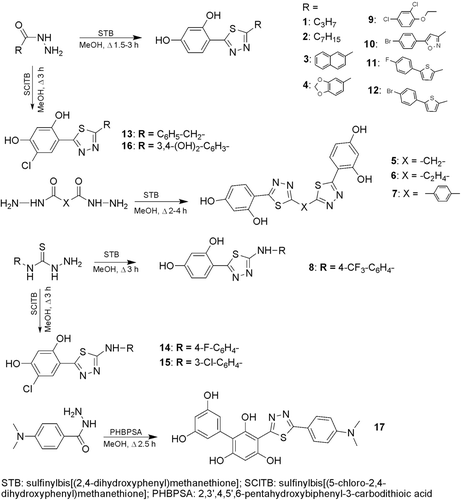
Abstract
In the present study, new (1,3,4-thiadiazol-2-yl)benzene-1,3-diol based compounds have been synthesized and their potential anticholinesterases properties have been investigated using the modified of Ellman’s spectrophotometric method. The compounds were obtained by the reaction of hydrazides or thiosemicarbazides with aryl-modified sulfinylbis[(2,4-dihydroxyphenyl)methanethione]s. Their chemical structures were elucidated by IR, 1H-NMR, 13C-NMR and EI-MS spectral data and elemental analyses. Most of the compounds acted as acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) inhibitors in vitro, with IC50 values ranging from >500 to 0.053 μM and from >500 to 0.105 μM, respectively. The most potent compound 9 (IC50 = 0.053 μM) proved to be selective toward AChE, exhibiting selectivity ratios versus BuChE of ca. 950. The kinetic studies showed that it is a mixed-type of AChE inhibitor. Another compound (2) was active against both enzymes with IC50 values in the low nM range. The structure-activity relationships (SARs) of the compounds under consideration were discussed.
Introduction
Acetylcholinesterase (AChE) inhibition is a major pharmacological approach to the treatment of Alzheimer’s disease (ADCitation1,Citation2). A common method for the treatment of cognitive and behavioral symptoms of AD is the use of structurally diverse group of cholinesterase inhibitors (ChEIs) with an amine moiety and distinct modes of action. Available ChEI drugs are tacrine (CognexCitation3), donepezil (AriceptCitation4) galanthamine (Reminyl) - reversible inhibitorsCitation5 and rivastigmine (ExelonCitation6) which acts as a pseudo-irreversible ChEI, and differs in selectivity toward AChE and butyrylcholinesterase (BuChE).
Two major evidences highlight the central role of AChE in AD: (i) inhibitors, which increase synaptic levels of available ACh by preventing its degradation and temporarily retard the loss of cognitive function, (ii) AChE induces the expression of the β-amyloid (Aβ) precursor protein in glia and activates glial cellsCitation7. The second class of compounds are the only approved drugs for the symptomatic treatment of ADCitation8. Recent research has revealed that in severe AD brains the levels of AChE are considerably reduced, whereas BuChE activity increases. BuChE may then act as a compensatory mechanism for ACh metabolism, aggravating the toxicity of AβCitation9.
To obtain more metabolically stable cholinomimetic ligands, it is possible to replace the ester group with a series of five-membered rings like oxadiazoles, thiadiazoles, triazoles and tetrazolesCitation10. Some researchers have studied thiadiazoles extensively and the compounds bearing thiadiazole moiety have been reported to exhibit significant anticholinesterase activityCitation11,Citation12.
The antioxidant and muscarinic receptor binding properties of 3-(thiadiazolyl)pyridine 1-oxides were reported by Martinez et alCitation13. as potential acetylcholinesterase inhibitors. Sarkadi et al. studied 1-benzyl-4-[2-(5-phenyl-1,3,4-thiadiazol-2-yl) aminoethyl]piperidinesCitation14. The thiadiazolidin-3,5-dione (TDZD) derivatives were reported as the first non-ATP competitive inhibitors of glycogen synthase kinase 3β (GSK-3β) which is one of the most attractive therapeutic targets for the development of selective inhibitors of Alzheimer’s disease treatmentCitation15. Misra and co-wokers described thiadiazoloquinazolone derivatives as AChEICitation12,Citation16. Recently N-(benzothiazol-2-yl)-2-[(5-amino/methyl-1,3,4-thiadiazol-2-yl)thio]acetamides have been presented as AChEI with IC50 values in the low µM rangeCitation17.
