Abstract
With the aim of finding new anticonvulsant drugs, new 6-substituted-[1,2,4]triazolo[3,4-a] (tetrazolo[5,1-a]) phthalazine derivatives (1–34) have been designed and synthesized. All the compounds were evaluated for their anticonvulsant activities using the maximal electroshock test (MES). Most of the synthesized compounds exhibited potent anticonvulsant activities in the MES. The most promising compound 14 showed significant anticonvulsant activity in MES test with ED50 value of 9.3 mg/kg. It displayed a wide margin of safety with protective index much higher than the standard drug Carbamazepine. And the potency of compound 14 against seizures induced by Pentylenetetrazole, Isoniazid, Thiosemicarbazide and 3-Mercaptopropionic acid in the chemical-induced seizure tests suggested that compound 14 displayed wide spectrum of activity in several models.
Introduction
Epilepsy, a ubiquitous disease characterized by recurrent seizures, afflicts more than 60 million people worldwide according to epidemiological studiesCitation1. For epilepsy treatment, nearly 95% of clinically available drugs were approved before 1985 and they could provide satisfactory seizure control for 60–70% of patients. These drugs, however, also cause notable adverse side effects such as drowsiness, ataxia, gastrointestinal disturbance, hepatotoxicity and megaloblastic anemiaCitation2–4, and even life threatening conditionsCitation5. Research to find more effective and safer antiepileptic drugs is, therefore, imperative and challenging in medicinal chemistry.
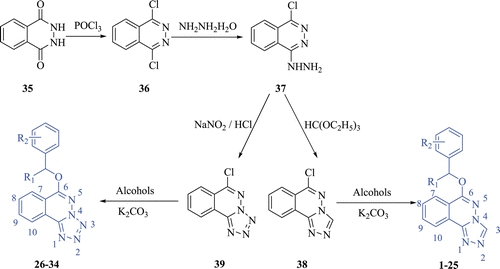
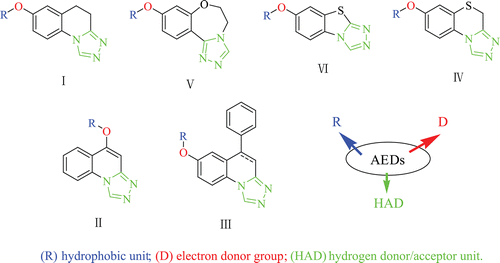
We previously described the synthesis and anticonvulsant activity evaluation of some compounds containing triazole, which exhibited potent activity in maximal electroshock test (MES) and in several chemical models such as Pentylenetetrazole- and Isoniazid-induced seizure tests ()Citation6–12. From the currently used AEDs, the major characteristics important in newly synthesized compounds are the inclusion of a hydrophobic unit (R), an electron donor group (D), a hydrogen donor/acceptor unit (HAD). With respect to the compounds mentioned above, obviously the R is the phenyl group and the substituents on it, the D is the oxygen atom and the HAD is the triazole. As part of our continuous efforts to find better anticonvulsant agents in this area, a series of 6-substituted-[1,2,4]triazolo[3,4-a](tetrazolo[5,1-a])phthalazine derivatives (1–34) were designed and synthesized in this study, which also possessed the three parts mentioned above.
Figure 1. Structures of some compounds in containing triazole and the sketch map. (R) hydrophobic unit; (D) electron donor group; (HAD) hydrogen donor/acceptor unit.
All the 34 new compounds were synthesized and evaluated as anticonvulsant agents in experimental epilepsy models. The rotarod assay was performed in mice to evaluate the neurotoxicity of the compounds. The anti-MES activity and the neurotoxicity of the marketed agent carbamazepine were evaluated in our laboratory under the same conditions for the purpose of comparison.
Experimental protocols
Melting points were determined in open capillary tubes and were uncorrected. IR spectra were recorded (in KBr) on IR Prestige-21. 1H NMR spectra were measured on an AV-300 (Bruker, Switzerland), and all chemical shifts were given in parts per million relative to tetramethysilane. Mass spectra were measured on a HP1100LC (Agilent Technologies, USA). Elemental analyses were performed on a 204Q CHN (Perkin Elmer, USA). The chemicals were purchased from Aldrich Chemical Corporation.
Chemistry
Synthesis of 1,4-dichlorophthalazine (36)
Compound 35 (8.5 g, 52.5 mmol) was dissolved in phosphorus oxychloride (45 mL) and stirred under reflux for 4 h. Then the solvent was removed under vacuum. The residue was dissolved in dichloromethane (200 mL) and stirred rapidly, and the solution was neutralized by the addition of solid and aqueous sodium hydrogen carbonate (cautiously). When effervescence had ceased, the organic layer was separated and the aqueous layer was extracted with dichloromethane (200 mL twice). The combined organic layers were dried over MgSO4, filtered and evaporated. The residue was purified by silica gel column chromatography with ethyl acetate: petroleum ether (1:8) and gained 9.0 g.
Synthesis of 1-hydrazine-4-chrorophthalazin (37)
A solution of compound 36 (5 g, 25.1 mmol) in THF (60 mL) was added dropwise to a solution of hydrazine hydrate (6.28 g, 125.6 mmol) in THF (10 mL) at room temperature. The mixture was stirred and heated at 60°C for 1 h, then half of the solvent was removed under reduced pressure and the solution was poured into petroleum ether. The precipitate was filtered and washed with petroleum ether, then kept below 0°C. The compound 37 obtained was pure enough for the following step.
Synthesis of 6-chloro-[1,2,4]triazolo[3,4-a]phthalazine (38)
In a round-bottomed flask, compound 37 (2.2 g, 11.3 mmol) was dissolved in Triethoxymethane (60 mL), and the solution was refluxed for 2 h, then the solvent was removed under reduced pressure. The residue was purified by silica gel chromatography using dichloromethane.
The general procedure for the synthesis of compounds 1–25
Compound 38 (0.65 g, 3.2 mmol), K2CO3 (0.44 g, 3.2 mmol), benzyltriethylammonium chloride (TEBA) (0.23 g, 1 mmol) and corresponding alcohol (0.70 mmol) were suspended in acetonitrile (50 mL) reacted with stirring and refluxing for about 3 h. After the acetonitrile was removed under reduced pressure, the solid residue was purified by silica gel chromatography with ethyl acetate: petroleum ether (2:3) to obtain compounds 1–25. The yield, melting point and spectral data of each compound were given below.
6-(Benzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (1)
Yield: 87%, m.p. 170–172°C. 1H-NMR (CDCl3): δ 8.93 (s, 1H, 3-H), 8.90 (d, 1H, J = 9.0 Hz, 10-H), 8.27 (d, 1H, J = 9.0 Hz, 7-H), 8.00 (t, 1H, J = 9.0 Hz, 9-H), 7.85 (t, 1H, J = 9.0 Hz, 8-H), 7.55 (d, 2H, J = 9.0 Hz, 2′6′-H), 7.49-7.37 (m, 3H, 3′, 4′, 5′-H), 5.57 (s, 2H, CH2). IR (KBr) cm−1: 1532 (C=N). MS (APCI) m/z 277 (M+1). Anal. Calc. for C16H12N4O: C, 69.55; H, 4.38; N, 20.28. Found: C, 69.32; H, 4.41; N, 20.33.
