Abstract
Some enzymes are known to be drug target inhibitions of which can be critical for organisms. PON has a critical role to prevent atherogenesis by inhibiting lipid peroxidation. It is well known that paraoxonase 1 (PON1) plays an important function on high-density lipoprotein (HDL) structure to prevent lipid oxidation not only of low-density lipoprotein, but also of HDL itself. We investigated in vitro effects of some medical drugs on PON1 activity from human serum. Ki constants for oxytetracycline hydrochloride, netilmycin sulfate, lincomycin hydrochloride, clindamycin phosphate, and streptomycin sulfate were found as 0.2, 3.73, 18.30, 35.80, and 56.30 mM, respectively. Our results indicate that these commonly used drugs inhibit the activity of the enzyme at very low doses with different inhibition mechanisms.
Introduction
As it is well known, almost all reactions are catalyzed by enzymes in metabolism. Chemicals, toxic compounds and drugs act through inhibition of specific enzymes in living metabolism. Especially, some enzymes are considered to be drug targets such as carbonic anhydrase, glucose 6-phosphate dehydrogenase, and paraoxonase (PON).Citation1–3 Thus, inhibition of these enzymes can cause critical results for living systems such as low quality of life and more. For example, deficiency or inhibition of PON activity leads to the development of a wide variety of diseases such as cardiovascular disease, diabetes mellitus, rheumatism, alzheimer and many others as organophosphate toxicities. PON1 has a special importance in this respect. It is abundantly found associated with HDL in human serum and has a great importance against atherosclerosis.Citation4 The concentrations of the enzyme are known to be inversely correlated with the development of atherosclerosis.Citation4 The protective mechanism of PON1 is probably related to its ability to hydrolyze some oxidized phospholipids and cholesteryllinoleate hydroperoxides, which are present in oxidatively modified low-density lipoprotein (LDL).Citation5,Citation6 It is considered that this antioxidant effect of PON1 occurs from the free sulphydryl group (cystein 284) in its structureCitation7–9 ().
Scheme 1. Fundamental representation of paraoxonase 1 (PON1) structure. Cys-284 residue is considered to provide the antioxidant effects at the three-dimensional structure of PON1 enzyme.
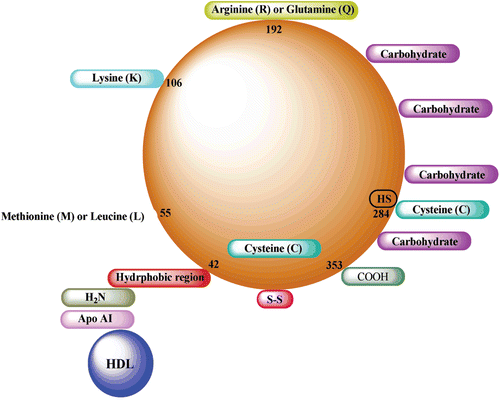
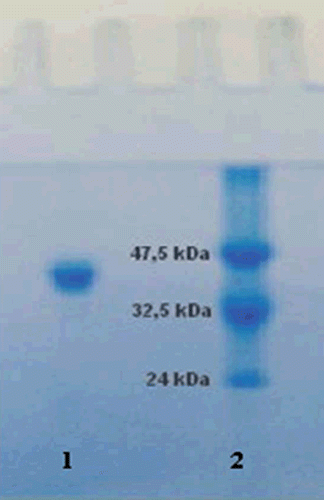
Figure 1. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of purified paraoxonase 1 (PON1). Lane 1: standard proteins (kD): aldolase (47,500), triosephosphateisomerase (32,000), soy bean trypsin inhibitor (24,000) Da. Lane 2: PON1 from human serum via Sephadex G-200 gel filtration chromatography.
Various factors can cause decreases in enzyme concentrations in the serum. This situation in serum leads to LDL oxidation and atherosclerotic lesions. For example, Gursu and Ozdin investigated the relationship between the levels of MDA, end product of lipid peroxidation, and PON1 levels in smokers. They used 49 male subjects aged 45 ± 5 years (22 chronic smokers, 27 controls) in the study. They found that smoking reduced serum PON1 activity and concentration and increased lipid peroxidation levels.Citation10 In another study, it was showed that some antibiotics inhibit the PON1 activity.Citation11 Although in recent years, some studies have focused on the enzyme–drug interactions, which are critical for human health, there are still few studies on PON1 enzyme and drug interactions. In vitro and in vivo studies on PON1 and other enzymes have been carried out by our group for a long time.Citation3,Citation9,Citation12
In this study, a high-density lipoprotein (HDL) associated enzyme, PON (arylesterase, EC 3.1.8.1, PON1), was purified from human serum and in vitro effects of netilmycin sulfate, lincomycin hydrochloride, streptomycin sulfate, Oxytetracycline hidroklorid, penicillin G potassium, and clindamycin phosphate were investigated.