In the previous studies, we found a series of 4-(5-phenyl)-1,3,4-thiadiazol-2-yl)benzene-1,3-diols substituted in the phenyl ring as in vitro AChE and BuChE inhibitors of high potencyCitation18. Some of them were efficient against both enzymes, others were characterized by high selectivity to AChE over to BuChE. With the aim of gaining insights into the structure–activity relationships and possibly identifying new leads in the AChE/BuChE inhibitors, in the present work we synthesized some new compounds from the (1,3,4-thiadiazol-2-yl)benzene-1,3-diol group. We included derivatives with 5-heterocyclic, aryl and alkyl substituents attached directly to the 1,3,4-thiadiazole ring or by -NH- group as well as the compounds with modified resorcinol moiety. The inhibition mechanism for the chosen compounds was studied.
Materials and methods
Chemistry
The IR spectra were recorded with a Perkin-Elmer FT-IR 1725X spectrophotometer (Perkin-Elmer Ltd., Beaconsfield, Buckinghamshire, England) (in KBr) or Varian 670 FT-IR spectrometer (ATR). The spectra were made in the range of 600–4000 cm−1. 1H NMR and 13C NMR spectra were recorded in DMSO-d6 using a Varian Mercury 400 or a Bruker DRX 500 (Bruker Daltoncs, Inc. Billerica, MA) instrument. Chemical shifts (δ, ppm) were given in relation to tetramethylsilane (TMS). The spectra MS (EI, 70 eV) were recorded using the apparatus AMD-604 (Intectra GmbH, Harpstedt, Germany). Elemental analyses (C, H, N) were conducted using a Perkin-Elmer 2400 instrument (Perkin Elmer, Waltham, MA) and were found to be in good agreement (±0.4%) with the calculated values. The melting point (mp) was determined using a Büchi B-540 (Flawil, Switzerland) melting point apparatus.
The purity of the compounds was examined by a liquid chromatograph Knauer (Berlin, Germany) with a dual pump, a 20-μL simple injection valve and a UV–visible detector (330 nm). The Hypersil Gold C18 (1.9 μm, 100 × 2.1 mm) column was used as the stationary phase. The mobile phase included different contents of methanol and acetate buffer (pH 4, 20 nM) as the aqueous phase. The flow rate was 0.5 mL min−1 at room temperature. The retention time of an unretained solute (t0) was determined by the injection of a small amount of acetone dissolved in water. Log k values for 70% of methanol (v/v) in the mobile phase are presented. Log k values were calculated as log k = log (tR – to)/to, where: tR = retention time of a solute, to = retention time of an unretained solute.
Synthesis of compounds
4-(5-Propyl-1,3,4-thiadiazol-2-yl)benzene-1,3-diol (1)
A mixture of butyrohydrazide (0.025 mol) (Alfa Aesar) and STB (0.025 mol) in methanol (120 mL) was refluxed (3 h). The hot mixture was filtered, water (40 mL) was added to the filtrate and the mixture was left at room temperature (24 h). Recrystallization from MeOH/H2O (4:3) (70 mL) afforded 1.
Yield: 65%; HPLC: log k = −0.26; m.p.: 243–245°C; anal. calc. for C11H12N2O2S (236.29): C, 55.91; H, 5.12; N, 11.86; found: C, 56.02; H, 5.10; N, 11.80; 1H NMR (500 MHz, DMSO-d6, ppm) δ: 11.04 (s, 1H, HO-C(3)), 9.97 (s, 1H, HO-C(1)), 7.94 (m, 1H, H-C(5)), 6.47 (m, 1H, H-C(2)), 6.42 (m, 1H, H-C(6)), 3.04 (t, J = 7.46 Hz, 2H, CH2), 1.76 (sextet, J = 7.43 Hz, 2H, CH2), 0.97 (t, J = 7.35 Hz, 3H, CH3); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 168.2, 162.6, 160.8, 156.2, 129.0, 108.4, 108.1, 102.4, 30.7 (CH2), 22.8 (CH2), 13.4 (CH3); IR (ATR, cm−1): 3162 (OH), 2962 (H-Ar), 1626 (C=N), 1598, 1552, 1529 (C=C), 1468, 1421, 1330, 1250, 1228, 1171 (C-O), 1133, 1054 (N=C-S-C=N), 986, 967, 845, 801, 745, 690 (C-S-C), 674; EI-MS (m/z, %): 236 (58), 235 (5), 221 (18), 210 (5), 209 (10), 208 (100), 207 (5), 184 (4), 167 (6), 153 (16), 136 (14), 135 (10), 80 (4), 69 (4), 52 (4), 41 (4).