6-(2-Fluorobenzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (2)
Yield: 89%, m.p. 177–179°C. 1H-NMR (CDCl3): δ 8.87 (s, 1H, 3-H), 8.62 (d, 1H, J = 9.0 Hz, 10-H), 8.17 (d, 1H, J = 9.0 Hz, 7-H), 7.89 (t, 1H, J = 9.0 Hz, 9-H), 7.78 (t, 1H, J = 9.0 Hz, 8-H), 7.58 (t, 1H, J = 9.0 Hz, 5′-H), 7.37-7.42 (m, 1H, 4′-H), 7.25-7.16 (m, 2H, 3′-H), 5.60 (s, 2H, CH2). IR (KBr) cm−1: 1519 (C=N). MS (APCI) m/z 295 (M+1). Anal. Calc. for C16H11FN4O: C, 65.30; H, 3.77; N, 19.04. Found: C, 65.28; H, 3.82; N, 19.22.
6-(3-Fluorobenzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (3)
Yield: 88%, m.p. 169–171°C. 1H-NMR (CDCl3): δ 8.86 (s, 1H, 3-H), 8.63 (d, 1H, J = 9.0 Hz, 10-H), 8.20 (d, 1H, J = 9.0 Hz, 7-H), 7.96 (t, 1H, J = 9.0 Hz, 9-H), 7.81 (t, 1H, J = 9.0 Hz, 8-H), 7.43-7.38 (m, 1H, 2′-H), 7.32-7.24 (m, 2H, 4′ and 5′-H), 7.12-7.09 (m, 1H, 6′-H), 5.53 (s, 2H, CH2). IR (KBr) cm−1: 1512 (C=N). MS (APCI) m/z 295 (M+1). Anal. Calc. for C16H11FN4O: C, 65.30; H, 3.77; N, 19.04. Found: C, 65.31; H, 3.82; N, 19.15.
6-(4-Fluorobenzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (4)
Yield: 89%, m.p. 200–202°C. 1H-NMR (CDCl3): δ 8.92 (d, 1H, J = 9.0 Hz, 10-H), 8.90 (s, 1H, 3-H), 8.27 (d, 1H, J = 9.0 Hz, 7-H), 8.02 (t, 1H, J = 9.0 Hz, 9-H), 7.88 (t, 1H, J = 9.0 Hz, 8-H), 7.70 (s, 1H, 2′ or 6′-H), 7.55 (d, 1H, J = 9.0 Hz, 3′ or 5′-H), 7.47 (d, 1H, J = 9.0 Hz, 3′ or 5′-H), 7.36-7.31 (m, 1H, 2′ or 6′-H), 5.32 (s, 2H, CH2). IR (KBr) cm−1: 1499 (C=N). MS (APCI) m/z 295 (M+1). Anal. Calc. for C16H11FN4O: C, 65.30; H, 3.77; N, 19.04. Found: C, 65.22; H, 3.91; N, 19.17.
6-(2-Chlorobenzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (5)
Yield: 91%, m.p. 166–167°C. 1H-NMR (CDCl3): δ 8.89 (s, 1H, 3-H), 8.72 (d, 1H, J = 9.0 Hz, 10-H), 8.24 (d, 1H, J = 9.0 Hz, 7-H), 7.95 (t, 1H, J = 9.0 Hz, 9-H), 7.80 (t, 1H, J = 9.0 Hz, 8-H), 7.62 (t, 1H, J = 9.0 Hz, 5′-H), 7.50-7.47 (m, 1H, 4′-H), 7.39-7.33 (m, 2H, 3′ and 6′-H), 5.66 (s, 2H, CH2). IR (KBr) cm−1: 1532 (C=N). MS (APCI) m/z 311 (M+1). Anal. Calc. for C16H11ClN4O: C, 61.84; H, 3.57; N, 18.03. Found: C, 61.81; H, 3.65; N, 18.11.
6-(3-Chlorobenzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (6)
Yield: 90%, m.p. 176–178°C. 1H-NMR (CDCl3): δ 8.85 (s, 1H, 3-H), 8.58 (d, 1H, J = 9.0 Hz, 10-H), 8.18 (d, 1H, J = 9.0 Hz, 7-H), 7.89 (t, 1H, J = 9.0 Hz, 9-H), 7.77 (t, 1H, J = 9.0 Hz, 8-H), 7.53 (s, 1H, 2′-H), 7.44-7.29 (m, 2H, 4′,5′ and 6′-H), 5.49 (s, 2H, CH2). IR (KBr) cm−1: 1511 (C=N). MS (APCI) m/z 311 (M+1). Anal. Calc. for C16H11ClN4O: C, 61.84; H, 3.57; N, 18.03. Found: C, 61.90; H, 3.67; N, 18.04.
6-(4-Chlorobenzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (7)
Yield: 89%, m.p. 210–212°C. 1H-NMR (CDCl3): δ 8.88 (s, 1H, 3-H), 8.78 (d, 1H, J = 9.0 Hz, 10-H), 8.22 (d, 1H, J = 9.0 Hz, 7-H), 7.98 (t, 1H, J = 9.0 Hz, 9-H), 7.82 (t, 1H, J = 9.0 Hz, 8-H), 7.49 (d, 2H, J = 9.0 Hz, 2′ and 6′-H), 7.42 (d, 2H, J = 9.0 Hz, 3′ or 5′-H), 5.52 (s, 2H, CH2). IR (KBr) cm−1: 1524 (C=N). MS (APCI) m/z 311 (M+1). Anal. Calc. for C16H11ClN4O: C, 61.84; H, 3.57; N, 18.03. Found: C, 61.77; H, 3.49; N, 18.07.
6-(2-Bromobenzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (8)
Yield: 78%, m.p. 177–179°C. 1H-NMR (CDCl3): δ 8.90 (s, 1H, 3-H), 8.55 (d, 1H, J = 9.0 Hz, 10-H), 8.17 (d, 1H, J = 9.0 Hz, 7-H), 7.88 (t, 1H, J = 9.0 Hz, 9-H), 7.74 (t, 1H, J = 9.0 Hz, 8-H), 7.64-7.59 (m, 1H, 3′ and 6′-H), 7.38 (d, 1H, J = 9.0 Hz, 4′-H), 7.28-7.23 (m, 1H, 5′-H), 5.56 (s, 2H, CH2). IR (KBr) cm−1: 1509 (C=N). MS (APCI) m/z 355 (M+1). Anal. Calc. for C16H11BrN4O: C, 54.10; H, 3.12; N, 15.77. Found: C, 54.01; H, 3.23; N, 15.81.
6-(3-Bromobenzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (9)
Yield: 84%, m.p. 186–187°C. 1H-NMR (CDCl3): δ 8.89 (s, 1H, 3-H), 8.78 (d, 1H, J = 9.0 Hz, 10-H), 8.22 (d, 1H, J = 9.0 Hz, 7-H), 7.98 (t, 1H, J = 9.0 Hz, 9-H), 7.82 (t, 1H, J = 9.0 Hz, 8-H), 7.56-7.51 (m, 2H, 2′ and 4′-H), 7.18-7.12 (m, 2H, 5′ and 6′-H), 5.53 (s, 2H, CH2). IR (KBr) cm−1: 1525 (C=N). MS (APCI) m/z 355 (M+1). Anal. Calc. for C16H11BrN4O: C, 54.10; H, 3.12; N, 15.77. Found: C, 54.04; H, 3.21; N, 15.79.