Materials and methods
Materials
Materials, including diethylaminoethyl cellulose (DEAE)-Sephadex A50, Sephadex G-200, Paraoxon, protein assay reagents, and chemicals for electrophoresis, were obtained from Sigma Chem. Co. (Sigma-Aldrich Chemie GmbH, Export Department, Taufkirchen, Germany). All other chemicals used were of analytical grade and were obtained from either Sigma-Aldrich or Merck (Merck KGaA Frankfurter strasse, Darmstadt, Germany). Antibiotics and the ohter drugs were provided from the University Hospital Pharmacy (Atatürk University, Erzurum, Turkey).
PON activity assay
PON activity was determined spectrophotometrically using the same procedure in our previous studies.Citation9 The enzyme assay was based on the estimation of p-nitrophenol at 412 nm. To calculate the activity the molar extinction coefficient of p-nitrophenol was utilized. It is e = 18,290 M−1 cm−1 at pH 10.5.Citation13 One enzyme unit was defined as the amount of enzyme that catalyzes the hydrolysis of 1 μmol of substrate at 25°C.
Partial purification
Eighteen milliliters of human serum was precipitated with ammonium sulphate. The precipitation intervals for PON were 60–80%.Citation14 The precipitate was collected by centrifugation at 15,000 rpm for 20 min and redissolved in 100 mM sodium (Na)-phosphate buffer (pH 7.0).
DEAE-Sephadex A50 anion exchange chromatography
The enzyme solution, which had been dialyzed in the presence of 1 mMNa-phosphate buffer (pH 7.0) at 4°C, was loaded onto the DEAE-Sephadex A50 anion exchange column (3 cm2 × 30 cm), which had been equilibrated with 100 mM Na-phosphate buffer (pH 7.0). The column was washed with 100 mM Na-phosphate buffer (pH 7.0), and then elution was performed with a linear gradient of 0–1.5 M sodium chloride. Eluted fractions were collected and enzyme activity was checked at 412 nm. Tubes with enzyme activity were combined. All purification procedures were performed at 4°C.
Gel filtration chromatography
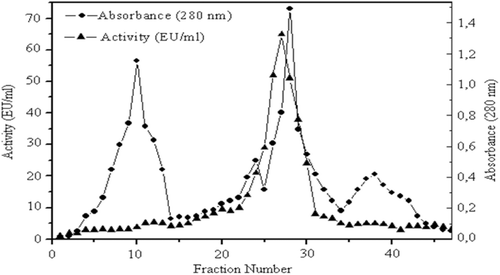
Enzyme solution from the DEAE-SephadexA50 column were mixed with glycerol and loaded onto the Sephadex G-200 chromatography (60 cm × 2 cm2), which had been equilibrated with 100 mM Na-phosphate buffer (pH 7.0). Elution was performed with the same buffer. For each fraction qualitative protein assay was performed at 280 nm and activity determination at 412 nm spectrophotometrically. Active fractions were combined for other kinetic studies.Citation9
Quantitative protein assay
The protein quantity was determined at 595 nm spectrophotometrically. The method has been performed as proposed by Bradford using bovine serum albumin as the standard.Citation15,Citation16
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed after purification of the enzyme. It was performed with 3 and 8% acrylamide concentrations for the stacking and separating gels, respectively, and 0.1% SDS.Citation17 Sample (20 μg) was applied to the electrophoresis medium. Gels were stained for 1.5 h in 0.1% Coomassie Brilliant Blue R-250, 50% methanol, and 10% acetic acid, and then destained with several changes of the same solvent without dye. The electrophoretic pattern was photographed () should be from this section.
