Abstract
The ability of a novel bispyridinium oxime K203 to reactivate VX agent-inhibited acetylcholinesterase was compared with the reactivating efficacy of four commonly used oximes (obidoxime, trimedoxime, methoxime, HI-6) using in vivo model. Our results showed that the reactivating efficacy of the oxime HI-6 is higher than the reactivating efficacy of the other oximes studied including the oxime K203 although the differrences between the oxime HI-6 and some other oximes are not significant, especially in the blood. Based on the obtained data, we can conclude that the antidotal treatment involving the oxime HI-6 brings the higher benefit for the antidotal treatment of acute poisonings with VX agent than other oximes.
Keywords::
Introduction
Highly toxic organophosphorus compounds called nerve agents are considered to be the most dangerous chemical warfare agents. They exert their toxic effects by the phosphonylation and subsequent inactivation of acetylcholinesterase (AChE, EC 3.1.1.7) leading to overstimulation of post-synaptic cholinergic receptors due to the accumulation of the neurotransmitter acetylcholine in synapses of the central and peripheral nervous systems. The widespread overstimulation of cholinergic receptors is manifested as salivation, lacrimation, sweating, diarrhea, urination, muscular twitching and fibrilation and ultimately tonic/clonic convulsionsCitation1,Citation2.
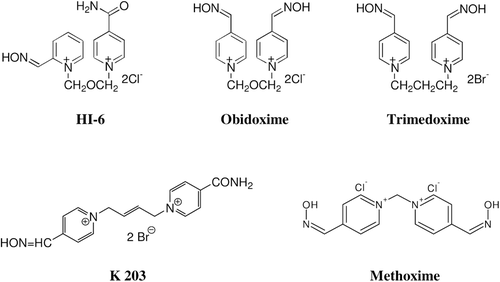
The current standard antidotal treatment of poisoning with nerve agents usually consists of a muscarinic cholinergic receptor antagonist to block the effects of acetylcholine-induced overstimulation of muscarinic receptor sites, while the oxime (compound with nucleophilic oximate anion) repairs biochemical lesions by dephosphonylating nerve agent-inhibited AChE and restoring its activityCitation1,Citation3,Citation4. In the last century, several oximes (pralidoxime, obidoxime, trimedoxime, methoxime, HI-6) were developed as antidotes against nerve agents, however, their potency to counteract the acute toxic effects of nerve agents differs depending on the type of nerve agentCitation4,Citation5. Therefore, the antidotal treatment of acute poisonings with some nerve agents still remains a serious problem and the development of new and more effective AChE reactivators is still very important. The novel bispyridinium asymmetric oxime K203 [1-(4-carbamoylpyridinium)-4-(4-hydroxy- iminomethylpyridinium)-but-2-ene dibromide] () was primarily synthesized at our departmentCitation6 to increase the efficacy of antidotal treatment of tabun poisoning that was shown to be resistant to conventional oxime therapy due to the conformational changes of AChE-tabun complex prior aging process in AChE active siteCitation7. As the oxime K203 was found to be promising reactivator of tabun-inhibited AChECitation8,Citation9, we decided to evaluate the reactivating and therapeutic efficacy of K203 against other nerve agents including VX agent, because we are still searching for a broad-spectrum oxime able to sufficiently counteract acute toxicity of all nerve agents regardless of their chemical structure.
VX agent [O-ethyl S-(2-diisopropylaminoethyl) methyl phosphonothioate] is a lipophilic liquid nerve agent with an extremely high toxicity. Thanks to its very high lipophilicity, it can easily and quickly penetrate the skin and, therefore, especially percutaneous exposure to VX agent is very dangerousCitation10. The main aim of this study was to determine the reactivating efficacy of a novel bispyridinium oxime K203 in comparison with currently available oximes (HI-6, obidoxime, trimedoxime, methoxime) against VX agent in rats.
Material and methods
Animals
Male albino Wistar rats weighing 200–240 g were purchased from VELAZ (Prague, Czech Republic). They were housed in propylene cages (56 × 36 × 19 cm3, six rats per cage) in climate- and access-controlled rooms (22 ± 2°C and 50 ± 10% relative humidity). The day/night cycle was 12/12 h. Food and tap water were available ad libitum. The rats were divided into groups of eight animals (n = 8). Handling with the experimental animals was done under the supervision of the Ethics Committee of the Faculty of Military Health Sciences in Hradec Kralove (Czech Republic).