4-[5-(Naphthalen-2-yl)-1,3,4-thiadiazol-2-yl]benzene-1,3-diol (3)
A mixture of naphthalene-2-carbohydrazide (0.005 mol) (Alfa Aesar) and STB (0.0037 mol) in methanol (35 mL) was refluxed (1.5 h). The hot mixture was filtered, water (50 mL) was added to the filtrate and the mixture was left at room temperature (24 h). Recrystallization from MeOH/H2O (1:1) (25 mL) afforded 3.
Yield: 74%; HPLC: log k = 0.514; m.p.: 248–250°C; anal. calc. for C18H12N2O2S (320.37): C, 67.48; H, 3.78; N, 8.74; found: C, 67.41; H, 3.80; N, 8.70; 1H NMR (500 MHz, DMSO-d6, ppm) δ: 11.14 (s, 1H, HO-C(3)), 10.09 (s, 1H, HO-C(1)), 8.58 (s, 1H, H-Ar), 8.19 (dd, J = 8.58 Hz and J = 1.78 Hz, 1H, H-Ar), 8.14 (m, 3H, H-Ar), 8.02 (m, 1H, H-CAr), 7.64 (m, 2H, H-Ar), 6.56 (d, J = 2.3 Hz, 1H, H-C(2)), 6.50 (dd, J = 8.66 Hz and J = 2.31 Hz, 1H, H-C(5)); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 166.3, 162.9, 161.4, 156.5, 133.8, 132.9, 129.0, 128.9, 128.6, 127.8, 127.7, 127.5, 127.4, 127.1, 123.9, 108.5, 108.4, 102.2; IR (KBr, cm−1): 3397, 3151 (OH), 1631 (C=N), 1600, 1527, 1473 (C=C), 1431, 1334, 1257, 1182 (C-O), 1140, 1052 (N=C-S-C=N), 1022, 986, 968, 939, 898, 852, 806, 743, 682 (C-S-C), 640, 611; EI-MS (m/z, %): 320 (M+, 100), 186 (4), 185 (24), 171 (16), 167 (42), 160 (5), 154 (11), 153 (30), 149 (4), 140 (6), 139 (4), 135 (7), 127 (25), 126 (9), 119 (4), 107 (6), 86 (4), 77 (5), 69 (4), 63 (4), 52 (6), 51 (5), 40 (9), 39 (6).
4-[5-(1,3-Benzodioxol-5-yl)-1,3,4-thiadiazol-2-yl]benzene-1,3-diol (4)
A mixture of 1,3-benzodioxole-5-carbohydrazide (0.005 mol) (Alfa Aesar) and STB (0.0037 mol) in methanol (30 mL) was refluxed (3 h). The hot mixture was filtered, water (30 mL) was added to the filtrate and the mixture was left at room temperature (24 h). Recrystallization from MeOH/H2O (2:1) (30 mL) afforded 4.
Yield: 82%; HPLC: log k = 0.118; m.p.: 308–310°C; anal. calc. for C15H10N2O4S (314.32): C, 57.32; H, 3.21; N, 8.91; found: C, 57.40; H, 3.19; N, 8.95; 1H NMR (500 MHz, DMSO-d6, ppm) δ: 11.06 (s, 1H, HO-C(3)), 10.04 (s, 1H, HO-C(1)), 8.02 (d, J = 8.67 Hz, 1H, H-C(5)), 7.55 (d, J = 1.74 Hz, 1H, H-C(4′)), 7.50 (dd, J = 8.07 Hz and J = 1.78 Hz, 1H, H-C(6′)), 7.07 (d, J = 8.10 Hz, 1H, H-C(7′)), 6.51 (d, J = 2.32 Hz, 1H, H-C(2)), 6.47 (dd, J = 8.68 Hz and J = 2.32 Hz, 1H, H-C(6)), 4.16 (s, 2H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 165.8, 162.2, 161.2, 156.2, 149.4, 148.1, 128.8, 124.2, 122.3, 109.0, 108.4, 108.3, 106.7, 102.3, 101.8; IR (KBr, cm−1): 3484, 3394, 3047 (OH), 3047 (H-Ar), 2921 (CH), 1612 (C=N), 1509 (C=C), 1448, 1321, 1285, 1263, 1229, 1178 (C-O), 1128, 1099, 1038, 1006, 986, 971, 937, 876, 858, 805, 725, 670 (C-S-C); EI-MS (m/z, %): 314 (M+, 100), 285 (4), 179 (14), 178 (21), 167 (36), 165 (16), 153 (8), 148 (7), 147 (16), 146 (15), 135 (9), 134 (5), 121 (8), 119 (4), 108 (5), 107 (9), 106 (4), 80 (5), 79 (4), 75 (5), 69 (7), 65 (5), 63 (15), 62 (7), 52 (9), 51 (6), 39 (8), 38 (4).