In vitro drug studies
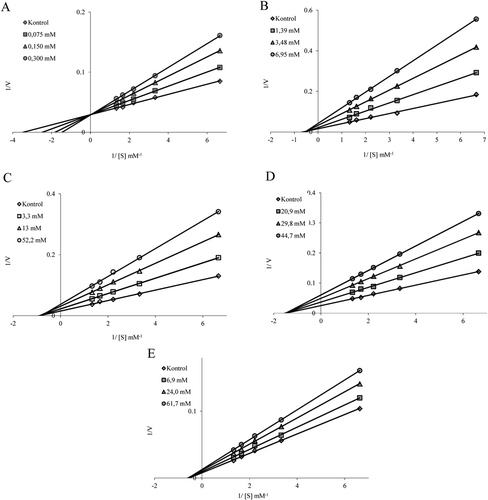
We examined the inhibitory effects of oxytetracycline hydrochloride, netilmycin sulfate, lincomycin hydrochloride, clindamycin phosphate, and streptomycin sulfate. PON activities were measured in the presence of different drug concentrations. Control activity was assumed to be 100% in the absence of inhibitor. Experiments of all compounds were tested in triplicate at each concentration used and Activity%-[Drug] graphs were drawn for each drug. Determination of Ki values was performed by using three different inhibitor concentrations. In these experiments, paraoxone was used as substrate at five different concentrations (0.15, 0.3, 0.45, 0.6, and 0.75 mM). Lineweaver–Burk curves were drawn and average Ki and inhibitor type were determined.Citation18
Results
Purification of human PON1
Purification of human serum PON1 (hPON1) was achieved using three procedures: ammonium sulfate fractionation (60–80%), DEAE Sephadex A50 anion exchange and Sephadex G-200 gel filtration chromatography. This procedure was the same as we used in our previous studies.Citation9 The enzyme was purified ~240-fold with a yield of 44.8%. The specific activity of the purified enzyme was found as 3967.2 U/mg proteins ( and ). Single protein band was obtained for hPON1 and the molecular weight was calculated as 43 kDa on SDS-PAGE (), which is in agreement with other studies.Citation9,Citation19
Table 1. Purification steps of PON1 enzyme from human serum by ammonium sulfate precipitation, anion exchange chromatography, and gel filtration chromatography.
Figure 2. Elutiongraph of paraoxonase 1 from human serum by Sephadex G-200 gel filtration chromatography. Fractions from the diethylaminoethyl cellulose-Sephadex column were mixed with glycerol and loaded onto the Sephadex G-200 column (60 cm × 2 cm),which had been equilibrated with 100 mM Na-phosphatebuffer (pH7.0). Elution was performed with the same buffer. Fractions were analyzed for both protein amount (280 nm) and enzyme activity (412 nm).
Inhibition studies
The inhibitory constants (Ki) of the drugs are presented in and . From these results, oxytetracycline hydrochloride and netilmycin sulfate were considered potential inhibitors. Oxytetracycline hydrochloride inhibited the enzyme in competitive manner and other drugs had noncompetitive effect.
Table 2. Ki values and inhibition types for five different medical drugs.
Figure 3. Determination of inhibitiontypesand Ki values of the drugs using Lineweaver–Burk curves. For the determination of Ki values, three different inhibitor concentrations were tested for each drug. Paraoxone was used as substrate at five different concentrations. Control activity was assumed to be 100% in the absence of inhibitor. Activity assays were performed as described in Materials and methods section.
Discussion
PON1 is an HDL-associated esterase/lactonase, and its activity is inversely associated with the risk of cardiovascular diseases.Citation20–22 PON1 plays an important role in the prevention of atherosclerosis development. Antiatherogenic properties of PON1 include protection of LDL, HDL, and macrophages against oxidative stress.Citation8,Citation23 Recently, it has been demonstrated that these anti-atherogenic properties of PON1 are all related to its lipolactonase activity.Citation24 PON1 prevents the accumulation of oxidized lipids in LDL particles, and thus, prevents vascular diseases.Citation25 Following this observation, it was reported that PON1 reduces the biological activity of minimally oxidized LDL.Citation26 Beside these antioxidant and antiatherogenic properties, PON1 is also a detoxifier that can hydrolyze toxic organophosphates. Whatever the roles of PON1 in lipid metabolism, low levels of PON1 appear to be a risk factor for vascular diseases. The role of PONs in pharmacokinetics will obviously be important and merits much more research effort. Nowadays, studies on PON1 have become very popular among scientists. However, there are few studies on the interactions between PON activity and drugs. For instance, Sinan et al. investigated the in vitro effects of gentamycin sulfate and cefazolin sodium on purified human serum PON1 enzyme and human liver PON1 activity in human hepatoma HepG2 cells. In that study, they found that gentamycin sulfate and cefazolin sodium were potent inhibitors for human serum PON1, and IC50 values were 0.887 and 0.0084 mM, respectively.Citation14 