Abstract
Human skin fibroblast collagenase also known as Matrix Metalloproteinase-1 (MMP-1) is a key enzyme in remodeling and degradation of extracellular matrix, and the inhibitors of human MMP-1 are effective drug candidates for the treatment of cancer. In this study, we report an improved method for high-level expression of soluble human MMP-1 catalytic domain (cd-MMP-1) in E.coli. The enzymatic activity is found maximum at pH 7.5 and temperature 40°C with a Km value of 13.02 µM. Effects of 17 structure-related flavonoids on MMP-1 activity are evaluated using a fluorescent assay, 6 inhibitors are identified with IC50 < 10 µM. Fisetin is the most active agent with an IC50 value of 1.35 µM and is identified as a mixed type inhibitor. Our improved soluble cd-MMP-1 expression method provides a basis for inhibitors identification and may be beneficial to discover novel anti-cancer agent targeting human MMP-1.
Introduction
Matrix metalloproteinases (MMPs) are a large family of calcium-dependent, zinc-containing endopeptidases that are involved in the tissue remodeling and degradation of extracellular matrix (ECM) and play key roles in cell development, bone remodeling, wound healing, and angiogenesisCitation1–3. Matrix metalloproteinases inhibitors (MMPIs) have been identified as potential therapeutic candidates for cancer metastasis, arthritis, chronic inflammation and wrinkle formationCitation4,Citation5. Therefore, developing of MMP inhibitors may provide a new treatment for these important diseases. Recently, a few inhibitors screened out from a large number of compounds for inhibition potency have been filed as investigational new drug candidates to treat disease. Rapid and cost-effective identification of such novel lead compound need the simple and easy-handling preparation of functional target protein. In general, heterologous expression in E.coli is chosen in priority to produce target recombinant MMPs, because the bacterial expression system is easy to engineer, practical and immediate to obtain target proteins for inhibitors screeningCitation6.
Human skin fibroblast collagenase also known as Matrix Metallo Proteinase-1 (MMP-1) is a member of the MMPs family. In humans, MMP-1 is considered as a prototype for all interstitial collagenases and plays an important role in the turnover of collagen fibrils in most tissuesCitation7,Citation8. Various forms of human MMP-1 have been cloned and expressed, such as in fibroblastsCitation9, CHO cellsCitation10,Citation11, COS cellCitation12, Methylotrophic yeastCitation13 and E.coliCitation14–16. However, the recombinant expression of MMP-1 in mammalian cells and yeast were low and the expression of MMP-1 in E.coli was always as inclusion bodies and should be refolded to obtain activity.
MMP-1 is an attractive therapeutic target for cancer invasion and metastasis. In order to fit for the demands for MMP-1 inhibitor screening and the further structural and biochemical characterization of enzyme-inhibitor complexes studies, we reported an improved method for high level expression of soluble human MMP-1 catalytic domain (cd-MMP-1) in E.coli. The purified cd-MMP-1 was highly active in digesting substrates and has similar activity as other MMP-1 reported previouslyCitation13.
Flavonoids represent one of the most prevalent classes of natural products that are widely distributed in fruits, vegetables and beverages. Flavonoids have shown antitumor, anti-inflammation, and antioxidant effectsCitation17. Natural occurring flavonoids have been shown to suppress MMP-2 and MMP-9 expression and activity in cancer cells. However, there is little study focused on the human MMP-1. In the present study, 17 structure-related flavonoids are used to investigate the inhibitory activities on cd-MMP-1 activity in vitro. We find that six flavonols markedly inhibited cd-MMP-1 enzymatic activity with IC50 less than 10 µM and we also investigate the inhibition mechanism of the most potent compound.
Material and methods
Reagent
All of flavonoids were obtained from Sigma-Aldrich, J&K or Aladdin reagent (Shanghai). The purity of the compounds was ≥95%. Restriction enzymes were from NEB (Beijing). pET-15b was purchased from novagen. Fluorogenic peptide substrate was from Anaspec (USA). MMP inhibitor II (Product number 444247) and MMP inhibitor III (product number 444264) were from Merck.