4,4′-[5,5′-methylenedi-(1,3,4-thiadiazole-2-yl)]di(benzene-1,3-diol)
A mixture of propanedihydrazide (0.01 mol) (Alfa Aesar) and STB (0.015 mol) in methanol (80 mL) was refluxed (4 h). The removed compound was filtered and washed with water. Recrystallization from MeOH/H2O (1:1) (40 mL) afforded 5.
Yield: 84%; HPLC: log k = −0.446; m.p.: 277–279°C; anal. calc. for C17H12N4O4S2 (400.43): C, 50.99; H, 3.02; N, 13.99; found: C, 51.18; H, 3.00; N, 14.05; 1H NMR (500 MHz, DMSO-d6, ppm) δ: 11.02 (s, 2H, HO-C(3)), 10.09 (s, 2H, HO-C(1)), 8.02 (d, 2H, J = 8.71 Hz, H-C(5)), 6.49 (d, 2H, J = 2.32 Hz, H-C(2)), 6.45 (dd, 2H, J = 8.70 Hz and J = 2.31 Hz, H-C(6)), 5.03 (s, 2H, CH2); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 163.9 (2C), 161.2 (2C), 156.2 (2C), 128.8 (2C), 123.1 (2C), 108.3 (2C), 108.2 (2C), 102.3 (2C), 29.3 (CH2); IR (KBr, cm−1): 3257 (OH), 1630 (C=N), 1522, 1470 (C=C), 1414, 1327, 1281, 1246, 1209, 1188 (C-O), 1136, 1108 (CAr-H), 986, 969, 852 (CAr-H), 799, 749, 730, 669 (C-S-C), 627, 601; EI-MS (m/z, %): 400 (M+, 100), 371 (4), 238 (4), 234 (13), 233 (16), 232 (12), 208 (4), 207 (7), 204 (5), 167 (12), 154 (4), 153 (43), 136 (12), 135 (45), 108 (14), 107 (12), 106 (7), 97 (9), 84 (8), 80 (10), 79 (7), 69 (12), 66 (5), 65 (6), 63 (6), 52 (17), 50 (4), 45 (5), 39 (13).
4,4′-{[5,5′-(ethane-1,2-diyl)]di(1,3,4-thiadiazol-2-yl)}di(benzene-1,3-diol)
A mixture of succinic dihydrazide butanedihydrazide (0.01 mol) (Aldrich) and STB (0.015 mol) in methanol (80 mL) was refluxed (2 h). The removed compound was filtered and washed with water. Recrystallization from MeOH/H2O (1:1) (50 mL) afforded 6.
Yield: 89%; HPLC: log k = 0.802; m.p.: 316–319°C; anal. calc. for C18H14N4O4S2 (414.46): C, 52.16; H, 3.40; N, 13.52; found: C, 52.25; H, 3.42; N, 13.48; 1H NMR (500 MHz, DMSO-d6, ppm) δ: 10.97 (s, 2H, HO-C(3)), 10.01 (s, 2H, HO-C(1)), 7.95 (d, 2H, J = 8.69 Hz, H-C(5)), 6.48 (d, 2H, J = 2.31 Hz, H-C(2)), 6.42 (dd, 2H, J = 8.68 Hz and J = 2.32 Hz, H-C(6)), 3.60 (s, 4H, CH2); 13C NMR (500 MHz, DMSO-d6, ppm) δ: 166.4, 163.3, 161.0, 156.2, 128.9, 108.4, 108.2, 102.3, 28.6 (2C, CH2); IR (KBr, cm−1): 3556 (OH), 3038, 2942 (CAr-H), 1605 (C=N, C=C), 1521, 1475 (C=C), 1432, 1343, 1319, 1277, 1214, 1184 (C-O), 1122 (CAr-H), 1084, 987, 970, 867 (CAr-H), 813, 723, 708, 663 (C-S-C), 618; EI-MS (m/z, %): ESI-MS (m/z): 413.1 [M-H]-, 827.1 [2M-H]-.
4-(5-(4-(Trifluoromethyl)phenylamino)-1,3,4-thiadiazol-2-yl)benzene-1,3-diol (8)
A mixture of 4-(4-(trifluoromethyl)phenyl)-3-thiosemicarbazide (0.004 mol) (Alfa Aesar) and STB (0.003 mol) in methanol (30 mL) was refluxed (3h). The hot mixture was filtered, water (50 mL) was added to the filtrate and it was left at room temperature (24 h). Recrystallization from MeOH/H2O (2:1) (30 mL) afforded 8.