In another study, the in vitro and in vivo effects of intravenous anesthetics, etomidate, propofol, and ketamine, on human serum PON1 activity were investigated. They evidenced that the three anesthetics dose-dependently decreased in vitro hPON1 activity. Inhibition types were found to be different for each drug under in vitro conditions. In vivo studies were also performed on three patients for each drug and these anesthetics significantly inhibited PON1 enzyme.Citation9 In addition, Ekinci and Beydemir investigated the in vitro effects of some antibiotics, teicoplanin, rifamycin, tobramycin, ceftriaxone sodium, cefuroxime sodium, ceftazidime, ornidazole and amikacin sulfate, on human serum PON1 activity.Citation11 Today, the entire world knows that smoking is a critical reason of cardiovascualar diseases. There are some studies about smoking and its effects on various enzymes. For instance, it was shown that cigarette smoke extract inhibited human plasma PON activity in a dose- and time-dependent manner. It was found that 250 μL of cigarette smoke extract inhibited approximately 50% of PON activity when incubated at 37°C for 6 h. Indeed, the rate of 50% inhibition is a large proportion in terms of human healty. Cigarette smoking increases the risk of atherosclerosis by decreasing plasma HDL (good cholesterol) levels, making the LDL (bad cholesterol) of cigarette smokers more susceptible to oxidation.Citation27–29
In general, drugs and toxic substances display their biological effects by interaction with specific enzymes. Some enzymes are taget for many chemical compounds and drugs. There are a lot of studies on organic compound synthesis and drug design, but a few examples are available about functions of pharmaceutical drugs in living metabolism. Studies on the interactions between drugs and specific enzymes have attracted increased attention in the recent years. For instance, inhibition and activation effects of various drugs on the activities of different enzymes have been studied in our laboratory up to now.Citation30–39 Considering the above mentioned information, we aimed in the present study to purify PON1 from human serum and to investigate the kinetic interactions between the enzyme and some antibiotic drugs, namely oxytetracycline hydrochloride, netilmycin sulfate, lincomycin hydrochloride, clindamycin phosphate, streptomycin sulfate. To this end, hPON1 was purified using simple methods. We then examined the effects of the antibiotics and some drugs on human PON1 activity.The drugs were determined to decrease the enzyme activity at various concentrations. Of course, chemical compounds and drugs can be effective due to some functional groups as hydroxyl, amine, amide and free chloride groups on target enzymes. In light of the above-mentioned information it is clear that the molecule with phenolic hydroxyl group, oxytetracycline hydrochloride, is more potent inhibitor compared with others (). This result is in agreement with our previous study. In our previous study, teicoplanin and rifamycin drugs which have phenolic hydroxyl group were determined as the most effective inhibitors. The similarity of these groups is important in terms of inhibition mechanism of the PON1 enzyme.Citation11 As known,PON1 has both esterase and lactonase activity. Hence, some chemicals which contain ester and lacton groups of its structure can behave as a substrate like paraoxon for the enzyme. On the other hand, these chemicals can inhibit the activity of the enzyme with their big structures or some functional groups. 6-Clyndamycin phosphate compound contains a phosphate ester on its structure and so it has the lowest inhibition of other compounds (). Inhibition range of the drugs is given as oxytetracyclinehidrokloride > netilmycin sulfate > lincomycin hydrochloride > clindamycin fosfate = streptomycin sulfate. According to the crystal structure of the enzyme, His 115 and His 134 can be seen as a ligand in the active site.Citation7 Interestingly, oxytetracyclinehidrochloride inhibits the enzyme in competitive manner by binding to the active site of the enzyme ( and ). This molecule has a more acidic proton than water. Other drugs inhibit the enzyme in uncompetitive manner by binding to anywhere outsite the enzyme active site ( and ).
Figure 4. Structures of the oxytetracycline hydrochloride, netilmycin sulfate, lincomycin hydrochloride, clindamycin phosphate, and streptomycin sulfate, respectively.