6-(2-Methoxybenzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (10)
Yield: 78%, m.p. 187–189°C. 1H-NMR (CDCl3): δ 8.87 (s, 1H, 3-H), 8.59 (d, 1H, J = 9.0 Hz, 10-H), 8.19 (d, 1H, J = 9.0 Hz, 7-H), 7.87 (t, 1H, J = 9.0 Hz, 9-H), 7.76 (t, 1H, J = 9.0 Hz, 8-H), 7.53 (d, 1H, J = 9.0 Hz, 6′-H), 7.37 (t, 1H, J = 9.0 Hz, 5′-H), 7.05 (t, 1H, J = 9.0 Hz, 4′-H), 6.97 (d, 1H, J = 9.0 Hz, 3′-H), 5.56 (s, 2H, CH2), 3.88 (s, 3H, -OCH3). IR (KBr) cm−1: 1512 (C=N). MS (APCI) m/z 307 (M+1). Anal. Calc. for C17H14N4O2: C, 66.66; H, 4.61; N, 18.29. Found: C, 66.71; H, 4.69; N, 18.21.
6-(3-Methoxybenzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (11)
Yield: 80%, m.p. 156–158°C. 1H-NMR (CDCl3): δ 8.86 (s, 1H, 3-H), 8.63 (d, 1H, J = 9.0 Hz, 10-H), 8.22 (d, 1H, J = 9.0 Hz, 7-H), 7.96 (t, 1H, J = 9.0 Hz, 9-H), 7.80 (t, 1H, J = 9.0 Hz, 8-H), 7.37 (t, 1H, J = 9.0 Hz, 5′-H), 7.13-7.7.08 (m, 2H, 2′ and 6′-H), 6.95 (d, 1H, 4′-H), 5.56 (s, 2H, CH2), 3.90 (s, 3H, -OCH3). IR (KBr) cm−1: 1541 (C=N). MS (APCI) m/z 307 (M+1). Anal. Calc. for C17H14N4O2: C, 66.66; H, 4.61; N, 18.29. Found: C, 66.72; H, 4.65; N, 18.22.
6-(4-Methoxybenzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (12)
Yield: 88%, m.p. 198–200°C. 1H-NMR (CDCl3): δ 8.86 (d, 1H, J = 9.0 Hz, 10-H), 8.58 (s, 1H, 3-H), 8.17 (d, 1H, J = 9.0 Hz, 7-H), 7.90 (t, 1H, J = 9.0 Hz, 9-H), 7.74 (t, 1H, J = 9.0 Hz, 8-H), 7.47 (d, 2H, J = 9.0 Hz, 2′ and 6′-H), 6.96 (d, 2H, J = 9.0 Hz, 3′ and 5′-H), 5.46 (s, 2H, CH2), 3.85 (s, 3H, -OCH3). IR (KBr) cm−1: 1519 (C=N). MS (APCI) m/z 307 (M+1). Anal. Calc. for C17H14N4O2: C, 66.66; H, 4.61; N, 18.29. Found: C, 66.71; H, 4.59; N, 18.19.
6-(2-(Trifluoromethyl)benzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (13)
Yield: 89%, m.p. 188–190°C. 1H-NMR (CDCl3): δ 8.88 (s, 1H, 3-H), 8.71 (d, 1H, J = 9.0 Hz, 10-H), 8.24 (d, 1H, J = 9.0 Hz, 7-H), 7.94 (t, 1H, J = 9.0 Hz, 9-H), 7.81 (t, 1H, J = 9.0 Hz, 8-H), 7.62 (t, 1H, J = 9.0 Hz, 5′-H), 7.51-7.49 (m, 1H, 4′-H), 7.40-7.33 (m, 2H, 3′ and 6′-H), 5.64 (s, 2H, CH2). IR (KBr) cm−1: 1518 (C=N). MS (APCI) m/z 345 (M+1). Anal. Calc. for C17H11F3N4O: C, 59.31; H, 3.22; N, 16.27. Found: C, 59.29; H, 3.19; N, 16.32.
6-(3-(Trifluoromethyl)benzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (14)
Yield: 87%, m.p. 164–166°C. 1H-NMR (CDCl3): δ 8.86 (d, 1H, J = 9.0 Hz, 10-H), 8.62 (s, 1H, 3-H), 8.19 (d, 1H, J = 9.0 Hz, 7-H), 7.95 (t, 1H, J = 9.0 Hz, 9-H), 7.81 (s, 1H, 2′-H), 7.79 (t, 1H, J = 9.0 Hz, 8-H), 7.76 (d, 1H, J = 9.0 Hz, 4′-H), 7.68 (d, 1H, J = 9.0 Hz, 6′-H), 7.58 (d, 1H, J = 9.0 Hz, 5′-H), 5.59 (s, 2H, CH2). IR (KBr) cm−1: 1517 (C=N). MS (APCI) m/z 345 (M+1). Anal. Calc. for C17H11F3N4O: C, 59.31; H, 3.22; N, 16.27. Found: C, 59.35; H, 3.23; N, 16.38.
6-(4-(Trifluoromethyl)benzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (15)
Yield: 87%, m.p. 210–212°C. 1H-NMR (CDCl3): δ 8.89 (s, 1H, 3-H), 8.79 (d, 1H, J = 9.0 Hz, 10-H), 8.23 (d, 1H, J = 9.0 Hz, 7-H), 7.99 (t, 1H, J = 9.0 Hz, 9-H), 7.83 (t, 1H, J = 9.0 Hz, 8-H), 7.50 (d, 2H, J = 9.0 Hz, 2′ and 6′-H), 7.45 (d, 2H, J = 9.0 Hz, 3′ or 5′-H), 5.54 (s, 2H, CH2). IR (KBr) cm−1: 1503 (C=N). MS (APCI) m/z 345 (M+1). Anal. Calc. for C17H11F3N4O: C, 59.31; H, 3.22; N, 16.27. Found: C, 59.33; H, 3.29; N, 16.34.
6-(4-Methylbenzyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (16)
Yield: 88%, m.p. 196–198°C. 1H-NMR (CDCl3): δ 8.87 (s, 1H, 3-H), 8.72 (d, 1H, J = 9.0 Hz, 10-H), 8.21 (d, 1H, J = 9.0 Hz, 7-H), 7.94 (t, 1H, J = 9.0 Hz, 9-H), 7.78 (t, 1H, J = 9.0 Hz, 8-H), 7.43 (d, 2H, J = 9.0 Hz, 2′ and 6′-H), 7.25 (d, 2H, J = 9.0 Hz, 3′ or 5′-H), 5.50 (s, 2H, CH2), 2.45 (s, 3H, CH3). IR (KBr) cm−1: 1507 (C=N). MS (APCI) m/z 291 (M+1). Anal. Calc. for C17H14N4O: C, 70.33; H, 4.86; N, 19.30. Found: C, 70.31; H, 4.96; N, 19.32.