Yield: 72%; HPLC: log k = 0.292; m.p.: 234–236°C; anal. calc. for C15H10F3N3O2S (353.32): C, 50.99; H, 2.85; N, 11.89; found: C, 51.08; H, 2.86; N, 11.81; 1H NMR (500 MHz, DMSO-d6, ppm) δ: 10.89 (s, 1H, HO-C(3)), 10.68 (s, 1H, HO-C(1)), 9.23 (s, 1H, NH), 7.86 (m, 3H, H-CAR), 7.71 (m, 2H, H-CAR), 6.46 (d, J = 2.39 Hz, 1H, H-C(2)), 6.41 (dd, J = 8.62 Hz and J = 2.39 Hz, 1H, H-C(6)); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 162.9, 160.4, 155.6, 155.4, 144.2, 128.5, 126.3, 126.2, 121.3, 121.0, 116.7, 116.6, 108.4, 108.1, 102.4; IR (KBr, cm−1): 3400, 3255 (OH, NH), 3024 (CAR-H), 1619 (C=N, C=C), 1573 (C=C), 1476, 1440, 1413, 1330 (CF3), 1247, 1216, 1168 (C-O), 1061 (N=C-S-C=N), 1012, 987, 970, 937, 832, 805, 736, 679 (C-S-C); EI-MS (m/z, %): 353 (M+, 100), 352 (9), 334 (4), 219 (4), 218 (34), 198 (7), 191 (4), 153 (5), 150 (4), 145 (5), 136 (5), 94 (6), 66 (4).
4-{5-[5-(4-Bromophenyl)-1,2-oxazol-3-yl]-1,3,4-thiadiazol-2-yl}benzene-1,3-diol (10)
A mixture of 5-(4-bromophenyl)isoxazole-3-carboxylic acid hydrazide (0.01mol) (Alfa Aesar) and STB (0.01 mol) in methanol (45 mL) was refluxed (3 h). The removed solid was filtered and washed with water. Recrystallization from MeOH (40 mL) afforded 10.
Yield: 62%; HPLC: log k = 0.692; m.p.: 345–347°C; anal. calc. for C17H10BrN3O3S (416.25): C, 49.05; H, 2.42; N, 10.09; found: C, 49.25; H, 4.43; N, 10.06; 1H NMR (500 MHz, DMSO-d6, ppm): δ 11.37 (s, 1H, HO-C(3)), 10.18 (s, 1H, HO-C(1)), 8.16 (d, 1H, J = 8.62 Hz, H-C(5)), 7.98 (m, 2H, H-C(2′, 6′)), 7.83 (s, 1H, H-isoxazole), 7.80 (m, 2H, H-C(3′, 5′), 6.54 (d, 1H, J = 2.28 Hz, H-C(2)), 6.49 (dd, J = 8.71 and 2.27 Hz, 1H, H-C(6)); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 169.7, 163.8, 161.9, 156.9, 156.8, 154.9, 132.4 (2C), 129.1, 127.9 (2C), 125.4, 124.5, 108.6, 107.9, 102.3, 99.2; IR (ATR, cm−1): 3358 (OH), 2916 (H-Ar), 2850 (CH), 1605 (C=N), 1525 (C=C), 1474, 1444, 1421, 1392, 1351, 1272, 1219, 1187 (C-O), 1107, 1039 (N=C-S-C=N), 1008, 947, 933, 870, 831, 797, 685 (C-S-C); EI-MS (m/z, %): 416 (M+, 21), 415 (100), 235 (6), 208 (6), 207 (5), 185 (39), 183 (40), 167 (18), 157 (10), 155 (10), 153 (10), 135 (15), 108 (4), 107 (5), 89 (13), 75 (5), 69 (4), 52 (5), 39 (74).
4-{5-[5-(4-Fluorophenyl)thiophen-2-yl]-1,3,4-thiadiazol-2-yl}benzene-1,3-diol (11)
A mixture of 5-(4-fluorophenyl)thiophene-2-carboxylic acid hydrazide (0.01mol) (Alfa Aesar) and STB (0.01 mol) in methanol (55 mL) was refluxed (3 h). The removed solid was filtered and washed with water. Recrystallization from MeOH (40 mL) afforded 11.