Chemicals
VX agent was obtained from the Technical Institute in Brno (Czech Republic) in compliance with permission for the handling of chemical warfare agents and it was 96% pure. Its purity was assayed by acidimetric titration. All oximes (obidoxime, trimedoxime, methoxime, the oxime HI-6, the oxime K203) were synthesized at our Department of Toxicology of the Faculty of Military Health Sciences (Czech Republic) and they were more than 98% pureCitation6,Citation11. Their purity was analyzed using a HPLC techniqueCitation12. All other drugs and chemicals of analytical grade were obtained commercially and used without further purification. All substances were administered intramuscularly (i.m.) at a volume of 1 mL/kg body weight (b.w.).
In vivo experiments
Before starting the evaluation of reactivating efficacy of oximes, the acute toxicity of tested oximes was evaluated in rats by the assessment of their LD50 values and their 95% confidence limits using probit-logarithmical analysis of death occuring within 24 h after i.m. administration of each oxime at five different doses with eight animals per doseCitation13.
To evaluate the reactivating efficacy of the oximes, the rats were injected i.m. with either atropine (21 mg/kg) alone or atropine (21 mg/kg) in combination with one of the oximes studied in equitoxic doses corresponding to 5% of their LD50 5 min before the rats received VX agent i.m. at a dose of 13.9 µg/kg (LD50). The prophylactic administration of antidotes was used because this procedure is suitable for a mechanistic study that compares the reactivating efficacy of various oximes. The technique should give better results than the treatment of animals after poisoning and reduce the influence of aging of nerve agent-AChE complexCitation14. The rats were decapitated and exsanguinated to obtain the blood 30 min subsequent to VX agent poisoning. The blood was hemolyzed in Tris-HCl buffer (0.02 mol/L, pH 7.6, 1:20). The diaphragm was removed and homogenized in Tris-HCl buffer (0.02 mol/L, pH 7.6, 1:10) to determine cholinesterase (ChE) activity by standard spectrophotometric methodCitation15. Acetylthiocholine was used as a substrate (Tris-HCl buffer, 0.1 mol/L, pH 7.6). Helios Alpha, the spectrophotometer was used for determination of absorbancy at 436 nm. The absorbance change per time was recorded and the activity of ChE was calculated and expressed as µkat/kg or L (µmol substrate hydrolyzed/kg wet tissue or L blood within 1 sec). The untreated control values for blood and diaphragm ChE activity were obtain from rats administered with saline instead of VX agent and antidotes (saline control). The percentage of reactivation was calculated using the ChE activity values: {1–[((saline control) – (oxime + atropine))/((saline control) – (atropine control))]} × 100Citation14.
Statistical significance was determined by the use of one-way ANOVA test with Scheffe’s post hoc test and differences were considered significant when p < 0.05. Statistical evaluation was determined with the relevant computer programsCitation13.
Results
The acute i.m. toxicity of tested oximes is summarized in . The results show that the acute toxicity of a novel bispyridinium oxime K203 is lower than the acute toxicity of obidoxime and trimedoxime but it is significantly higher (p < 0.05) than the acute toxicity of methoxime and the oxime HI-6. According to our results, the oxime HI-6 can be considered to be the least toxic for rats.
Table 1. LD50 values of oximes following i.m. administration in rats.
The ability of oximes to reactivate VX agent-inhibited ChE in rat blood and diaphragm in vivo is shown in . In the blood, the reactivating efficacy of the oxime K203 corresponds to the reactivating efficacy of trimedoxime and methoxime but its ability to reactivate VX-inhibited ChE is slightly lower in comparison with obidoxime and significantly lower (p < 0.05) compared to the oxime HI-6. In the diaphragm, the reactivating efficacy of the oxime K203 as well as other oximes studied is low with the exception of the oxime HI-6 that was able to sufficiently reactivate VX-inhibited AChE in the diaphragm. Thus, the oxime HI-6 was only able to sufficiently reactivate VX-inhibited ChE in peripheral compartment.
Table 2. Percentage of reactivation of VX agent-inhibited ChE by oximes in rat blood and diaphragm in vivo.
