura H, Sasaki H, Chin M, Sugama K, Katsuhara T, Mitsuhashi H. Five cyclopentanoid monoterpenes from Rehmannia glutinosa. Phytochemistry 1989; 28: 2385–2391.
- Yan MM, Zhao DQ, Shao S, Liu WH, Bi SN, Wang HJ. [Studies on the constituents from the herba of Erigeron acer]. Zhong Yao Cai 2008;31:1334–1336.
- De-Eknamkul W, Potduang B. Biosynthesis of beta-sitosterol and stigmasterol in Croton sublyratus proceeds via a mixed origin of isoprene units. Phytochemistry 2003;62:389–398.
- Naas R, Rimpler H. Distribution of iridoids in different populations of Physostegia virginiana and some related remarks on iridoids from Avicennia officinalis and Scrophularia ningpoensis. Phytochemistry 1996; 41: 489–498.
- Nes W, Norton R, Benson M. Carbon-13 NMR studies on sitosterol biosynthesized from [13C] mevalonates. Phytochemistry 1992; 31: 805–811.
- Ghosh A, Misra S, Dutta K, Choudhury A. Pentacyclic triterpenoids and sterols from seven spieces of Mangrove. Phytochemistry 1985; 24: 1725–1727.
- Green B, Bentley MD, Chung BY, Lynch NG, Jensen BL. Isolation of Betulin and Rearrangement to Allobetulin. A Biomimetic Natural Product Synthesis. J Chem Educ 2007; 84: 1985
- Müller K. 5-Lipoxygenase and 12-lipoxygenase: attractive targets for the development of novel antipsoriatic drugs. Arch Pharm (Weinheim) 1994;327:3–19.
- Pontiki E, Hadjipavlou-Litina D. Antioxidant and anti-inflammatory activity of aryl-acetic and hydroxamic acids as novel lipoxygenase inhibitors. Med Chem 2006;2:251–264.
- Liégeois C, Lermusieau G, Collin S. Measuring antioxidant efficiency of wort, malt, and hops against the 2,2′-azobis(2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. J Agric Food Chem 2000;48:1129–1134.
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 2005;53:4290–4302.
- Sacchetti G, Medici A, Maietti S, Radice M, Muzzoli M, Manfredini S et al. Composition and functional properties of the essential oil of amazonian basil, Ocimum micranthum Willd., Labiatae in comparison with commercial essential oils. J Agric Food Chem 2004;52:3486–3491.
- Sökmen M, Serkedjieva J, Daferera D, Gulluce M, Polissiou M, Tepe B et al. In vitro antioxidant, antimicrobial, and antiviral activities of the essential oil and various extracts from herbal parts and callus cultures of Origanum acutidens. J Agric Food Chem 2004;52:3309–3312.
- Dukic N, Bozin, Sokovic, M, Simin N. Antimicrobial and Antioxidant Activities of Melissa officinalis L. (Lamiaceae) Essential Oil. J. Agric. Food Chem. 2004; 52: 2485–2499.
- Rajic Z, Hadjipavlou-Litina D, Pontiki E, Kralj M, Suman L, Zorc B. The novel ketoprofen amides–synthesis and biological evaluation as antioxidants, lipoxygenase inhibitors and cytostatic agents. Chem Biol Drug Des 2010;75:641–652.
- Damtoft S. Biosynthesis of catalpol. Phytochemistry 1994; 35: 1187–1189.
- Jensen SR. Plant iridoids, their biosynthesis and distribution in angiosperms. In (Eds. Harborne &Barbarán). Annual Proceedings of the Phytochemical Society of Europe. Ecological Chemistry and Biochemistry of Plant Terpenoids. Oxford University Press; 1991, pp. 133–158.
- Huang ST, Wang CY, Yang RC, Chu CJ, Wu HT, Pang JH. Wogonin, an active compound in Scutellaria baicalensis, induces apoptosis and reduces telomerase activity in the HL-60 leukemia cells. Phytomedicine 2010;17:47–54.
- Peltason L, Bajorath J. SAR index: quantifying the nature of structure-activity relationships. J Med Chem 2007;50: 5571–5578.
- Wawer M, Peltason L, Weskamp N, Teckentrup A, Bajorath J. Structure-activity relationship anatomy by network-like similarity graphs and local structure-activity relationship indices. J Med Chem 2008;51:6075–6084.
- Heilmann J, Calis I, Kirmizibekmez H, Schühly W, Harput S, Sticher O. Radical scavenger activity of phenylethanoid glycosides in FMLP stimulated human polymorphonuclear leukocytes: structure-activity relationships. Planta Med 2000;66:746–748.
- Ling SK, Tanaka T, Kouno I. Effects of iridoids on lipoxygenase and hyaluronidase activities and their activation by beta-glucosidase in the presence of amino acids. Biol Pharm Bull 2003;26:352–356.
- Park KS, Kim BH, Chang IM. Inhibitory potencies of several iridoids on cyclooxygenase-1, cyclooxygnase-2 enzymes activities, tumor necrosis factor-a and nitric oxide production in vitro. Evid Based Complement Alternat Med 2010;7:41–45.