6-(Cyclohexyloxy)-[1,2,4]triazolo[3,4-a]phthalazine (17)
Yield: 67%, m.p. 139–142°C. 1H-NMR (CDCl3): δ 8.95 (s, 1H, 3-H), 8.43 (d, 1H, J = 9.0 Hz, 10-H), 8.18 (d, 1H, J = 9.0 Hz, 7-H), 7.90 (t, 1H, J = 9.0 Hz, 9-H), 7.73 (t, 1H, J = 9.0 Hz, 8-H), 5.24-5.16 (m, 1H, CH), 2.36-2.09 (m, 2H, CH2), 1.88-1.25 (m, 8H, (CH2)4). IR (KBr) cm−1: 1509 (C=N). MS (APCI) m/z 269 (M+1). Anal. Calc. for C15H16N4O: C, 67.15; H, 6.01; N, 20.88. Found: C, 67.21; H, 6.19; N, 20.77.
6-(1-Phenylethoxy)-[1,2,4]triazolo[3,4-a]phthalazine (18)
Yield: 71%, m.p. 68–70°C. 1H-NMR (CDCl3): δ 8.56 (s, 1H, 3-H), 8.19 (d, 1H, J = 8.4 Hz, 10-H), 7.91 (d, 1H, J = 8.4 Hz, 7-H), 7.50-7.44 (m, 2H, 8 and 9-H), 7.42 (d, 2H, J = 7.8 Hz, 2′ and 6′-H), 7.13 (t, 2H, J = 7.8 Hz, 3′ and 5′-H), 7.08 (d, J = 7.8 Hz, 4′-H), 6.01 (q, 1H, J = 6.6 Hz, -OCH), 1.62 (d, 3H, J = 6.6 Hz, -CH3). IR (KBr) cm−1: 1513 (C=N). MS (APCI) m/z 269 (M+1). Anal. Calc. for C17H14N4O: C, 70.33; H, 4.86; N, 19.30. Found: C, 70.37; H, 4.91; N, 19.29.
6-(1-(4-Fluorophenyl)ethoxy)-[1,2,4]triazolo[3,4-a]phthalazine (19)
Yield: 71%, m.p. 68–70°C. 1H-NMR (CDCl3): δ 8.78 (s, 1H, 3-H), 8.52 (d, 1H, J = 8.4 Hz, 10-H), 8.22 (d, 1H, J = 8.4 Hz, 7-H), 7.87 (t, 1H, J = 8.6 Hz, 9-H), 7.77 (t, 1H, J = 8.6 Hz, 8-H), 7.53-7.49 (m, 2H, 3′ and 5′-H), 7.07-7.09 (t, 2H, J = 7.8 Hz, 2′ and 6′-H), 6.24 (q, 1H, J = 6.5 Hz, -OCH), 1.62 (d, 3H, J = 6.5 Hz, -CH3). IR (KBr) cm−1: 1509 (C=N). MS (APCI) m/z 309 (M+1). Anal. Calc. for C17H13FN4O: C, 66.23; H, 4.25; N, 18.17. Found: C, 66.12; H, 4.34; N, 18.21.
6-(1-(4-Cholorophenyl)ethoxy)-[1,2,4]triazolo[3,4-a]phthalazine (20)
Yield: 78%, m.p. 130–132°C. 1H-NMR (CDCl3): δ 8.77 (s, 1H, 3-H), 8.52 (d, 1H, J = 8.4 Hz, 10-H), 8.21 (d, 1H, J = 8.4 Hz, 7-H), 7.85 (t, 1H, J = 8.6 Hz, 9-H), 7.78 (t, 1H, J = 8.6 Hz, 8-H), 7.45 (d, 2H, J = 7.8 Hz, 3′ and 5′-H), 7.34 (d, 2H, J = 7.8 Hz, 2′ and 6′-H), 6.24 (q, 1H, J = 6.6 Hz, -OCH), 1.62 (d, 3H, J = 6.6 Hz, -CH3). IR (KBr) cm−1: 1521 (C=N). MS (APCI) m/z 325 (M+1). Anal. Calc. for C17H13ClN4O: C, 62.87; H, 4.03; N, 17.25. Found: C, 62.79; H, 4.12; N, 17.33.
6-(1-(4-Methoxyphenyl)ethoxy)-[1,2,4]triazolo[3,4-a]phthalazine (21)
Yield: 86%, m.p. 84–86°C. 1H-NMR (CDCl3): δ 8.78 (s, 1H, 3-H), 8.54 (d, 1H, J = 8.6 Hz, 10-H), 8.24 (d, 1H, J = 8.4 Hz, 7-H), 7.90 (t, 1H, J = 8.6 Hz, 9-H), 7.75 (t, 1H, J = 8.4 Hz, 8-H), 7.45 (d, 2H, J = 7.8 Hz, 3′ and 5′-H), 6.90 (d, 2H, J = 7.8 Hz, 2′ and 6′-H), 6.22 (q, 1H, J = 6.6 Hz, -OCH), 3.80 (s, 3H, -OCH3), 1.79 (d, 3H, J = 6.6 Hz, -CH3). IR (KBr) cm−1: 1512 (C=N). MS (APCI) m/z 321 (M+1). Anal. Calc. for C18H16N4O2: C, 67.49; H, 5.03; N, 17.49. Found: C, 67.54; H, 5.23; N, 17.33
6-(1-4-Tolylethoxy)-[1,2,4]triazolo[3,4-a]phthalazine (22)
Yield: 81%, m.p. 68–70°C. 1H-NMR (CDCl3): δ 8.76 (s, 1H, 3-H), 8.52 (d, 1H, J = 8.6 Hz, 10-H), 8.23 (d, 1H, J = 8.4 Hz, 7-H), 7.83 (t, 1H, J = 8.6 Hz, 9-H), 7.76 (t, 1H, J = 8.4 Hz, 8-H), 7.40 (d, 2H, J = 7.8 Hz, 3′ and 5′-H), 7.18 (d, 2H, J = 7.8 Hz, 2′ and 6′-H), 6.22 (q, 1H, J = 6.6 Hz, -OCH), 2.33 (s, 3H, -CH3), 1.77 (d, 3H, J = 6.6 Hz, -CH3). IR (KBr) cm−1: 1509 (C=N). MS (APCI) m/z 305 (M+1). Anal. Calc. for C18H16N4O: C, 71.04; H, 5.30; N, 18.41. Found: C, 71.11; H, 5.26; N, 18.45.
6-(Benzhydryloxy)-[1,2,4]triazolo[3,4-a]phthalazine (23)
Yield: 84%, m.p. 188–190°C. 1H-NMR (CDCl3): δ 8.76 (s, 1H, 3-H), 8.58 (d, 1H, J = 7.2 Hz, 10-H), 8.36 (d, 1H, J = 7.2 Hz, 7-H), 7.90 (t, 1H, J = 7.2 Hz, 9-H), 7.80 (t, 1H, J = 7.2 Hz, 8-H), 7.51 (d, 4H, J = 8.4 Hz, (2′ and 6′-H)2), 7.41-7.30 (m, 6H, (3′, 4′ and 5′-H)2), 7.24 (s, 1H, -OCH). IR (KBr) cm−1: 1516 (C=N). MS (APCI) m/z 353 (M+1). Anal. Calc. for C22H16N4O: C, 74.98; H, 4.58; N, 15.90. Found: C, 75.01; H, 4.62; N, 15.78.