Cloning of the cd-MMP-1 into expression vectors
The cDNA encodes the proenzyme form of human fibroblast collagenase (proMMP-1) was kindly provided by Prof. Christopher M. Overall (Centre for Blood Research, University of British Columbia), the catalytic domain of human MMP-1 (Phe100-Gln268) (cd-MMP-1) was sub-cloned into vector pET-15b using NdeI/BamHI restrict enzyme sites. The primers used for PCR amplification were listed as follows: (sense) 5′-TCCGTTTCATATGTTTGTCCTCACTGAG-3′, (anti-sense) 5′-AATGGATCCT CACTGGACAGGATTTTGG-3′, and the nucleotide sequence of the insert was confirmed by DNA sequencing. For expression of the recombinant protein, pET15b-cd-MMP-1 was transformed into E.coli strain BL21(DE3).
Expression of cd-MMP-1 in E. coli
The 169-residue cd-MMP-1 was expressed in E.coliBL21(DE3) containing the plasmid described above. An overnight culture was started in Luria broth (LB) with 100 μM ampicillin from a signal colony. The culture was dilute to 200-fold using the same media and continually incubated at 37°C with shaking, until OD600 was between 0.8 and 1.0. IPTG was added to a final concentration of 0.5 mM and the incubation was continued for 8 h. The cells were harvested by centrifugation at 4000 rpm for 20 min and frozen at −80°C overnight for next purification.
Purification of cd-MMP-1
The frozen cell pellet was suspended in 20 ml buffer A (50 mM Tris-HCl, 0.15 M NaCl, 10 mM CaCl2, 10 μM ZnCl2, 1 mM DTT, 0.02% NaN3, pH 7.5), the cell suspension was lysed by sonication, and the disrupted cell lysate was centrifuged on 10,000 rpm for 30 min. The supernatant was load on a nickel-nitrilotriacetic acid (Ni-NTA) resin (Qiagen) column (1 mL) pre-equilibrated with buffer A. After 4 h, the column was washed with buffer A and buffer B (20 mM imidazole in Buffer A), the protein of interest was eluted with 10 mL elution buffer (50 mM Tris-HCl, 0.15 M NaCl, 10 mM CaCl2, 10 μM ZnCl2, 0.02% NaN3, 300 mM imidazole pH 7.5). The fractions containing the target protein were identified by SDS-PAGE. Imidazole was removed by dialysis against the corresponding buffer (50 mM Tris-HCl, 0.15 M NaCl, 10 mM CaCl2, 10 μM ZnCl2, 0.02% NaN3, pH 7.5). The proteins were concentrated with a 10-kDa cut-off membrane (Amicon) at 4°C and the concentration was measured by the standard Bradford method.
cd-MMP-1 activity assay
The activity of cd-MMP-1 was measured using a fluorescence-based assay. It was performed in white 96-well half area microplate (Greiner) in a final volume of 100 μL. The reaction buffer contained 7.6 nM cd-MMP-1 in assay buffer (50 mM Tris-HCl, 0.15 M NaCl, 10 mM CaCl2, 10 µM ZnCl2, pH 7.5). The reaction was initiated by the addition of 50 μl of quenched fluorogenic peptide substrate: Dnp-Pro-beta-cyclohexyl-Ala-Gly-Cys(Me)-His-Ala-Lys(Nma)-NH2 (final concentration 5 μM). The cd-MMP-1 activity was determined in an Synergy™ 2 Multi-Mode Microplate Reader(BioTek) at an excitation wavelength of 360 nm and an emission wavelength of 460 nm.
Effect of pH on cd-MMP-1 enzymatic activity
The pH optima of purified cd-MMP-1 were investigated using a pH range of 6.0–9.0. The buffer system used was a constant ionic strength containing 0.15 M NaCl, 10 mM CaCl2 and 10 µM ZnCl2, the protein concentration was 7.6 nM. The activities were determined as mentioned in cd-MMP-1 activity assay section.
Effect of temperature on cd-MMP-1 enzymatic activity
To investigate the effect of temperature on MMP-1 enzymatic activity, the assay contents were equilibrated for 20 min in Biotek Synergy™ 2 at appropriate temperatures. The temperature range used was from 10 to 50°C. The reaction was started by addition of a substrate solution and fluorescence was read for 20 min.
