Abstract
Apocynin (APO), curcumin (CUR) and vanillin (VAN) are o-methyl catechols widely studied due their antioxidant and antitumour properties. The effect of treatment with these o-methyl catechols on tamoxifen (TAM)-induced cytotoxicity in normal and tumour cells was studied. The cytotoxicity of TAM on red blood cells (RBC) was performed by haemoglobin or K+release and on polymorphonuclear leukocytes (PMNs) by trypan blue dye exclusion method. Cytotoxic activity was assessed in human chronic myeloid leukemia (K562) cell line by (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide). According the release of haemoglobin and K+, the CUR showed a decrease in TAM cytotoxicity on RBC; however, in PMN, APO, CUR and VAN showed increased of these cells viability. VAN presented the highest cytotoxicity on K562 cells, followed by APO and CUR. These results point the potential therapeutic value of these o-methyl catechols with TAM, particularly of CUR, which potentiates the cytotoxic effects of TAM on K562 cells and also decreases TAM-associated cytotoxicity on RBC and PMN.
Keywords::
Introduction
Curcuma longa L., traditionally known as turmeric, is one of the most widely used herbs in traditional medicine, due to its aromatic and therapeutic properties, especially in China and India, from the earliest times.
Curcumin (CUR) [1,7-bis(4-hydroxy-3methoxyphenyl)-1,6-heptadiene-3,5-dione] () is among the most promising and effective agentsCitation1. The multiple therapeutic effects of CUR are due to its ability to modulate the activities of various enzymes as well as gene expression in tumour cells, thereby affecting cell proliferation and apoptosisCitation2.
Apocynin (APO) (1-[4-hydroxy-3-methoxyacetophenone]) () is a compound extracted from the roots of Picrorhiza kurroa, a plant native to the mountains of India, Nepal, Tibet and Pakistan. APO has the ability to inhibit the enzyme complex NADPH oxidaseCitation3.
Vanillin (VAN) (4-hydroxy-3-methoxybenzaldehyde) () is widely used due to its pleasant aroma and flavour. It can be found in the berries and seeds of Vanilla planifoliaCitation4. Some of the mechanisms proposed for the antitumoural activity of VAN involve its ability to suppress the enzymatic activity of matrix metalloproteinase-9 and inhibit DNA-PKCitation5,Citation6.
Cancer is considered a public health problem in developed and developing countriesCitation7. According to the latest report from the International Agency for Research on Cancer/WHO, the incidence was estimated at 12.7 million new cases annually, with cancer causing the deaths of 7.6 million people worldwide per yearCitation8.
Tamoxifen (TAM) is a synthetic no steroidal anti-estrogen, it is commonly prescribed to treat patients with breast cancerCitation9. TAM belongs to the family of selective modulators of estrogen receptors. These are molecules that bind with high selectivity and affinity to estrogen receptors, resulting in molecular and biological activityCitation1. Additionally, TAM has been proposed to function via an interaction with lipids and proteinsCitation10, inhibition of protein kinase C and cAMP phosphodiesterase or induction of apoptosis; however, the exact mechanisms of TAM action have not been fully elucidatedCitation11.
Most chemotherapeutic agents not only induce cell death in tumour cells but also induce severe damage to normal cells, causing serious side effects as well as major difficulties in treatment adherence. Documented adverse effects of chronic TAM use include hyperplasia, formation of endometrial polypsCitation12, adenocarcinoma, ovarian cyst formation, thromboembolism, liver cancerCitation13 and retinopathyCitation14.
In recent years, an increasing attention has been focused some natural compounds, because their chemopreventive activity and, when combined with TAM, minimize its cytotoxic affects on normal cells without decreasing its antitumour activity. Among such compounds are α-tocopherol and α-tocopherol acetateCitation10, green teaCitation15 and taurineCitation16. Indeed, many natural products have been studied as potential chemopreventive agents in combination with antitumour compounds. Apart from exerting protective effects on normal cells, such compounds may also enhance the anti-proliferative activity of antitumour drugs.
Based on these data, this work aims to evaluate the effects of the combined treatment of the o-methyl catechols CUR, APO or VAN with TAM on normal red blood cells (RBC) and polymorphonuclear leukocytes (PMNs), as well as cytotoxic activity through use of the human chronic myeloid leukemia K562 cell line.