6-(Bis(4-fluorophenyl)methoxy)-[1,2,4]triazolo[3,4-a]phthalazine (24)
Yield: 82%, m.p. 180–182°C. 1H-NMR (CDCl3): δ 8.77 (s, 1H, 3-H), 8.60 (d, 1H, J = 8.0, 10-H), 8.32 (d, 1H, J = 7.8 Hz, 7-H), 7.94 (t, 1H, J = 8.0 Hz, 9-H), 7.82 (t, 1H, J = 7.8 Hz, 8-H), 7.48-7.44 (m, 4H, (3′ and 5′-H)2),7.22 (s, 1H, -OCH), 7.06 (t, 4H, J = 8.2 Hz, (2′ and 6′-H)2). IR (KBr) cm−1: 1524 (C=N). MS (APCI) m/z 389 (M+1). Anal. Calc. for C22H14N4O: C, 68.04; H, 3.63; N, 14.43. Found: C, 68.23; H, 3.51; N, 14.39.
6-((4-Chlorophenyl)(phenyl)methoxy)-[1,2,4]triazolo[3,4-a]phthalazine (25)
Yield: 69%, m.p. 182–185°C. 1H-NMR (CDCl3): δ 8.76 (s, 1H, 3-H), 8.54 (d, 1H, J = 7.8 Hz, 10-H), 8.32 (d, 1H, J = 7.6 Hz, 7-H), 7.87 (t, 1H, J = 7.8 Hz, 9-H), 7.80 (t, 1H, J = 7.6 Hz, 8-H), 7.52-7.33 (m, 9H, Ar-H), 7.20 (s, 1H, -OCH). IR (KBr) cm−1: 1532 (C=N). MS (APCI) m/z 387 (M+1). Anal. Calc. for C22H15ClN4O: C, 68.31; H, 3.91; N, 14.48. Found: C, 68.23; H, 3.78; N, 14.61.
Synthesis of 6-chlorotetrazolo[5,1-a]phthalazine (39)
Compound 37 (3 g, 15.4 mmol) was dissolved in 50 ml of 30% H2SO4, then a solution of NaNO2 (1.06 g, 15.4 mmol) in 15 ml H2O was added dropwise to the mixture under ice-bath, be sure that the reaction temperature was below 5°C. Then the mixture was stirred at room temperature for 2 h (TLC monitoring). The mixture was extracted twice with dichloromethane (60 ml). The dichloromethane layer was washed three times with saturated aqueous NaCl (60 × 3) and dried over anhydrous MgSO4. After removing the solvents, 2.15 g light yellow product was gained, yield 68%.
The general procedure for the synthesis of compounds 26–34
Compound 38 (0.65 g, 3.2 mmol), K2CO3 (0.44 g, 3.2 mmol), benzyltriethylammonium chloride (TEBA) (0.23g, 1 mmol) and corresponding alcohol (0.70 mmol) were suspended in acetonitrile (50 mL), and reacted with stirring and refluxing for about 3 h. After the acetonitrile was removed under reduced pressure, the solid residue was purified by silica gel chromatography with ethyl acetate: petroleum ether (2:3) to obtain compounds 26–34. The yield, melting point and spectral data of each compound were given below.
6-(Benzyloxy)tetrazolo[5,1-a]phthalazine (26)
Yield: 89%, m.p. 165–167°C; 1H-NMR (CDCl3, 300 MHz) δ: 8.61 (d, 1H, J = 8.5 Hz, 9-H), 8.33 (d, 1H, J = 8.2 Hz, 6-H), 8.01 (t, 1H, J = 4.2 Hz, 8-H), 7.94 (t, 1H, J = 4.0 Hz, 7-H), 7.58 (d, 2H, J = 7.9 Hz, 3′ and 5′-H), 7.28-7.48 (m, 3H, 2′, 4′ and 6′-H), 5.68 (s, 2H, CH2). IR (KBr) cm−1: 1509 (C=N). MS (APCI) m/z: 278 (M+1); Anal. Calcd for C15H11N5O: C, 64.97; H, 4.00; N, 25.26. Found: C, 64.78; H, 4.13; N, 25.12.
6-(4-Fluorobenzyloxy)tetrazolo[5,1-a]phthalazine (27)
Yield: 86%, m.p. 164–166°C; 1H-NMR (CDCl3, 300 MHz) δ: 8.64 (d, 1H, J = 8.0 Hz, 9-H), 8.33 (d, 1H, J = 8.0 Hz, 6-H), 8.05 (t, 1H, J = 4.2 Hz, 8-H), 7.96 (t, 1H, J = 4.0 Hz, 7-H), 7.57 (d, 2H, J = 7.9 Hz, 3′ and 5′-H), 7.13 (d, 2H, J = 7.9 Hz, 2′ and 6′-H), 5.66 (s, 2H, CH2). IR (KBr) cm−1: 1523 (C=N). MS (APCI) m/z: 296 (M+1); Anal. Calcd for C15H10FN5O: C, 61.02; H, 3.41; N, 23.72. Found: C, 60.87; H, 3.53; N, 23.68.
6-(2-Chlorobenzyloxy)tetrazolo[5,1-a]phthalazine (28)
Yield: 89%, m.p. 162–164°C; 1H-NMR (CDCl3, 300 MHz) δ: 8.61 (d, 1H, J = 8.0 Hz, 9-H), 8.34 (d, 1H, J = 8.0 Hz, 6-H), 8.03 (t, 1H, J = 4.2 Hz, 8-H), 7.97 (t, 1H, J = 7.6 Hz, 7-H), 7.69-7.34 (m, 4H, 3′, 4′, 5′, and 6′-H), 5.77 (s, 2H, CH2). IR (KBr) cm−1: 1527 (C=N). MS (APCI) m/z: 312 (M+1); Anal. Calcd for C15H10ClN5O: C, 57.79; H, 3.23; N, 22.47. Found: C, 57.81; H, 3.39; N, 22.23.
6-(4-Chlorobenzyloxy)tetrazolo[5,1-a]phthalazine (29)
Yield: 89%, m.p. 190–192°C; 1H-NMR (CDCl3, 300 MHz) δ: 8.65 (d, 1H, J = 8.0 Hz, 9-H), 8.33 (d, 1H, J = 8.0 Hz, 6-H), 8.06 (t, 1H, J = 4.2 Hz, 8-H), 7.94 (t, 1H, J = 7.6 Hz, 7-H), 7.57 (d, 2H, J = 7.9 Hz, 3′ and 5′-H), 7.42 (d, 2H, J = 7.9 Hz, 2′ and 6′-H), 5.66 (s, 2H, CH2). IR (KBr) cm−1: 1517 (C=N). MS (APCI) m/z: 312 (M+1); Anal. Calcd for C15H10ClN5O: C, 57.79; H, 3.23; N, 22.47. Found: C, 57.58; H, 3.42; N, 22.39.