VX agent-poisoned rats treated with atropine alone showed wide spectrum of clinical signs of poisoning including muscarinic (salivation, nose secretion) and niconitic (a decrease in muscular tonus and spontaneous motor activity, fasciculation, impairment of gait) signs within a few minutes. When the rats were treated with the oxime HI-6, the onset of VX-induced signs and symptoms was delayed and some VX-induced signs and symptoms were eliminated or at least reduced including the impairment of gait, fasciculation, nose secretion and lacrimation. When VX-poisoned rats were treated with other oximes studied, some VX-induced signs and symptoms (lacrimation, nose secretion) were reduced but not eliminated till the end of experiments.
Discussion
Based on the previously published results, the novel oxime K203 seems to be effective reactivator of tabun-inhibited AChE and it is considered to be suitable oxime for the antidotal treatment of acute tabun poisoningsCitation8,Citation9. However, due to the threat of misuse of different nerve agents for military as well as terrorist purposes, the broad-spectrum oxime, sufficiently effective against all nerve agents regardless of their chemical structure, is necessary to reach the satisfactorily effective antidotal treatment of nerve agent exposures. As no broad spectrum oxime has been developed till nowCitation4,Citation16,Citation17, it is important to know if some of newly developed oximes is able to sufficiently protect organisms against all nerve agents. Therefore, the evaluation of the ability of novel oximes to reactivate nerve agent-inhibted AChE and to protect against acute signs and symptoms of nerve agents regardless of their chemical structure is necessary.
In this paper, the potency of the novel oxime K203 to reactivate VX agent-inhibited AChE was evaluated in comparison with chosen currently available oximes including the oxime HI-6 that is considered to be the best oxime against VX agentCitation18,Citation19 although there are some authors who prefer methoximeCitation20. Our results demonstrate that the oxime K203 did not reach not only the reactivating efficacy of the oxime HI-6 but also the reactivating efficacy of some other commonly used oximes. The difference between the reactivating efficacy of oximes roughly corresponds to the ability of oximes to eliminate or at least reduce acute toxic signs and symptoms that were observed in VX-poisoned rats. While the oxime HI-6 was able to eliminate some VX-induced sings and symptoms, the other oximes studied were only able to reduce them.
These results might not be explained solely by significant differences in the reactivating efficacy of the oximes studied in the peripheral as well as central compartmentCitation21 but also by other antidotal mechanisms observed after administration of the oximes, such as direct antimuscarinic action and restoration of neuromuscular transmission. The relatively high therapeutic potency of the oxime HI-6 may be due to various antidotal mechanisms based on reactivation of phosphonylated AChE, direct antimuscarinic and ganglion blocking actions, restoration of neuromuscular transmission, retardation of the formation of the aged inhibitor-enzyme complex and inhibition of acetylcholine releaseCitation22.
Thus, only the oxime HI-6 appears to be able to sufficiently protect experimental animals from VX-induced adverse effects and improve survival of VX-poisoned animals due to the highest ability to reactivate VX-inhibited AChE in the blood, diaphragm and brainCitation3,Citation4,Citation20. However, it is difficult to extrapolate the animal data to humans because in vitro reactivating data indicate that the reactivating potency of oximes may be different in humans and animal species. The published data dealing with in vitro reactivation of nerve agent-inhibited AChE showed marked differences among the species and indicate that the findings from animal expriments need careful evaluation before extrapolating these data to humansCitation23,Citation24.
Conclusion
The above described data confirm that HI-6 is a significantly more efficacious oxime than other currently available oximes in the case of the antidotal treatment of severe VX poisoning. In addition, the newly developed oxime K203 seems to be significantly less efficacious to reactivate VX-inhibited ChE in rats than the oxime HI-6 and other currently available oximes. Therefore, it is not suitable for the replacement of the oxime HI-6 for the treatment of acute VX poisoning.
Acknowledgement
The authors wish to thank to Mrs Jana Uhlirova for her skilful assistance and Dr. Blaha for statistical evaluation of data.
Declaration of interest: The authors report no declarations of interest. The study was supported by the grant of Ministry of Defense “A long-term organization development plan 1011â.
References
- Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem 2004;38:151–216.
- Lotti M. Organophosphorus compounds. In: Spencer PS, Schaumburg HH. eds. Experimental and Clinical Neurotoxicology. New York: Oxford University Press; 2000, pp. 898–925.
- Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J Toxicol Clin Toxicol 2002;40:803–816.
- Marrs TC, Rice P, Vale JA. The role of oximes in the treatment of nerve agent poisoning in civilian casualties. Toxicol Rev 2006;25:297–323.