Effect of zinc and calcium ions on cd-MMP-1 activity
Since zinc and calcium ions are important for cd-MMP-1 structure and activity, we also investigate the effects of above two ions on our cd-MMP-1 catalytic activity. The effects of zinc and calcium ion concentrations on cd-MMP-1 activity were evaluated using 4.7 nM cd-MMP-1 in buffer containing 50 mM HEPES buffer (pH 7.5). In the presence of 5 mM CaCl2, the protein activity was determined with ZnCl2 concentration ranging from 100 nM to 1 mM. In the presence of 1 µM ZnCl2, the protein activity was determined with CaCl2 concentration ranging from 50 µM to 25 mM.
Effect of flavonoids on cd-MMP-1 activity
Seventeen structure-related flavonoids were dissolved in DMSO as stock solution. The enzyme was pre-incubated with varied concentration of compounds for 20 min at room temperature and the activity was tested as described above. The MMP inhibitor II (Product number 444247) and MMP inhibitor III (product number 444264) from Merck were used as positive control to evaluate the inhibitors screening system.
Results
Protein expression and purification
The expression vector encoding human MMP-1 catalytic domain (residues Phe100-Gln268) was constructed using plasmid of pET-15b. The expression of 169-residue cd-MMP-1 construct was carried out using E.coli.BL21(DE3) as host system in LB media with 100 μM ampicillin and was induced by IPTG. After cell harvest, lysing and purification, the purified soluble protein was obtained and generated band of 21 kDa (shown as ). The yield from the purification was about 12–15 mg per half liter (protein concentration was determined using the bradford method with BSA as standard).
Figure 1. SDS-PAGE analysis of cd-MMP-1 expression and purification procedures. Lane M, the standard protein mass markers; lane 1, the total cell lysate before induced; lane 2, the total cell lysate after induced; lane 3, pellet of cell lysate after induced; lane 4, supernatant of cell lysate after induced; lane 5, purified cd-MMP-1 protein.
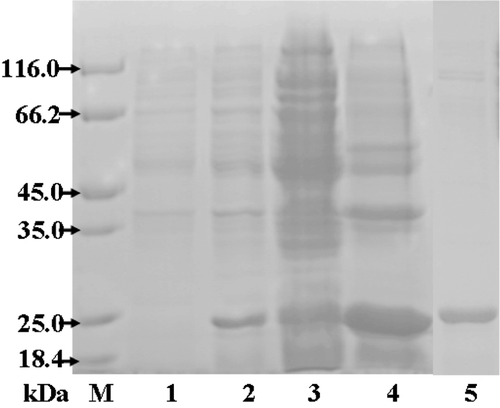
For all recombinant MMPs reported so far, MMP-1 was always expressed in E.coli as insoluble inclusions body. The protein refolding is always complicated and time-consumingCitation18. In present study, using soluble expression, we can get large amount of active cd-MMP-1 easily in a short time. The efficient preparation of active human cd-MMP-1 will facilitate the establishment of high-throughput screening assays for MMP-1 and further marking rapid and cost-effective identification of novel drug candidates for the treatment of diseases.
Characterization of cd-MMP-1
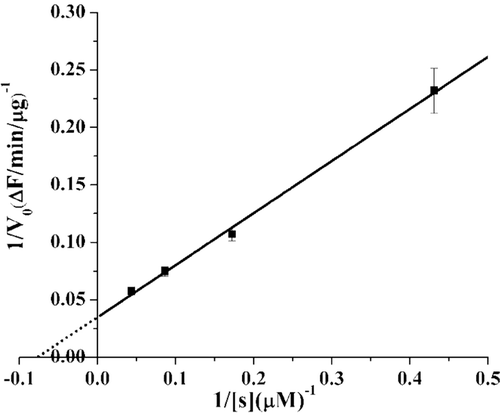
In order to verify the activity of our soluble cd-MMP-1, the data of the quenched peptide hydrolysis by cd-MMP-1 (3.8 nM) was measured at substrate concentration of 23.2, 11.6, 5.8, 2.32 µM at room temperature. The Km value was calculated as 13.02 µM, Vmax value was calculated as 28.72 ΔF/min/μg and Vmax/Km was 2.21 ΔF/min/μg/μM through the double-reciprocal form of the Michaelis–Menten equation, which plotting 1/v (reciprocal of rate) vs. 1/[s] (reciprocal of substrate concentration) ().
Figure 2. Measurement the Km of human cd-MMP-1. Lineweaver-Burk plot analysis yielded the Km of cd-MMP-1 for peptide substrate was 13.02 μM. The data points are means ± SE for triplicate experiments.