6-(3-Bromobenzyloxy)tetrazolo[5,1-a]phthalazine (30)
Yield: 81%, m.p. 180–183°C; 1H-NMR (CDCl3, 300 MHz) δ: 8.67 (d, 1H, J = 8.20 Hz, 9-H), 8.35 (d, 1H, J = 8.20 Hz, 6-H), 8.07 (t, 1H, J = 4.2 Hz, 8-H), 7.97 (t, 1H, J = 7.6 Hz, 7-H), 7.73-7.33 (m, 4H, Ar-H), 5.66 (s, 2H, CH2). IR (KBr) cm−1: 1519. MS (APCI) m/z: 356 (M+1); Anal. Calcd for C15H10BrN5O: C, 50.58; H, 2.83; N, 19.66. Found: C, 50.62; H, 2.91; N, 19.57.
6-(3-(Trifluoromethyl)benzyloxy)-tetrazolo[5,1-a]phthalazine (31)
Yield: 80%, m.p. 155–157°C; 1H-NMR (CDCl3, 300 MHz) δ: 8.87 (d, 1H, J = 9.00 Hz, 10-H), 8.35 (d, 1H, J = 9.00 Hz, 7-H), 8.05 (t, 1H, J = 9.0 Hz, 9-H), 7.98 (s, 1H, 2′-H), 7.79 (t, 1H, J = 9.0 Hz, 8-H), 7.76 (d, 1H, J = 9.0 Hz, 4′-H), 7.68 (d, 1H, J = 9.0 Hz, 6′-H), 7.58 (d, 1H, J = 9.0 Hz, 5′-H), 5.59 (s, 2H, CH2). IR (KBr) cm−1: 1527. MS (APCI) m/z: 346 (M+1); Anal. Calcd for C16H10F3N5O: C, 55.66; H, 2.92; N, 20.28. Found: C, 55.87; H, 2.91; N, 20.34.
6-(4-(Methyl)benzyloxy)-tetrazolo[5,1-a]phthalazine (32)
Yield: 85%, m.p. 153–155°C; 1H-NMR (CDCl3, 300 MHz) δ: 8.61 (d, 1H, J = 7.90 Hz, 9-H), 8.31 (d, 1H, J = 8.20 Hz, 6-H), 8.02 (t, 1H, J = 8.2 Hz, 8-H), 7.90 (t, 1H, J = 8.2 Hz, 7-H), 7.46 (d, 2H, J = 8.0 Hz, 3′ and 5′-H), 7.25 (d, 1H, J = 8.0 Hz, 2′ and 6′-H), 5.63 (s, 2H, CH2), 2.39 (s, 3H, CH3). IR (KBr) cm−1: 1523. MS (APCI) m/z: 292 (M+1); Anal. Calcd for C16H13N5O: C, 65.97; H, 4.50; N, 24.04. Found: C, 65.78; H, 4.65; N, 24.09.
6-(1-(4-Fluorophenyl)ethoxy)tetrazolo[5,1-a]phthalazine (33)
Yield: 82%, m.p. 113–115°C; 1H-NMR (CDCl3, 300 MHz) δ: 8.55 (d, 1H, J = 7.90 Hz, 9-H), 8.37 (d, 1H, J = 7.90 Hz, 6-H), 8.01-7.95 (m, 2H, Ar-H), 7.61-7.56 (m, 2H, Ar-H), 7.10-7.04 (m, 2H, Ar-H), 6.45 (q, 2H, J = 6.5 Hz CH2), 1.89 (d, 3H, J = 6.5 Hz, CH3). IR (KBr) cm−1: 1509. MS (APCI) m/z: 310 (M+1); Anal. Calcd for C16H12FN5O: C, 62.13; H, 3.91; N, 22.64. Found: C, 62.21; H, 4.02; N, 22.56.
6-(Bis(4-fluorophenyl)methoxy)tetrazolo[5,1-a]phthalazine (34)
Yield: 89%, m.p. 204–206°C; 1H-NMR (CDCl3, 300 MHz) δ: 8.65 (d, 1H, J = 7.90 Hz, 9-H), 8.47 (d, 1H, J = 7.90 Hz, 6-H), 8.07 (t, 1H, J = 7.20 Hz, 8-H), 7.99 (t, 1H, J = 7.20 Hz, 7-H), 7.54-7.49 (m, 4H, Ar-H), 7.44 (s, 1H, CH), 7.12-7.06 (m, 4H, Ar-H). IR (KBr) cm−1: 1544. MS (APCI) m/z: 390 (M+1); Anal. Calcd for C21H13F2N5O: C, 64.78; H, 3.37; N, 17.99. Found: C, 64.86; H, 3.45; N, 18.12.
Biological assays
Anticonvulsant effects in the maximal electroshock seizure (MES) test
The MES test and rotarod test were carried out according to procedures described in the Antiepileptic Drug Development Program (ADD) of the National Institutes of Health (USA) (15, 16). Seizures were elicited with a 60 Hz alternating current of 50 mA intensity in KunMing mice. The current was applied via corneal electrodes for 0.2 s. Protection against the spread of MES-induced seizures was defined as the abolition of the tonic maximal extension of the hind leg. At 0.5 h after the administration of the compounds, the activities were evaluated in MES test. In phase-I screening, each compound was administered at the dose levels of 30 and 100 mg/kg for evaluating the preliminary anticonvulsant activity. For determination of the median effective dose (ED50) and the median toxic dose (TD50), the phase-II screening was prepared. Groups of 10 mice were given a range of intraperitoneal doses of the tested compound until at least three points were established in the range of 10–90% seizure protection or minimal observed neurotoxicity. From the plot of this data, the respective ED50 and TD50 values, 95% confidence intervals, slope of the regression line, and the standard error of the slope were calculated by means of a computer program written at National Institute of Neurological Disorders and StrokeCitation13,Citation14.
Neurotoxicity (NT) screening
The neurotoxicity of the compounds was measured in Kunming mice by the rotarod test for three times. The mice were trained to stay on an accelerating rotarod of diameter 3.2 cm that rotates at 10 rpm. Trained animals were given i.p. injection of the test compounds. Neurotoxicity was indicated by the inability of the animal to maintain equilibrium on the rod for at least 1 min in each of the trialsCitation15,Citation16.
Sc-PTZ-induced seizures
At 0.5 h after the administration of the test compound, 85 mg/kg PTZ dissolved in saline was administered sc. The animals (10 KunMing mice in each group) were placed in individual cages and observed for 0.5 h. The numbers of clonic seizure (range from exaggerated twitches of the limbs to violent shaking or vibrating of the stiffened extremities) and tonic seizure (the extremities pull towards the body or rigidly push away from it, usually maximal extension of the hind leg) as well as the number of death were notedCitation15,Citation16.
Isoniazid-induced seizures test
At 0.5 h after the administration of the test compound, the animals (10 KunMing mice in each group) were given ISO by ip at a dose of 250 mg/kg at which 100% of the animals showed convulsive reactions. The mice were placed in individual cages and observed for 1 h. The numbers of clonic and tonic seizures as well as the number of death were notedCitation17.