Yield: 65%; HPLC: log k = 0.609; m.p.: 312–314°C; anal. calc. for C18H11FN2O2S2 (370.42): C, 58.36; H, 2.99; N, 7.56; found: C, 58.51; H, 3.01; N, 7.54; 1H NMR (500 MHz, DMSO-d6, ppm) δ: 11.17 (s, 1H, HO-C(3)), 10.09 (s, 1H, HO-C(1)), 8.06 (d, 1H, J = 8.71 Hz, H-C(5)), 7.82 (m, 2H, H-C(2′, 6′), 7.76 (d, 1H, J = 3.89 Hz, H-C(thioph)), 7.60 (d, 1H, J = 3.89 Hz, H-C(thioph)), 7.31 (m, 2H, H-C(3′, 5′)), 6.53 (d, 1H, J = 2.29 Hz, H-C(2)), 6.46 (dd, J = 8.69 and 2.29 Hz, 1H, H-C(6)); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 162.1, 161.4, 161.2, 160.1, 156.4, 144.5, 131.4, 130.7, 129.5, 128.9, 127.8, 127.7, 124.9, 116.3, 116.1, 108.5, 108.2, 102.3; IR (ATR, cm−1): 3157 (OH), 3097 (CAR-H), 2917 (CH), 2850 (CH), 1636 (C=N), 1601, 1547 (C=C), 1468, 1445, 1411, 1352, 1249, 1176 (C-O), 1098, 967, 912, 874, 823, 792, 681 (C-S-C); EI-MS (m/z, %): 370 (M+, 100), 234 (8), 221 (7), 203 (9), 167 (8), 153 (4), 133 (6), 69 (5).
4-{5-[5-(4-Bromophenyl)thiophen-2-yl]-1,3,4-thiadiazol-2-yl}benzene-1,3-diol (12)
A mixture of 5-(4-bromophenyl)thiophene-2-carboxylic acid hydrazide (0.01mol) (Aldrich) and STB (0.01 mol) in methanol (40 mL) was refluxed (3 h). The removed solid was filtered and washed with water. Recrystallization from MeOH (40 mL) afforded 12.
Yield: 62%; HPLC: log k = 0.899; m.p.: 253–254°C; anal. calc. for C18H11BrN2O2S2 (431.33): C, 50.12; H, 2.57; N, 6.49; found: C, 50.30; H, 2.59; N, 6.44; 1H NMR (500 MHz, DMSO-d6, ppm) δ: 11.21 (s, 1H, HO-C(3)), 10.12 (s, 1H, HO-C(1)), 8.06 (d, 1H, J = 8.70 Hz, H-C(5)), 7.78 (d, 1H, J = 3.91 Hz, H-C(thioph)), 7.73 (m, 2H, H-C(2′, 6′), 7.67 (m, 3H, H-C(3′, 5′, thioph)), 6.55 (d, 1H, J = 2.26 Hz, H-C(2)), 6.46 (dd, J = 8.70 and 2.27 Hz, 1H, H-C(6)); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 162.1, 161.4, 159.0, 156.3, 144.4, 132.2 (2C), 132.1, 131.8, 130.8, 128.9, 127.6 (2C), 125.6, 121.6, 108.4, 108.2, 102.3; IR (ATR, cm−1): 3352 (OH), 2946 (CAR-H), 2835 (CH), 1660, 1630 (C=N), 1603, 1529 (C=C), 1462, 1450, 1415, 1226, 1035, 685 (C-S-C); EI-MS (m/z, %): 431 (M+, 20), 430 (100), 297 (12), 295 (11), 283 (13), 281 (10), 266 (5), 265 (21), 264 (5), 263 (17), 216 (6), 199 (4), 172 (5), 171 (5), 167 (30), 158 (7), 153 (11), 140 (10), 135 (4), 107 (4), 69 (10), 40 (10).
4-(5-Benzyl-1,3,4-thiadiazol-2-yl)-6-chlorobenzene-1,3-diol (13)
A mixture of phenylacetic hydrazide (0.016 mol) (Aldrich) and SClTB (0.012 mol) in methanol (80 mL) was refluxed (3 h). The removed solid was filtered, washed with water. Recrystallization from MeOH/H2O (3:1) (10 mL) afforded 13.
Yield: 60%; HPLC: log k = 0.087; m.p.: 189–190°C; anal. calc. for C15H11ClN2O2S (318.78): C, 56.52; H, 3.48; N, 8.79; found: C, 56.59; H, 3.46; N, 8.76; 1H NMR (500 MHz, DMSO-d6, ppm) δ: 11.31 (s, 1H, HO-C(3)), 10.76 (s, 1H, HO-C(1)), 8.05 (s, 1H, H-C(5)), 7.35 (d, 4H, J = 4.42 Hz, H-C(Ar’)), 7.27 (m, 1H, H-C(Ar’)), 6.68 (s, 1H, C(2)), 4.45 (s, 2H, CH2); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 168.6, 162.1, 156.1, 154.6, 138.0, 128.9 (2C), 128.8 (2C), 127.8, 127.1, 111.8, 109.5, 103.6, 34.7 (CH2); IR (ATR, cm−1): 3100 (OH), 3028 (CAr-H), 2870, 2851 (CH), 1625 (C=N), 1604, 1587 (C=C), 1480, 1467, 1442, 1426, 1407, 1391, 1384, 1371, 1352, 1296, 1256, 1223, 1191 (C-O), 1162, 1095, 971, 871, 843 (CAr-H), 805, 730, 700, 678 (C-S-C); EI-MS (m/z, %): 318 (M+, 59), 317 (10), 169 (9), 150 (12), 149 (100), 148 (6), 141 (5), 122 (22), 121 (9), 117 (7), 116 (9), 91 (37), 89 (7), 78 (5), 69 (7), 65 (12), 63 (5), 51 (10), 39 (7).