Materials and methods
Chemicals
APO, CUR, VAN and (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (MTT), were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All of the reagents used for solutions were of analytical grade.
Cytotoxicity activity
Preparation of red blood cells
The heparinized venous blood was collected from healthy volunteers. Whole blood was centrifuged at 1200g at 4°C for 5 min to separate plasma from RBC. The RBC were washed three times in cold phosphate buffered saline (PBS) (0.15 mM NaCl, 10 mM sodium phosphate, pH 7.4), and the buffy coat was removed with each wash. The RBC were resuspended in 50 mM PBS (pH 7.4) to obtain a hematocrit of 1%Citation10. All experiments involving human blood were approved by the Ethics Committee of the University (protocol 128/2010).
Haemolysis measurements
In the presence or absence of TAM (28 µg/mL), each o-methyl catechols (concentration range: 0.06–1.66 µg/mL) was incubated with a 1% red blood cell suspension for 90 min at 37°C with constant shaking. After incubation, the red blood cell suspension was centrifuged at 1200g at 4°C for 5 min. Haemolysis was determined by measuring the absorbance at 540 nm in a microplate reader (Molecular Devices Spectra Max 190Citation10). The results were calculated and expressed as % inhibition.
K+ release
RBC were incubated with different concentrations of o-methyl catechols (0.06–1.66 µg/mL) in the presence or absence of TAM (28 µg/mL) for 90 min at 37°C with constant shaking. After the incubation, the RBC were centrifuged at 1200g at 4°C for 5 min, and K+ release was measured with an ion-selective electrode (Roche/AVL 9180Citation10). The results were calculated and expressed as % inhibition.
Cytotoxicity in polymorphonuclear leukocyte
Heparinized whole blood was collected, mixed with a solution of 1.5% dextran in a 2:1 ratio and then incubated at 37°C for 1 h. Following incubation, the mixture was centrifuged for 5 min at 750g at 4°C to isolate PMN. The supernatant was discarded, and the cell pellet was washed three times with 50 mM PBS (pH 7.4) in the absence of Ca2+. The cells were resuspended in PBS buffer (Dulbecco) and counted in a Newbauer chamberCitation17.
The cytotoxicity of TAM in PMNs was measured using the trypan blue dye exclusion test, in which viable cells do not incorporate the dye and appear brighter than dead cells upon observation with an optical microscope. The PMNs (1 × 106 cells/mL in PBS-Dulbecco) were incubated at 37°C for 30, 60 and 90 min with each o-methyl catechol (50 µg/mL) in the presence and absence of TAM (14 µg/mL). After incubation, the suspension was mixed with a solution of 0.5% trypan blue in a 1:1 ratio for 5 min. The cells were observed in a Newbauer chamber via microscopy. The total number of stained and unstained cells was calculated, and the results were expressed as the percentage of unviable cellsCitation18. As a control, an equal number of cells was suspended in PBS-Dulbecco and similarly analysed.
Cell culture
The Human Chronic Myeloid Leukemia (K562) cell line was obtained from American Type Cell Culture (Rockville, MD, USA). Cells were cultured in RPMI 1640 medium supplemented with 10% foetal bovine serum, 2 mM glutamine, 100 U/mL of penicillin and 100 mg/mL of amikacin at 37°C in a humidified atmosphere containing CO2 (5%).
Cell viability
Briefly, human chronic myeloid leukemia (K562) cells were plated at a density of 4 × 104 in 96-well tissue culture plates (Corning, NY). Cells were treated with the o-methyl catechols (3.1, 6.2 and 12.5 µg/mL) in the presence or absence of TAM (1.5, 3.2 and 6.4 µg/mL) for different time intervals (24, 48 and 72 h). Cell proliferation and viability were determined by the MTT method. For this assay, following treatment, 0.5 mg/mL MTT in fresh medium was added to the cultures. After 4 h of incubation, the formazan crystals were dissolved in 100 µL of a 10% sodium dodecyl sulfate/10 mM HCl solution, and the absorbance was measured at 570 nm in a microplate reader (BioTek Instruments). Experiments were done in triplicate. For the CUR groups, a cell-free blank was performed to subtract for background absorbance.
Values for % inhibition were calculated as follows:
Where, Ac is the control absorbance and At is the test absorbance.
Statistical analysis
Results are presented as the mean ± SD (n = 3). All experiments were done in triplicate. Statistical comparisons were made by the ANOVA method using a 95% confidence interval, and differences were considered statistically significant at p < 0.05.