3-MP induced seizures test
At 0.5 h after the administration of the test compound, 60 mg/kg of 3-MP in saline solution was injected by sc to mice (10 KunMing mice in each group). The mice were placed in individual cages and observed for 0.5 h. The numbers of clonic and tonic seizures as well as the number of death were notedCitation18.
Thiosemicarbazide-induced seizures test
At 0.5 h after the administration of the test compound, the animals (10 KunMing mice in each group) were given an i.p. dose of TSC (50 mg/kg). The mice were placed in individual cages and observed for 2.5 h. The number of clonic seizures, tonic seizures and the lethality were recordedCitation19.
Results and discussion
Chemistry
All the target compounds 1–34 were synthesized according to . Compound 36, a key substrate for the whole reaction, was prepared by an established procedureCitation20. The starting material 2,3-dihydrophthalazine-1,4-dione (35) reacted with the refluxing phosphorus oxychloride to yield compound 36. Compound 36 reacted further with hydrazine hydrate in tetrahydrofuran to afford 1-hydrazine-4-chrorophthalazin (37). Cyclization of compound 37 with triethyl orthoformate yielded compound 38. The target compounds 1–25 were gained from compound 38 by reacting with corresponding alcohol in acetonitrile in the presence of K2CO3. And via the diazotization of compound 37 with NaNO2 at 5°C in 30% H2SO4, 6-chloro-tetrazolo[5,1-a] phthalazine (39) was obtainedCitation21. The target compounds 26–34 were gained from compound 39 by reacting with corresponding alcohol in acetonitrile in the presence of K2CO3.
Biological evaluation
Phase I evaluation of anticonvulsant activity
As we know, there are two models in vivo, the maximal electroshock seizure (MES), the subcutaneous Pentylenetetrazole (sc-PTZ) models represent the two animal seizure models most widely used in the search for new AEDsCitation22,Citation23. In the present study, we used the MES seizure model for screening the anticonvulsant activity of target compounds. Compounds 1–25 were prepared to evaluate the influence of the triazole ring and 26–34 were prepared to evaluate the influence of the tetrazole ring on anticonvulsant activity. Their preliminary anticonvulsant activities were obtained and listed in . After i.p. injection in mice using doses of 30 and 100 mg/kg, the following observations can be made. At the dose of 100 mg/kg, compounds 4, 6, 8, 9, 13, 14, 15, 16, 18, 20–22, 24, 25 and 34 showed complete protection. Compounds 4, 13, 14, 15, 19 and 24 exhibited complete protection at 30 mg/kg.
Table 1. Phase I evaluation of anticonvulsant activity in mice (i.p.).
Phase II evaluation of anticonvulsant activity
On the basis of the considerable anticonvulsant activity suggested in phase I testing, compounds 4, 13, 14, 15, 19 and 24 were subjected to phase II trials for quantification of their anticonvulsant activity (indicated by ED50) and neurotoxicity (indicated by TD50) in mice. Results of the quantitative test for selected compounds, along with the data on the current antiepileptic drug, were shown in . Among the tested compounds, 6-(3-(Trifluoromethyl)benzyloxy)-[1,2,4]triazolo[3,4-a] phthalazine (14) was the most active and promising compound in this work. It possessed strong anti-MES activity with ED50 of 9.3 mg/kg, which was much better than currently used antiepileptic drug Carbamazepine. The neurotoxicity caused by it was minimal and was markedly lower than the drug compared. It showed a protective index of 91.7, which was many folds higher than the current antiepileptic drug having the PI value of 6.9. Furthermore, compounds 4, 13, 15 and 24 also showed higher protective index than Carbamazepine.
Table 2. Quantitative anticonvulsant data in mice (i.p.).
Structure activity relationships discussions
Analysing the activities of the synthesized compounds, the following structure activity relationships (SAR) were obtained. We can divide all the compounds into two groups, in the first group compounds (1–25) with triazole ring compounds: among compounds 1–16, R1 was H and R2 was substituted part, effects of different substitutions at benzyl were observed. The bioevaluation led to an understanding of the importance of the position of the substituted group at the phenyl. For example, the activity order of the F position on the phenyl ring was p-F > m-F > o-F; the activity order of the Cl position on the phenyl ring was m-Cl > o-Cl > p-Cl; the activity order of the -OCH3 position was o-OCH3 > m-OCH3 > p-OCH3; and the activity order of the –CF3 position wasm-CF3 > p-CF3 > o-CF3. Among compounds 18–22: R1 was CH3 and R2 was substituted part, effects of different substitutions at benzyl were observed. Among the five para-position compounds (18–22), the similar anticonvulsant activities were observed. Among compound 23–25: R1 was a substituted phenyl and R2 were H, p-F and p-Cl respectively, the activity order of three compounds was p-F > p-Cl > H. We also can see that the other nine compounds (26–34) exhibited lower anticonvulsant activity with tetrazole ring structure,
Chemicals induced seizures
In this study, the majority of synthesized compounds were highly potent in the MES test, and the MES test is known to be sensitive to sodium channel inhibitors (e.g. phenytoin, carbamazepine). To further investigate the effects of the anticonvulsant activity in several different models and speculate about the possible mechanism of anticonvulsant action, compound 14 was tested against convulsions induced by chemical substances, including PTZ, ISO, 3-MP, and TSC. Compound 14 was administered to mice at 30 mg/kg i.p., which was higher than its ED50 value and far below its TD50 value. The reference drug Carbamazepine was also administered at 30 mg/kg i.p..
In the sc-PTZ model, Carbamazepine inhibited the clonic seizures, tonic seizures and death at the rates of 0%, 100% and 100%, respectively. While compound 14 inhibited the clonic seizures, tonic seizures and lethality at the rates of 80%, 100% and 100% induced by sc-PTZ (), respectively, which revealed that compound 14 possessed excellent activity against sc-PTZ. Compound 14, exhibiting high anticonvulsant activity in the MES and sc-PTZ models which are most widely used in the search for new AEDs, suggested that it really possesses a good anticonvulsant profile. In the ISO model, both Carbamazepine and compound 14 inhibited the clonic seizures, tonic seizures and death induced by ISO at the rates of 50%, 100% and 100%, respectively (). PTZ and ISO have been reported to produce seizures by inhibiting γ-aminobutyric acid (GABA) neurotransmissionCitation24,Citation25. GABA is the main inhibitory neurotransmitter in the brain, and is widely implicated in epilepsy. Inhibition of GABAergic neurotransmission or activity has been shown to promote and facilitate seizuresCitation26, while enhancement of GABAergic neurotransmission is known to inhibit or attenuate seizures. The findings of the present study suggest that the newly synthesized compound 14 might inhibit or attenuate PTZ-induced seizures and ISO-induced seizures in mice by enhancing GABAergic neurotransmission.
Table 3. Effects of compound 13 on chemical-induced seizures in mice.