4-Chloro-6-(5-(4-fluorophenylamino)-1,3,4-thiadiazol-2-yl)benzene-1,3-diol (14)
A mixture of 4-fluorophenyl-3-thiosemicarbazide (0.003 mol) (Alfa Aesar) and SClTB (0.002 mol) in methanol (25 mL) was refluxed (3 h). The removed solid was filtered and washed with water. Recrystallization from MeOH (10 mL) afforded 14.
Yield: 64%; HPLC: log k = 0.146; m.p.: 261–262°C; anal. calc. for C14H9ClFN3O2S (337.76): C, 49.78; H, 2.69; N, 12.44; found: C, 49.84; H, 2.71; N, 12.38; 1H NMR (500 MHz, DMSO-d6, ppm) δ: 11.03 (s, 1H, HO-C(1)), 10.64 (s, 1H, HO-C(3)), 10.27 (s, 1H, NH), 7.92 (s, 1H, H-C(5)), 7.66 (m, 2H, CAR-H), 7.19 (m, 2H, CAr-H), 6.67 (s, 1H, H-C(2)); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 163.6, 160.4, 157.9, 155.7, 154.8, 137.4, 128.5, 108.9, 108.8, 115.7, 115.5, 111.6, 109.9, 103.6; IR (ATR, cm−1): 3374 (OH), 3020 (H-Ar), 1617 (C=N), 1567 (C=C), 1444, 1432, 1411, 1240, 1211, 1164 (C-O), 1059 (N=C-S-C=N), 1010, 988, 975, 942, 912, 828, 801, 726, 674 (C-S-C), 612, 587. EI-MS (m/z, %): 337 (M+, 100), 336 (9), 189 (6), 187 (13), 184 (5), 170 (8), 169 (18), 168 (65), 155 (8), 154 (8), 141 (20), 136 (19), 130 (8), 128 (20), 121 (5), 114 (9), 110 (16), 100 (15), 95 (20), 83 (20), 75 (14), 69 (15), 63 (5), 57 (6), 51 (8), 40 (16), 39 (5).
4-Chloro-6-(5-(3-chlorophenylamino)-1,3,4-thiadiazol-2-yl)benzene-1,3-diol (15)
A mixture of 4-(3-chlorophenyl-3-thiosemicarbazide (0.01 mol) (Alfa Aesar) and SClTB (0.01 mol) in methanol (50 mL) was refluxed (3 h). The hot mixture was filtered and water (50 mL) was added to the filtrate. The formed solid was filtered off and crystallized from methanol (4:1) (50 mL). The formed solid was boiled in chloroform (30 mL) and filtered. Recrystallization from MeOH/H2O (1:1) (50 mL) afforded 15.
Yield: 60%; HPLC: log k = 0.392; m.p.: 300–302°C; anal. calc. for C14H9C12O2S (354.21): C,47.47; H, 2.56; N, 11.86; found: C, 47.58; H, 2.54; N, 11.89; 1H NMR (500 MHz, DMSO-d6, ppm) δ: 11.10 (s, 1H, HO-C(1)), 10.68 (s, 1H, HO-C(3)), 10.49 (s, 1H, NH), 7.98 (m, 1H, H-C(5)), 7.95 (t, 1H, J = 1.93 Hz, H-C(2′)), 7.47 (m, 1H, H-C(6′)), 7.37 (t, 1H, J = 8.10 Hz, H-C(5′)), 7.04 (m, 1H, H-C(4′)), 6.70 (s, 1H, H-C(2)); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 163.9, 155.4, 153.9, 152.9, 142.1, 133.5, 130.6, 127.3, 121.0, 116.6, 115.7, 111.7, 109.8, 103.6; IR (ATR, cm−1): 3369 (OH), 3018 (Ar-H), 1619 (C=N), 1569 (C=C), 1440, 1438, 1421, 1235, 1220, 1168 (C-O), 1057 (N=C-S-C=N), 1009, 997, 985, 937, 922, 838, 810, 731, 664 (C-S-C), 620, 614, 594, 578; EI-MS (m/z, %): 354 (M+, 25), 353 (100), 321 (5), 319 (14), 189 (5), 187 (14), 184 (69), 171 (7), 170 (10), 169 (11), 159 (12), 157 (10), 155 (5), 152 (7), 149 (23), 130 (5), 128 (14), 127 (5), 122 (7), 113 (5), 111 (12), 100 (9), 90 (4), 69 (7).