Results
Cytotoxic activity in vitro in red blood cells
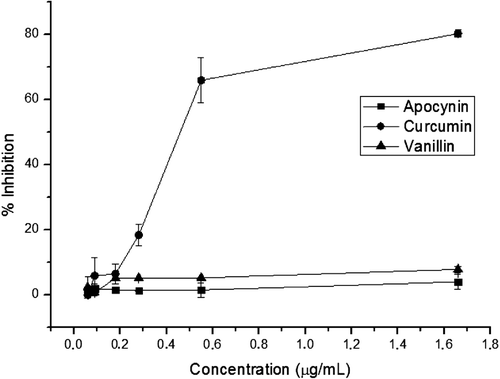
The haemolysis assay was used to determine the effects of each o-methyl catechols on the cytotoxicity of TAM in RBC. The ability of these compounds to inhibit haemolysis was calculated in reference to the positive control (TAM alone). The absorbance for TAM treatment alone was 1.31 ± 0.05, which was higher than that for the negative control (absorbance of 0.008 ± 0.0007, 1% RBC only). It observed CUR to be the only o-methyl catechols that presented a protective effect against the cytotoxicity of TAM in RBC (). This inhibitory effect of CUR on TAM-induced haemolysis was concentration-dependent. APO and VAN, at all tested concentrations showed no significant inhibitory activity (p > 0.05).
Figure 2. Effect of methoxy-catechols on tamoxifen (TAM) induced haemolysis. Red blood cells (1%) were incubated with the indicated concentrations of apocynin, curcumin and vanillin in the presence of TAM (28 µg/mL) at 37°C for 90 min. Haemolysis was determined by measuring the release of haemoglobin via spectrophotometry at a wavelength of 540 nm; (n = 3). All experiments were done in triplicate.
The concentration of K+ release was calculated as 4.13 mM ± 0.22 for the positive control (TAM alone). In comparison, the negative control (1% RBC only) exhibited a concentration of K+ release of 0.008 mM ± 0.0007 (). Consistent with the haemolysis assay, it observed that only CUR presented a concentration-dependent, cell-protective effect. At all concentrations tested, APO and VAN did not affect K+ release.
Figure 3. Inhibition of the tamoxifen (TAM)-induced release of K+ ions [mM] by methoxy-catechols. Red blood cells (1%) were incubated in the indicated concentrations of apocynin, curcumin or vanillin in the presence of TAM (28 µg/mL) at 37°C for 90 min. K+ release was measured by an ion-selective electrode; (n = 3). All experiments were done in triplicate.
![Figure 3. Inhibition of the tamoxifen (TAM)-induced release of K+ ions [mM] by methoxy-catechols. Red blood cells (1%) were incubated in the indicated concentrations of apocynin, curcumin or vanillin in the presence of TAM (28 µg/mL) at 37°C for 90 min. K+ release was measured by an ion-selective electrode; (n = 3). All experiments were done in triplicate.](/cms/asset/79adf54c-277c-48a9-9a0f-8fb6ed5b01d8/ienz_a_680064_f0003_b.gif)
Cytotoxicity on polymorphonuclear leukocyte
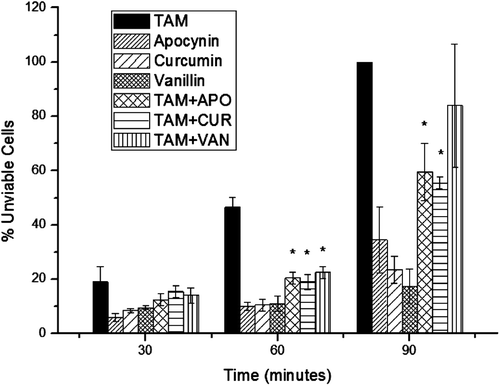
PMN viability was measured following incubation with APO, CUR or VAN (50 µg/mL) in the presence or absence of TAM (14 µg/mL) during different time intervals (). PMN death was observed to increase with the time of exposure to TAM. The percentage of unviable cells after 30 min was 19 ± 5.66, 46.5 ± 3.53 after 60 min, and 100 ± 0.0 after 90 min. After 30 min, it was observed that APO, CUR and VAN were able to reduce TAM-mediated cytotoxicity in PMN, with the percentage of unviable cells being 12.5 ± 2.12, 15.5 ± 2.12 and 14.0 ± 2.83, respectively, but did not significantly. It was observed for APO, CUR and VAN treatment at 60 min with the percentage of unviable cells being 20.5 ± 2.12, 19.0 ± 2.83 and 22.5 ± 2.12, respectively (p < 0.05). After 90 min, the o-methyl catechols exhibited a cytotoxic effect on PMN similar to that observed for TAM at the 30 min time point; however, APO and CUR still showed a protective effect against TAM-induced cytotoxicity at this time point, with the percentage of unviable cells calculated as 59.5 ± 10.6 for APO and 55.5 ± 2.12 for CUR (p < 0.05). VAN did not have a pronounced effect on TAM-induced cytotoxicity in PMNs after 90 min of treatment (% unviable cells = 77.0 ± 12.72).