The pH optima of purified cd-MMP-1 were determined around 7.5 from the activities at various pH values (). Cd-MMP-1 revealed low activity at low pH values and the activity gradually increased with increasing pH. Enzyme activity reached a maximum value at pH 7.5 and thereafter a drastic decrease occurred at higher pH.
Figure 3. Effect of pH, metal ion and temperature on cd-MMP-1 activity. (A) pH effect on the catalytic activity of cd-MMP-1. Activity of cd-MMP-1 was monitored in 50 mM Bis-Tris buffer (pH 6.0–7.0, squares), 50 mM HEPES buffer (pH 7.0–8.0, circles) or 50 mM Tris buffer (pH 7.5–9.0, triangles). The data points are means ± SE for triplicate experiments. (B) Temperature optimum profiles of purified cd-MMP-1. The data points are means ± SE for triplicate experiments. (C) Effect of zinc and calcium concentration on catalytic activities of cd-MMP-1. Hydrolysis of the fluorogenic peptide substrate was monitored in HEPES buffer (pH 7.5). Activities at varied ZnCl2 concentrations in the presence of 5 mM CaCl2 are shown as open circles and activities at varied CaCl2 concentrations in the presence of 1 µM ZnCl2 are shown as closed circles. The data points are means ± SE for triplicate experiments.
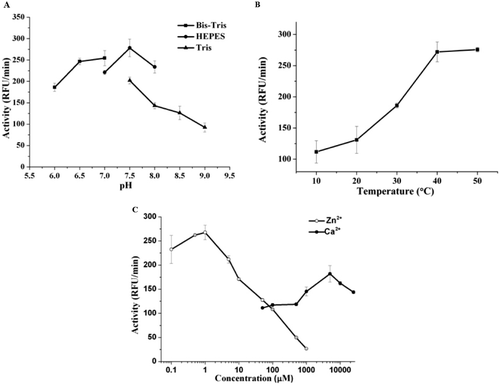
The temperature optimum profiles for purified cd-MMP-1 were shown in . Protein showed an gradual increase in activity from 10 to 20°C, then followed by a steep increase in activity from 20 to 40°C, and thereafter do not show any significant change in activity until 50°C. From the result, we can find that the optimum temperature of cd-MMP-1 is around 37°C, which is the body temperature of human being.
Since zinc and calcium ions are important in the structure and activity of cd-MMP-1 protein, we also investigated the effects of zinc and calcium ions on cd-MMP-1 activity (). In the present of 5 mM CaCl2, the optimal activity of cd-MMP-1 was achieved around 1 µM ZnCl2, while the activity of cd-MMP-1 decreased significantly if the concentration of ZnCl2 went higher. In the present of 1 µM ZnCl2, cd-MMP-1 shown the best activity at the concentration of CaCl2 around 5 mM. Our data shown that cd-MMP-1 required low concentration of zinc ions and higher concentration of calcium ions to maintain active, zinc concentration higher than 1µM could inhibit the cd-MMP-1 activity.
Inhibition of cd-MMP-1 by flavonoids
We evaluate the screening system by using the positive compounds. The inhibition effect of the positive compounds MMP inhibitor II and MMP inhibitor III from Merck were determined with an IC50 value of 13.05 nM and 10.20 nM, respectively which are consistent with the referencesCitation19,Citation20. For optimal signal to noise ratio, we chose 10 nM cd-MMP-1 and 10 µM of the compound concentration for the initial screening assay. 17 structure-related flavonoids including flavones, flavonols, flavanones, isoflavones, and flavanonol were used to investigate the inhibitory effect on cd-MMP-1 enzyme activity (). These screening procedures leading to the identification of six compounds (luteolin, fisetin, kaempferol, morin, myricetin and quercetin) with an IC50 value less than 10 µM. Among these six active compounds, fisetin, kaempferol, morin, myricetin and quercetin belong to flavonols and luteolin belong to flavones. Flavonoids are well-known antitumor, antioxidant, and anti-inflammation agents and previous structure-activity analysis has indicated that number and location of OH substitutions are important for the biological effects of flavonoidsCitation21. Obviously, the active flavonoids in this study contain four or more hydroxyl groups and hydroxylation at the 3′- and or the 4′-position of the B ring seemed of special importance. Among the five active flavonlos, the most potent two compounds fisetin and quercetin both have the 3′-position hydroxyl group. This substitution may beneficial for cd-MMP-1 inhibition activity of flavonoids. Some flavones tested in the present study showed low free inhibition activities against cd-MMP-1, such as apigenin and chrysin, however luteolin exhibited intense activity because of the 3′-position hydroxyl group. Flavanones, isoflavones and flavanonol selected in this study have no inhibition activities for cd-MMP-1. In this study, flavonoids glycoside (Quercitrin, Rutin, Naringin) did not inhibit the enzymatic activity of cd-MMP-1 because the glycoside may serve as negative moieties on MMP inhibitionCitation22.