- Dawson RM. Review of oximes available for treatment of nerve agent poisoning. J Appl Toxicol 1994;14:317–331.
- Musilek K, Holas O, Kuca K, Jun D, Dohnal V, Opletalova V et al. Synthesis of monooxime-monocarbamoyl bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against tabun- and paraoxon-inhibited acetylcholinesterase. J Enzym Inhib Med Chem 2008;23:70–76.
- Ekström F, Akfur C, Tunemalm AK, Lundberg S. Structural changes of phenylalanine 338 and histidine 447 revealed by the crystal structures of tabun-inhibited murine acetylcholinesterase. Biochemistry 2006;45:74–81.
- Kassa J, Karasova J, Musilek K, Kuca K. An evaluation of therapeutic and reactivating effects of newly developed oximes (K156, K203) and commonly used oximes (obidoxime, trimedoxime, HI-6) in tabun-poisoned rats and mice. Toxicology 2008;243:311–316.
- Kovarik Z, Vrdoljak AL, Berend S, Katalinic M, Kuca K, Musilek K et al. Evaluation of oxime k203 as antidote in tabun poisoning. Arh Hig Rada Toksikol 2009;60:19–26.
- Vachek J, Gersl V, Fusek J, Krs O, Skopec F, Bajgar J. Toxicities of O-alkyl S-(2-dialkylaminoethyl) methyl phosphonothiolates (V-compounds). Acta Medica (Hradec Kralove) 1996;39:67–71.
- Musilek K, Kuca K, Jun D, Dolezal M. Synthesis of bispyridinium compounds bearing propane linker and evaluation of their reactivation activity against tabun- and paraoxon-inhibited acetylcholinesterase. Lett Org Chem 2006;3:831–835.
- Jun D, Kuca K, Stodulka P, Koleckar V, Dolezal B, Simon P, Veverka M. HPLC analysis of HI-6 dichloride and dimethanesulfonate – antidotes against nerve agents and organophosphorus pesticides. Anal Lett 2007;40:2783–2787.
- Tallarida R, Murray R. Manual of Pharmacological Calculation with Computer Programs. New York:Springer-Verlag; 1987.
- Clement JG, Hansen AS, Boulet CA. Efficacy of HLö-7 and pyrimidoxime as antidotes of nerve agent poisoning in mice. Arch Toxicol 1992;66:216–219.
- Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
- Kassa J, Kuca K, Bartosova L, Kunesova G. The development of new structural analogues of oximes for the antidotal treatment of poisoning by nerve agents and the comparison of their reactivating and therapeutic efficacy with currently available oximes. Curr Org Chem 2007;11:267–283.
- Szinicz L, Worek F, Thiermann H, Kehe K, Eckert S, Eyer P. Development of antidotes: problems and strategies. Toxicology 2007;233:23–30.
- Lundy PM, Raveh L, Amitai G. Development of the bisquaternary oxime HI-6 toward clinical use in the treatment of organophosphate nerve agent poisoning. Toxicol Rev 2006;25:231–243.
- Lundy PM, Hamilton MG, Sawyer TW, Mikler J. Comparative protective effects of HI-6 and MMB-4 against organophosphorous nerve agent poisoning. Toxicology 2011;285:90–96.
- Shih TM, Skovira JW, O’Donnell JC, McDonough JH. Evaluation of nine oximes on in vivo reactivation of blood, brain, and tissue cholinesterase activity inhibited by organophosphorus nerve agents at lethal dose. Toxicol Mech Method 2009;19:386–400.
- Worek F, Widmann R, Knopff O, Szinicz L. Reactivating potency of obidoxime, pralidoxime, HI 6 and HLö 7 in human erythrocyte acetylcholinesterase inhibited by highly toxic organophosphorus compounds. Arch Toxicol 1998;72: 237–243.
- van Helden HP, Busker RW, Melchers BP, Bruijnzeel PL. Pharmacological effects of oximes: how relevant are they? Arch Toxicol 1996;70:779–786.
- Worek F, Reiter G, Eyer P, Szinicz L. Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Arch Toxicol 2002;76:523–529.
- Kuca K, Cabal J, Kassa J. In vitro reactivation of sarin-inhibited brain acetylcholinesterase from different species by various oximes. J Enzyme Inhib Med Chem 2005;20:227–232.