Figure 4. Inhibition of the tamoxifen (TAM)-induced cytotoxicity on polymorphonuclear leukocytes (PMNs) by methoxy-catechols. Samples of methoxy-catechols (50 µg/mL) in the absence or presence of TAM (14 µg/mL), were incubate in different times (30, 60 and 90 min). The PMN viability was analysed by trypan blue exclusion assay. *Significantly different from control cells treated with TAM (p < 0.05); (n = 3). All experiments were done in triplicate.
Cytotoxicity on K562 cells
It was investigated the effect of treatment with TAM and each o-methyl catechols on K562 cell viability at different time intervals (24, 48 and 72 h). The results are expressed in . After 24 h, the TAM or o-methyl catechols did not significantly result K562 cell viability. However, it was observed significant effects on cell death, when TAM/o-methyl catechols combination. With regards to CUR, increased death was only observed at the highest tested concentrations for both TAM and the o-methyl catechols (12.5 µg/mL CUR + 6.2 µg/mL TAM). For APO, synergistic effects on cell viability were observed at the two highest concentrations tested (6.2 µg/mL APO + 3.1 µg/mL TAM and 12.5 µg/mL APO + 6.2 µg/mL TAM). Significant effects were seen at the lowest tested concentrations for VAN (3.1 µg/mL VAN + 1.5 µg/mL TAM), however at the highest tested concentrations (6.2 µg/mL VAN + 3.1 µg/mL TAM, 12.5 µg/mL VAN + 6.2 µg/mL TAM), it was observed significant synergistic effects on cell death. Thus, after 48 h of treatment, the o-methyl catechols showed a significant decrease in K562 cell viability at the two highest concentrations tested (12.5 and 6.2 µg/mL o-methyl catechol). TAM treatment alone had a similar effect on K562 cells regardless of concentration. With regards to TAM concentration, significant effects were observed at the lowest concentrations (1.5 µg/mL TAM + 3.1 µg/mL o-methyl catechol), while at the two highest concentrations (3.1 µg/mL TAM + 6.2 µg/mL o-methyl catechol and 6.2 µg/mL TAM + 12.5 µg/mL o-methyl catechol), it was observed significant synergistic effect on cell death. Lastly, after 72 h of treatment, the o-methyl catechols alone showed a similar effect on cell death than 48 h. With regards to TAM concentration, the three o-methyl catechols at the all concentrations tested had significant synergistic effect (1.5 µg/mL TAM + 3.1 µg/mL o-methyl catechols, 3.1 µg/mL TAM + 6.2 µg/mL o-methyl catechols and 6.2 µg/mL TAM + 12.5 µg/mL o-methyl catechol). In the absence of TAM, VAN had the greatest effect on cell death, followed by CUR and APO. In the presence of TAM, VAN again exhibited the greatest effect on cell death, followed by APO and CUR. These data show that combined treatment with o-methyl catechols and TAM has a synergistic effect on K562 cell death in comparison to treatment with TAM or o-methyl catechols alone.
Table 1. Effects of TAM, APO, CUR and VAN (single or in combination) on the viability of K562 cells in vitro.
Discussion
Natural products are excellent candidates for new antitumour drugs, as they are widely-available. The o-methyl catechols used in this work constitute one of the largest groups of phytochemical that exhibit anticancer biological properties. Studies have related the high consumption of natural products that are rich in phenolic groups with lower rates of cancer incidence; however, studies have also shown that high concentrations of natural products are required to achieve effective antitumour activity. For this reason, natural products may be more useful in combination with other chemotherapeutic drugs, with the aim being to obtain synergistic antitumour effects. This would be greatly valuable in cancer therapy, as lower doses of chemotherapy could be used, and the harmful side effects caused by treatment could be reduced.