Table 1. Inhibitions activity flavonoids against human MMP-1 catalytic domain. The inhibition rate was determined at the concentration of 10 μM.
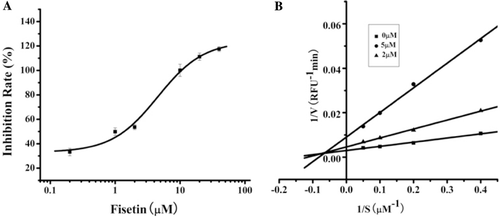
As presented in , fisetin was the most potent inhibitor with an IC50 = 1.35 µM. To further investigate the inhibition kinetics, the most potent inhibitor of cd-MMP-1, fisetin was using the Lineweaver-Burk method (). The concentration of quenched fluorogenic peptide substrate: Dnp-Pro-beta-cyclohexyl-Ala-Gly-Cys(Me)-His-Ala-Lys(Nma)-NH2 was from 2.5 to 20 μM. The velocities of reactions were measured without or with inhibitor (2 or 5 μM). The inhibition kinetics analyzed by Lineweaver-Burk plots indicates that fisetin is a mixed type inhibitor of cd-MMP-1. In mixed inhibition, the inhibitor binds to a site different from the active site where the substrate binds. Luteolin was reported as a non-competitive or mixed type inhibitor of MMP-9 and supposed binding MMP-9 outside the active siteCitation23. Fisetin may also employ the similar interaction mode with cd-MMP-1 as luteolin. Further structural information (like crystallography) is needed to determine the accurate interaction mode for fisetin towards cd-MMP-1. In summery, our result could help to provide a basis for the design of novel anti-cancer agents targeting human MMP-1.
Figure 4. Inhibition activity of fisetin against cd-MMP-1. (A) Effect of fisetin against cd-MMP-1, the data points are means ± SE for triplicate experiments. (B) Lineweaver-Burk plots for inhibitory activity of Fisetin (0, 2 and 5 μM) on cd-MMP-1 activity with the concentration of the substrate range from 2.5 μM to 20 μM. Each value was the mean of three independent experiments.
Conclusion
The efficient production of purified protein is important for biomedical applications such as small molecular drug discovery and enzyme-inhibitor complex structure determination. Large scale soluble expression is usually a bottleneck for the recombinant preparation of MMPs. Since the importance of MMP-1, human MMP-1 has been expressed use many methodsCitation9–15, however soluble and active MMP-1 are still difficult to achieve. Herein, we develop an efficient method for the high level production of soluble and active MMP-1 in E.coli. Our experiment results indicated that cd-MMP-1 prepared following our procedure has similar activity as other MMP-1 reported previouslyCitation13, and the yield is around 12–15 mg per half liter. The cd-MMP-1 protein can serve as a screening enzyme, because catalytic domain and full-length MMP-1 show similar activity. In general, the inhibitors of cd-MMP-1 may also inhibit the activity of full-length MMP-1 with the same potency and selectivityCitation24. 17 structure-related flavonoids were tested for their cd-MMP-1 inhibition activities and six compounds (luteolin, fisetin, kaempferol, morin, myricetin and quercetin) were active with an IC50 value less than 10 µM. Fisetin, the most potent inhibitor, was identified as a mixed type inhibitor of cd-MMP-1. High level expression of soluble cd-MMP-1 has allowed for kinetic study and inhibitors identification and could help to discover novel anti-cancer agent targeting human MMP-1.
Acknowledgments
This work was supported by the 111 Project (Grant B07023), the National Natural Science Foundation of China (Grants 10979072, 81102420), the Innovation Program of Shanghai Municipal Education Commission (Grant 10ZZ41), and the Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant 20090074120012).
Declaration of interest
The authors report no conflicts of interest.Reference
References
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001;17:463–516.