In the 3-MP induced seizure model, carbamazepine inhibited the clonic seizures, tonic seizures and death at the rates of 0%, 100% and 100%, respectively. In comparison, compound 14 showed the anticonvulsant effect higher than Carbamazepine in inhibiting the clonic seizures induced by 3-MP with the inhibition rate of 60% (). In the TSC-induced seizure model, the anticonvulsant effect was similar to that of the 3-MP induced seizure model. Compared with the control group, Carbamazepine showed inhibition of clonic and tonic seizures and death at the rates of 0%, 100% and 100%, respectively. Compound 14 showed inhibition of clonic seizure, tonic seizures and death at the rates of 60%, 100% and 80% induced by TSC (), respectively. 3-MP and TSC are competitive inhibitors of the GABA synthesis enzyme glutamate decarboxylase (GAD), and they inhibit the synthesis of GABA resulting in decrease of GABA levels in the brainCitation27. Compound 14 showed moderate antagonism to both 3-MP induced seizures and TSC-induced seizures.
Conclusion
In the present study we described the syntheses and anticonvulsant activity evaluation of 6-substituted-[1,2,4]triazolo[3,4-a](tetrazolo[5,1-a])phthalazine (1–34). Most of the synthesized compounds exhibited potent anticonvulsant activities in the maximal electroshock test (MES). The most promising compound 14 showed significant anticonvulsant activity in MES test with ED50 value of 9.3 mg/kg. It displayed a wide margin of safety with protective index much higher than the standard drug Carbamazepine. And the potency of compound 14 against seizures induced by Pentylenetetrazole, Isoniazid, Thiosemicarbazide and 3-Mercaptopropionic acid in the chemical-induced seizure tests, suggested that compound 14 displayed wide spectrum activity in several models.
Declaration of interest
This work was supported by the National Natural Science Foundation of China (No. 81160382).
References
- Strine TW, Kobau R, Chapman DP, Thurman DJ, Price P, Balluz LS. Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: results from the 2002 National Health Interview Survey. Epilepsia 2005;46:1133–1139.
- Mc Namara OJ, Brunton LL, Lazo JS, Parker KL, eds. (2006). The Pharmacological Basis of Therapeutics. New York: McGraw-Hill, 501–526.
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319.
- Penovich PE, Willmore LJ. Use of a new antiepileptic drug or an old one as first drug for treatment of absence epilepsy. Epilepsia 2009;50 Suppl 8:37–41.
- Al-Soud YA, Al-Masoudi NA, Ferwanah Ael-R. Synthesis and properties of new substituted 1,2,4-triazoles: potential antitumor agents. Bioorg Med Chem 2003;11:1701–1708.
- Xie ZF, Chai KY, Piao HR, Kwak KC, Quan ZS. Synthesis and anticonvulsant activity of 7-alkoxyl-4,5-dihydro-[1,2,4]triazolo[4,3-a]quinolines. Bioorg Med Chem Lett 2005;15:4803–4805.
- Guo LJ, Wei CX, Jia JH, Zhao LM, Quan ZS. Design and synthesis of 5-alkoxy-[1,2,4]triazolo[4,3-a]quinoline derivatives with anticonvulsant activity. Eur J Med Chem 2009;44:954–958.
- Zhang L, Guan LP, Sun XY, Wei CX, Chai KY, Quan ZS. Synthesis and anticonvulsant activity of 6-alkoxy-[1,2,4]triazolo[3,4-a]phthalazines. Chem Biol Drug Des 2009;73:313–319.
- Deng XQ, Wei CX, Li FN, Sun ZG, Quan ZS. Design and synthesis of 10-alkoxy-5, 6-dihydro-triazolo[4,3-d]benzo[f][1,4]oxazepine derivatives with anticonvulsant activity. Eur J Med Chem 2010;45:3080–3086.
- Zhang LQ, Guan LP, Wei CX, Deng XQ, Quan ZS. Synthesis and anticonvulsant activity of some 7-alkoxy-2H-1,4-benzothiazin-3(4H)-ones and 7-alkoxy-4H-[1,2,4]triazolo[4,3-d]benzo[b][1,4]thiazines. Chem Pharm Bull 2010;58:326–331.
- Deng XQ, Song MX, Wei CX, Li FN, Quan ZS. Synthesis and anticonvulsant activity of 7-alkoxy-triazolo-[3, 4-b]benzo[d]thiazoles. Med Chem 2010;6:313–320.
- Guan LP, Tian GR, Chai KY, Lee JS, Song MS, Quan ZS. Synthesis and anticonvulsant activity of 1-substituted-7-methoxy-1,2,4-triazolo [4, 3-a]quinoline. Turk J Chem 2008;32:181–189.
- Hester JB Jr, Von Voigtlander P. 6-Aryl-4H-s-triazolo[4,3-a][1,4]benzodiazepines. Influence of 1-substitution on pharmacological activity. J Med Chem 1979;22:1390–1398.
- Hester JB Jr, Rudzik AD, VonVoigtlander P. 2,4-Dihydro-6-phenyl-1H-s-triazolo[4,3-a][1,4]benzoidiazepin-1-ones with antianxiety and antidepressant activity. J Med Chem 1980;23:402–405.
- Krall RL, Penry JK, White BG, Kupferberg HJ, Swinyard EA. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia 1978;19:409–428.
- Porter RJ, Cereghino JJ, Gladding GD, Hessie BJ, Kupferberg HJ, Scoville B et al. Antiepileptic Drug Development Program. Cleve Clin Q 1984;51:293–305.
- Bernasconi R, Klein M, Martin P, Christen P, Hafner T, Portet C et al. Gamma-vinyl GABA: comparison of neurochemical and anticonvulsant effects in mice. J Neural Transm 1988;72:213–233.
- Arnoldi A, Bonsignori A, Melloni P, Merlini L, Quadri ML, Rossi AC et al. Synthesis and anticonvulsant and sedative-hypnotic activity of 4-(alkylimino)-2,3-dihydro-4H-1-benzopyrans and -benzothiopyrans. J Med Chem 1990;33:2865–2869.
- Lo¨scher W, Schmidt D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res 1988;2:145–181.
- Hill J, Ehrlich J. Nucleophilic Heteroaromatic Substitution. II. Phthalazines. J Org Chem 1971;36:3248–3251.
- Chimirri A, Zappala M, Gitto S, Quartarone S, Bevacqua F. Synthesis and structural features of 11H-tetrazolo[1,5-c][2,3]-benzodiazepines. Heterocycles 1999;51:1303–1209.
- White HS. Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia 2003;44 Suppl 7:2–8.
- Levy RH, Mattson RH, Meldrum BS, eds. (1995). Antiepileptic Drugs. New York: Raven Press, 99–110.
- Okada R, Negishi N, Nagaya H. The role of the nigrotegmental GABAergic pathway in the propagation of pentylenetetrazol-induced seizures. Brain Res 1989;480:383–387.
- Olsen RW. GABA-benzodiazepine-barbiturate receptor interactions. J Neurochem 1981;37:1–13.
- Gale K. GABA and epilepsy: basic concepts from preclinical research. Epilepsia 1992;33 Suppl 5:S3–12.
- LÖscher W. 3-Mercaptopropionic acid: convulsant properties, effects on enzymes of the gamma-aminobutyrate system in mouse brain and antagonism by certain anticonvulsant drugs, aminooxyacetic acid and gabaculine. Biochem Pharmacol 1979;28:1397–1407.