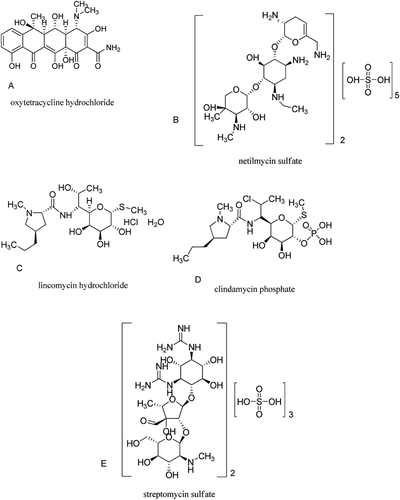
In conclusion, human serum PON1 was purified in three steps with a high specific activity and interactions between the enzyme and some antibiotic drugs, namely oxytetracycline hydrochloride, netilmycin sulfate, lincomycin hydrochloride, clindamycin phosphate, and streptomycin sulfate were investigated. These antibiotics have been used often in medicinal applications. PON enzyme is vital in cardiovascular cases and lipid peroxidation. For this reason, it is critical to investigate effects of these drugs on PON activity. Also, it is well-known that in general, drugs and toxic substances display their biological effects by interaction with specific enzymes. In this respect, determination of PON1-drug interaction is critical. Consequently, oxytetracycline hydrochloride is most effective of other compounds and it inhibited the enzyme activity in competitive manner. For other compounds, inhibition type determined as uncompetitive. We consider that more studies should be performed on inhibitors and activators of PON1. Besides, in vivo and genetic studies must be performed on this subject in the future because cardiovascular diseases are one of the most common diseases inaround the world.
Declaration of interest
This research was financed by a grant from Research Fund of Atatürk University. The authors are grateful to Atatürk University for financial support (Project no: BAP 2009/81).
References
- Ekinci D, Beydemir S, Alim Z. Some drugs inhibit in vitro hydratase and esterase activities of human carbonic anhydrase-I and II. Pharmacol Rep 2007;59:580–587.
- Beydemir S, Ciftçi M, Küfrevioglu OI. Purification and characterization of glucose 6-phosphate dehydrogenase from sheep erythrocytes and inhibitory effects of some antibiotics on enzyme activity. J Enzyme Inhib Med Chem 2002;17:271–277.
- Ekinci D, Beydemir S. Effect of some analgesics on paraoxonase-1 purified from human serum. J Enzyme Inhib Med Chem 2009;24:1034–1039.
- Mackness B, Mackness M, Aviram M, György P. (2008) The Paraoxonases: Their Role in Disease Development andXenobiotic Metabolism. Dordrecht, The Netherlands: Springer.
- Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest 1998;101:1581–1590.
- Deakin SP, James RW. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin Sci 2004;107:435–447.
- Harel M, Aharoni A, Gaidukov L, Brumshtein B, Khersonsky O, Meged R et al. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol 2004;11:412–419.
- Aviram M, Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med 2004;37:1304–1316.
- Alici HA, Ekinci D, Beydemir S. Intravenous anesthetics inhibit human paraoxonase-1 (PON1) activity in vitro and in vivo. Clin Biochem 2008;41:1384–1390.
- Gürsu MF, Özdin M. The investigation of levels of paraoxonase (PON 1) and malondialdehyde in smokers. Fırat Tıp Dergisi 2002;7:732–737.
- Ekinci D, Beydemir S. Evaluation of the impacts of antibiotic drugs on PON 1; a major bioscavenger against cardiovascular diseases. Eur J Pharmacol 2009;617:84–89.
- Ekinci D, Sentürk M, Beydemir S, Küfrevioglu OI, Supuran CT. An alternative purification method for human serum paraoxonase 1 and its interactions with sulfonamides. Chem Biol Drug Des 2010;76:552–558.
- Renault F, Chabrière E, Andrieu JP, Dublet B, Masson P, Rochu D. Tandem purification of two HDL-associated partner proteins in human plasma, paraoxonase (PON1) and phosphate binding protein (HPBP) using hydroxyapatite chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 2006;836:15–21.
- Sinan S, Kockar F, Arslan O. Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglikozide derived antibiotics. Biochimie 2006;88:565–574.
- Bradford MM. A rapid and sensitive method for the quantition of microgram quantities of protein utilizing the principle of protein-dyebinding. Anal Biochem 1976;72: 248–254.
- Yilmaz H, Ciftci M, Beydemir S, Bakan E. Purification of glucose 6-phosphate dehydrogenase from chicken erythrocytes. investigation of some kinetic properties. Prep Biochem Biotechnol 2002;32:287–301.
- Laemmli DK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970; 227:680–683.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–666.
- Pla A, Rodrigo L, Hernández AF, Gil F, Lopez O. Effect of metal ions and calcium on purified PON1 and PON3 from rat liver. Chem Biol Interact 2007;167:63–70.