- Sang QX, Jin Y, Newcomer RG, Monroe SC, Fang X, Hurst DR et al. Matrix metalloproteinase inhibitors as prospective agents for the prevention and treatment of cardiovascular and neoplastic diseases. Curr Top Med Chem 2006;6:289–316.
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161–174.
- Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2002;2:657–672.
- Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov 2007;6:480–498.
- Katsuno H, Shirakawa R, Miyazaki K, Ozeki Y, Yasumitsu H. Production of active MMP7 in E. coli and its application for metalloproteinase inhibitors screening. Protein Pept Lett 2010;17:568–572.
- Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994;94:2493–2503.
- Saarialho-Kere UK, Chang ES, Welgus HG, Parks WC. Distinct localization of collagenase and tissue inhibitor of metalloproteinases expression in wound healing associated with ulcerative pyogenic granuloma. J Clin Invest 1992;90:1952–1957.
- Grant GA, Eisen AZ, Marmer BL, Roswit WT, Goldberg GI. The activation of human skin fibroblast procollagenase. Sequence identification of the major conversion products. J Biol Chem 1987;262:5886–5889.
- Murphy G, Allan JA, Willenbrock F, Cockett MI, O’Connell JP, Docherty AJ. The role of the C-terminal domain in collagenase and stromelysin specificity. J Biol Chem 1992;267:9612–9618.
- Cockett MI, Bebbington CR, Yarranton GT. The use of engineered E1A genes to transactivate the hCMV-MIE promoter in permanent CHO cell lines. Nucleic Acids Res 1991;19:319–325.
- Murphy G, Cockett MI, Stephens PE, Smith BJ, Docherty AJ. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J 1987;248:265–268.
- Rosenfeld SA, Ross OH, Hillman MC, Corman JI, Dowling RL. Production and purification of human fibroblast collagenase (MMP-1) expressed in the methylotrophic yeast Pichia pastoris. Protein Expr Purif 1996;7:423–430.
- Iyer S, Wei S, Brew K, Acharya KR. Crystal structure of the catalytic domain of matrix metalloproteinase-1 in complex with the inhibitory domain of tissue inhibitor of metalloproteinase-1. J Biol Chem 2007;282:364–371.
- Chung L, Shimokawa K, Dinakarpandian D, Grams F, Fields GB, Nagase H. Identification of the (183)RWTNNFREY(191) region as a critical segment of matrix metalloproteinase 1 for the expression of collagenolytic activity. J Biol Chem 2000;275:29610–29617.
- Gehring MR, Condon B, Margosiak SA, Kan CC. Characterization of the Phe-81 and Val-82 human fibroblast collagenase catalytic domain purified from Escherichia coli. J Biol Chem 1995;270:22507–22513.
- Chang TL. Inhibitory effect of flavonoids on 26S proteasome activity. J Agric Food Chem 2009;57:9706–9715.
- Ou L, Ma J, Zheng X, Chen X, Li G, Wu H. The expression and refolding of isotopically labeled recombinant Matrilysin for NMR studies. Protein Expr Purif 2006;47:367–373.
- Pikul S, McDow Dunham KL, Almstead NG, De B, Natchus MG, Anastasio MV et al. Discovery of potent, achiral matrix metalloproteinase inhibitors. J Med Chem 1998;41:3568–3571.
- Covington MD, Burghardt RC, Parrish AR. Ischemia-induced cleavage of cadherins in NRK cells requires MT1-MMP (MMP-14). Am J Physiol Renal Physiol 2006;290:F43–F51.
- Shen SC, Ko CH, Tseng SW, Tsai SH, Chen YC. Structurally related antitumor effects of flavanones in vitroand in vivo: involvement of caspase 3 activation, p21 gene expression, and reactive oxygen species production. Toxicol Appl Pharmacol 2004;197:84–95.
- Ko CH, Shen SC, Lee TJ, Chen YC. Myricetin inhibits matrix metalloproteinase 2 protein expression and enzyme activity in colorectal carcinoma cells. Mol Cancer Ther 2005;4:281–290.
- Ende C, Gebhardt R. Inhibition of matrix metalloproteinase-2 and -9 activities by selected flavonoids. Planta Med 2004;70:1006–1008.
- Ye QZ, Hupe D, Johnson L. Catalytic domains of matrix metalloproteinases: a molecular biology approach to drug discovery. Curr Med Chem 1996;3:407–418.