CUR, a diferuloylmethane derived from the plant Curcuma longa, has several antitumour effects. In particular, it has been demonstrated to be an anti-proliferative agent and induces tumour cell apoptosis. CUR is known to function by directly interfering with cell survival signalling pathways such as the nuclear factor-kappa-B (NF-κB) pathwayCitation19. The cytotoxic activity of CUR has been shown in multiple tumour cell lines. CUR has been widely studied in recent years in combination with other chemotherapeutic agents. The cytotoxic effects of gemcitabine are potentiated by CUR in pancreatic cancer through the suppression of NF-κB-regulated gene products or through effects on other cell signalling pathwaysCitation20. CUR and epigallocatechin gallate have been shown to act synergistically, decreasing proliferation in oral cancerCitation21. Recent studies have shown that other o-methyl catechols have effects on tumour cells. For example, 4-methylcatechol (a metabolite of quercetin) has been shown to induce the intrinsic apoptotic pathway in metastatic melanoma cellsCitation22. Serving as an example of the beneficial effects of CUR in the medical clinic, the combination of CUR and TAM has been shown to increase phosphatidylserine flipping, mitochondria depolarization and the generation of reactive oxygen species in chemo-resistant melanomaCitation23. Part of the cytotoxic activity of VAN on tumour cells is also due to its inhibition of NF-κB activation, which results in enhanced TRAIL-induced apoptotic cell deathCitation24 as well as cytolytic and cytostatic effectsCitation25. Through the use of models phagocytic and non-phagocytic cell models, the effects of APO have been attributed to its ability to efficiently inhibit the NADPH-oxidase complex, resulting in the production of reactive oxygen speciesCitation3.
TAM has several detrimental effects on RBC, causing haemolytic anemia in vivo and haemolysis in vitroCitation10. These effects are mainly due to the ability of TAM to induce a structural disruption of biomembranes. Using RBC as a model, we demonstrated that CUR presented dose-dependent decreases in TAM-associated haemolysis. This effect was not observed with the other methoxy-catechols tested, VAN and APO. These results are in agreement with those we obtained through the measurement of potassium ion release. In PMN, treatment with TAM resulted in time-dependent increases in toxicity; however, the three o-methyl catechols were able to protect against PMN cell death, particularly after 60 min of treatment.
These studies demonstrated the synergistic effect of the combination of TAM with the o-methyl catechols on K562 tumour cell death.
This effect was concentration-and time-dependent. After 72 h of treatment, a synergistic effect was observed even at the lowest tested concentrations of TAM and the o-methyl catechols. Interesting to note the effect of APO, a compound that has not been studied about its effects on tumour cells. APO had the least effect of the three compounds on K562 cell death but, when combined with TAM, showed greater increases in cell death than CUR/TAM.
VAN presented the highest cytotoxic activity (followed by CUR and APO). In association with TAM, VAN presented the higher cytotoxic activity on tumour cells (K562), followed by APO and CUR. In addition, their combination with TAM produced not an additive effect, but a synergistic effect.
Several papers have shown the effect of TAM or CUR on K 562 cells. Although a K562 cell does not present estrogen receptors, some papers has showed the ability of TAM to induce apoptosis in this line cells by mechanisms independent of its ability to modulate estrogen receptors. The estrogen receptor expression is not considered a prerequisite for the antitumour activity of TAM, since some tumours even without these receptors are able to respond to the treatment. Other mechanisms other than binding to estrogen receptors may contribute to TAM antitumour activityCitation26. Akeli et al.Citation27 demonstrated high affinity binding sites for TAM in K562 cells and also the ability of TAM in the inhibition of collagenolytic activity in these cells. This process is involved in the invasion process and the metastasizing of cancer cells. Candi et al.Citation28 demonstrated that the treatment of K562 cells with TAM induced an 8-fold increase in the number of apoptotic cells after 48 h of exposure. The authors also demonstrated that the mechanism of cell death TAM-induced was not related to its estrogen modulation in K562 cells, since these cells were more sensitive to TAM than human breast MCF-7G cells. Macarrone et al.Citation29 demonstrated that TAM induced a remarkable increase in the activity and expression of nitric oxide synthase in K562 cells, suggesting that the free radical nitric oxide might play a role in the execution of programmed cell death induced by the drug. Also, the authors showed the enhancement of the activity and expression of the 5-lipoxygenase, that is involved in apoptosis.