- Gaidukov L, Tawfik DS. High affinity, stability, and lactonase activity of serum paraoxonase PON1 anchored on HDL with ApoA-I. Biochemistry 2005;44:11843–11854.
- Getz GS, Reardon CA. Paraoxonase, a cardioprotective enzyme: continuing issues. Curr Opin Lipidol 2004;15:261–267.
- Mackness MI, Durrington PN, Mackness B. Paraoxonase 1 activity, concentration and genotype in cardiovascular disease [Hyperlipidaemia and cardiovascular disease]. Curr Opin Lipidol 2004;15:399–404.
- Rozenberg O, Shih DM, Aviram M. Paraoxonase 1 (PON1) attenuates macrophage oxidative status: studies in PON1 transfected cells and in PON1 transgenic mice. Atherosclerosis 2005;181:9–18.
- Rosenblat M, Karry R, Aviram M. Paraoxonase 1 (PON1) is a more potent antioxidant and stimulant of macrophage cholesterol efflux, when present in HDL than in lipoprotein-deficient serum: relevance to diabetes. Atherosclerosis 2006;187:74–81.
- Mackness M, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density-lipoprotein. FEBS Lett 1991;286:152–154.
- Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM et al. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest 1995;96:2882–2891.
- Nishio E, Watanabe Y. Cigarette smoke extract inhibits plasma paraoxonase activity by modification of the enzyme’s free thiols. Biochem Biophys Res Commun 1997;236:289–293.
- Fortmann SP, Haskell WL, Williams PT. Changes in plasma high density lipoprotein cholesterol after changes in cigarette use. Am J Epidemiol 1986;124:706–710.
- Scheffler E, Huber L, Frühbis J, Schulz I, Ziegler R, Dresel HA. Alteration of plasma low density lipoprotein from smokers. Atherosclerosis 1990;82:261–265.
- Bayram E, Senturk M, Kufrevioglu OI, Supuran CT. In vitro inhibition of salicylic acid derivatives on human cytosolic carbonic anhydrase isozymes I and II. Bioorg Med Chem 2008;16:9101–9105.
- Ekinci D, Cavdar H, Durdagi S, Talaz O, Sentürk M, Supuran CT. Structure-activity relationships for the interaction of 5,10-dihydroindeno[1,2-b]indole derivatives with human and bovine carbonic anhydrase isoforms I, II, III, IV and VI. Eur J Med Chem 2012;49:68–73.
- Abdülkadir Coban T, Beydemir S, Gülcin I, Gücin I, Ekinci D, Innocenti A et al. Sildenafil is a strong activator of mammalian carbonic anhydrase isoforms I-XIV. Bioorg Med Chem 2009;17:5791–5795.
- Ekinci D, Beydemir S. Risk assessment of pesticides and fungicides for acid–base regulation and salt transport in rainbow trout tissues. Pestic Biochem Phys 2010;97:66–70.
- Isgör MM, Beydemir S. Some cardiovascular therapeutics inhibit paraoxonase 1 (PON1) from human serum. Eur J Pharmacol 2010;645:135–142.
- Aksakal E, Ekinci D, Erdogan O, Beydemir S, Alim Z, Ceyhun SB. Increasing stocking density causes inhibition of metabolic-antioxidant enzymes and elevates mRNA levels of heatshock protein 70 in rainbow trout. Livest Sci 2011;141:69–75.
- Siktar E, Ekinci D, Siktar E, Beydemir S, Gulcin I, Gunay M. Protective role of L-carnitinesupplementationagainstexhaustiveexercise-inducedoxidativestress in rats. Eur J Pharm 2011;668:407–413.
- Senturk M, Ekinci D, Alici HA, Beydemir S. Paraoxonase-1, an organophosphate detoxifier and cardioprotective enzyme, is inhibited by anesthetics: an in vitro and in vivo insight. Pestic Biochem Phys 2011;101:206–211.
- Ceyhun SB, Aksakal E, Ekinci D, Erdogan O, Beydemir S. Influence of cobalt and zinc exposure on mRNAexpression profiles of metallothionein and cytocrome P450 in rainbow trout. Biol Trace Elem Res 2011;144:781–789.
- Balaydin HT, Soyut H, Ekinci D, Goksu S, Beydemir S, Menzek A, Sahin E. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols including natural products. J Enzym Inhib Med Chem 2012;27:43–50.