CUR induced DNA damage and triggered apoptosisCitation30, cell death by apoptotic and autophagic mechanismsCitation31 and induce heat shock responseCitation32 on K562 cells. Few papers have evaluated the effect of APO and VAN on tumour cells.
As mentioned above, TAM and CUR triggered apoptosis on K562 cell. The increase in this effect by the presence of both compounds on the K562 cell appears to be the main mechanism involved in the synergistic effect observed, whereas apoptosis is a major event related to diminished viability of tumour cells.
The association of CUR and TAM in breast cancer cells has been already studied. Gasperi et al.Citation33 showed that CUR and TAM co-treatment was found to synergistically inhibit survival in myrAKT MCF-7 cells. One mechanism of the resistance to TAM treatment is due the aberrant activation AKt pathway, that is characterized by high levels of the pro-survival transcription factor NF-κB. As CUR is recognized able to inhibit the activation of NF-κB, the cells remain more sensitive to TAM. The authors agree that the identification of alternative approaches for suppressing NF-κB activity could prove beneficial for enhancing response to TAM. As CUR and VAN present the ability of inhibition of NF-κB, this effect could be a starting point to explain the synergism with TAM described in this work.
These results point to the potential therapeutic value of TAM in association with the tested o-methyl catechols, particularly CUR, which potentiates the cytotoxic effects of TAM on tumour cells (K562) while also decreasing TAM-associated cytotoxicity in normal, healthy RBC and PMN. Further studies will be necessary to clarify the mechanisms by which the o-methyl catechols and TAM act synergistically in these processes.
Acknowledgments
This manuscript is as part of a published of academic thesis and all the details and results presented here were send to patent deposit in Brazil and must be protected according to the Brazilian agency regulation (protocol number: 0000221107823603).
Declaration of interest
The authors report no conflicts of interest.
References
- Singh S, Khar, A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem 2006;6:259–270.
- Zhang C, Li B, Zhang X, Hazarika P, Aggarwal BB, Duvic M. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients’ PBMCs: potential role for STAT-3 and NF-κB signaling. J Invest Dermatol 2010;130:2110–2119.
- Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm 2008;2008:106507.
- Pacheco SMV, Damasio F. Vanilina: origem, propriedades e produção. Quim Nova 2010;32:215–219.
- Liang JA, Wu SL, Lo HY, Hsiang CY, Ho TY. Vanillin inhibits matrix metalloproteinase-9 expression through down-regulation of nuclear factor-κB signaling pathway in human hepatocellular carcinoma cells. Mol Pharmacol 2009;75:151–157.
- Durant S, Karran P. Vanillins–a novel family of DNA-PK inhibitors. Nucleic Acids Res 2003;31:5501–5512.
- Guerra MR, Gallo CVM, Azevedo G, Mendonça S. Risco de câncer no Brasil: tendências e estudos epidemiológicos mais recentes. Rev Bras Canc 2005;51:227–234.
- Instituto Nacional de Câncer, Ministério da Saúde. (2009). Estimativa/2010: incidência de câncer no Brasil. Rio de Janeiro. [Online]. Available at: http://www.inca.gov.br/ Accessed on 08 June. 2011.
- Petinari L, Kohn LK, de Carvalho JE, Genari SC. Cytotoxicity of tamoxifen in normal and tumoral cell lines and its ability to induce cellular transformation in vitro. Cell Biol Int 2004;28:531–539.
- Silva MMC, Madeira VMC, Almeida LM, Custodio JBA.Hemolysis of human erythrocytes induced by tamoxifen is related to disruption of membrane structure. Biochim Biophys Acta 1999;1464:41–61.
- Engelk M, Bojarski P, Bloss R, Diehl H. Tamoxifen perturbs lipid bilayer order and permeability: comparison of DSC, fluorescence anisotropy, laurdan generalized polarization and carboxyfluorescein leakage studies. Biophys Chem 2001;90:157–173.
- Senkus-Konefka E, Konefka T, Jassem J. The effects of tamoxifen on the female genital tract. Cancer Treat Rev 2004;30:291–301.
- Mocanu EV, Harrison RF. Tamoxifen in gynaecology. Gynaec Pract2004;4:37–45
- Huang CC, Cheng HH, Lin KL, Cheng JS, Tsai JY, Liao WC et al. Tamoxifen-induced [Ca2+]i rise and apoptosis in corneal epithelial cells. Toxicology 2009;255:58–64.
- El-Beshbishy HA. Hepatoprotective effect of green tea (Camellia sinensis) extract against tamoxifen-induced liver injury in rats. J Biochem Mol Biol 2005;38:563–570.
- Tabassum H, Rehman H, Banerjee BD, Raisuddin S, Parvez S. Attenuation of tamoxifen-induced hepatotoxicity by taurine in mice. Clin Chim Acta 2006;370:129–136.
- Cíz M, Lojek A. Improved dextran preparation of human leucocytes for chemiluminescence analysis of the oxidative burst of polymorphonuclear cells. Clin Lab Haematol 1997;19:49–51.
- Phillips HJ. Dye exclusion test for viability. (1973). In: Kruse PF Jr, Patterson MH Jr, (Eds.). Tissue culture: methods and application. New York: Academic Press, 406–408.
- Bava SV, Sreekanth CN, Thulasidasan AK, Anto NP, Cheriyan VT, Puliyappadamba VT et al. Akt is upstream and MAPKs are downstream of NF-κB in paclitaxel-induced survival signaling events, which are down-regulated by curcumin contributing to their synergism. Int J Biochem Cell Biol 2011;43:331–341.
- Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-κB-regulated gene products. Cancer Res 2007;67:3853–3861.
- Khafif A, Schantz SP, Chou TC, Edelstein D, Sacks PG. Quantitation of chemopreventive synergism between (-)-epigallocatechin-3-gallate and curcumin in normal, premalignant and malignant human oral epithelial cells. Carcinogenesis 1998;19:419–424.
- Payton F, Bose R, Alworth WL, Kumar AP, Ghosh R. 4-Methylcatechol-induced oxidative stress induces intrinsic apoptotic pathway in metastatic melanoma cells. Biochem Pharmacol 2011;81:1211–1218.
- Chatterjee SJ, Pandey S. Chemo-resistant melanoma sensitized by tamoxifen to low dose curcumin treatment through induction of apoptosis and autophagy. Cancer Biol Ther 2011;11:216–228.
- Lirdprapamongkol K, Sakurai H, Suzuki S, Koizumi K, Prangsaengtong O, Viriyaroj A et al. Vanillin enhances TRAIL-induced apoptosis in cancer cells through inhibition of NF-κB activation. In Vivo 2010;24:501–506.
- Ho K, Yazan LS, Ismail N, Ismail M. Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol 2009;33:155–160.
- Rotstein S, Blomgren H, Petrini B, Wasserman J, von Stedingk LV. Influence of adjuvant tamoxifen on blood lymphocytes. Breast Cancer Res Treat 1988;12:75–79.
- Akeli MG, Madoulet C, Rallet A, Jardillier JC. Inhibition of collagenolytic activity in human leukemic K562 cells by tamoxifen. Leuk Res 1991;15:1153–1157.
- Candi E, Melino G, De Laurenzi V, Piacentini M, Guerrieri P, Spinedi A et al. Tamoxifen and somatostatin affect tumours by inducing apoptosis. Cancer Lett 1995;96:141–145.
- Maccarrone M, Fantini C, Ranalli M, Melino G, Agrò AF. Activation of nitric oxide synthase is involved in tamoxifen-induced apoptosis of human erythroleukemia K562 cells. FEBS Lett 1998;434:421–424.
- Lu JJ, Cai YJ, Ding J. The short-time treatment with curcumin sufficiently decreases cell viability, induces apoptosis and copper enhances these effects in multidrug-resistant K562/A02 cells. Mol Cell Biochem 2012;360:253–260.
- Jia YL, Li J, Qin ZH, Liang ZQ. Autophagic and apoptotic mechanisms of curcumin-induced death in K562 cells. J Asian Nat Prod Res 2009;11:918–928.
- Teiten MH, Reuter S, Schmucker S, Dicato M, Diederich M. Induction of heat shock response by curcumin in human leukemia cells. Cancer Lett 2009;279:145–154.
- Gasperi M, Cavazos D, Graffenried L. Curcumin modulates tamoxifen response in resistant breast cancer cells. Cancer Res 2009;69:Abstract nr 3098.