4-Chloro-6-(5-(3,4-dihydroxyphenyl)-1,3,4-thiadiazol-2-yl)benzene-1,3-diol (16)
A mixture of 3,4-dihydroxybenzhydrazide (0.015 mol) (Alfa Aesar) and SClTB (0.015 mol) in methanol (75 mL) was refluxed (3 h). The hot mixture was filtered, water (40 mL) was added to the filtrate and it was left at room temperature (24 h). Recrystallization from MeOH (40 mL) afforded 16.
Yield: 62%; HPLC: log k = 0.11; m.p.: 280–282°C; anal. calc. for C14H9ClN2O4S (336.75): C, 49.93; H, 2.69; N, 8.32; found: C, 50.03; H, 2.68; N, 8.36; 1H NMR (500 MHz, DMSO-d6, ppm) δ: 11.30 (s, 1H, HO-C(1)), 10.83 (s, 1H, HO-C(3)), 9.58 (s, 1H, HO-C(3′)), 9.41 (s, 1H, HO-C(4′)), 8.10 (s, 1H, C(5)), 7.43 (d, J = 2.18 Hz, 1H, H-C(2′)), 7.26 (dd, J = 8.18 Hz, J = 2.16 Hz, 1H, H-C(6′)), 6.87 (d, J = 8.20, 1H, H-C(5′)), 6.77 (s, 1H, H-C(2)); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 166.6, 160.2, 156.4, 154.8, 148.5, 145.8, 127.7, 121.4, 119.5, 116.3, 114.1, 111.8, 109.5, 104.0; IR (ATR, cm−1): 3350 (OH), 3093 (CH), 2851 (CH), 1626 (C=N), 1605 (C=C), 1502 (C=C), 1461, 1450, 1400, 1385, 1319, 1297, 1280, 1253, 1231, 1187 (C-O), 1157, 1110, 978, 903, 875, 852, 827, 791, 721, 684 (C-S-C); EI-MS (m/z, %): 336 (M+, 100), 303 (8), 302 (8), 201 (19), 187 (6), 168 (6), 167 (18), 153 (12), 136 (5), 135 (9), 121 (4), 63 (4).
3-{5-[4-(Dimethylamino)phenyl]-1,3,4-thiadiazol-2-yl}biphenyl-2,3′,4,5′,6-pentol (17)
A mixture of 4-dimethylaminobenzhydrazide (0.014 mol) (Aldrich) and 2,3′,4,5′,6-pentahydroxybiphenyl-3-carbodithioic acid (0.014 mol) in methanol (70 mL) was refluxed (2.5 h). The removed compound was filtered and washed with water. Recrystallization from MeOH (40 mL) afforded 17.
Yield: 65%; HPLC: log k = -0.26; m.p.: 236–238°C; anal. calc. for C22H19N3O5S (437.47): C, 60.40; H, 4.38; N, 9.61; found: C, 60.54; H, 4.36; N, 9.57;1H NMR (500 MHz, DMSO-d6, δ): 12.66 (s, 1H, HO), 11.33 (s, 1H, HO), 9.80 (s, 1H, HO), 8.98 (s, 2H, HO), 7.82 (d, J = 8.90 Hz, 2H, H-C(Ar)), 6.82 (d, J = 9.00 Hz, 2H, H-C(Ar)), 6.26 (s, 1H, H-C(Ar)), 6.16 (d, J = 2.18 Hz, 2H, H-C(Ar)), 6.12 (t, J = 2.15 Hz, 1H, H-C(Ar)), 3.01 (s, 6H, CH3); IR (ATR, cm−1): 3200 (OH), 2930 (Ar-H), 1640, 1630 (C=N), 1605 (C=C), 1483, 1468, 1461, 1443, 1426, 1413, 1390, 1371, 1334, 1249, 1231, 1200, 1156 (C-O), 1070, 1043 (N=C-S-C=N), 1006, 945, 883, 806, 689 (C-S-C); EI-MS (m/z, %): 437 (M+, 25), 219 (35), 178 (6), 177 (18), 164 (18), 163 (7), 150 (9), 148 (17), 147 (12), 146 (72), 145 (100), 132 (9), 131 (9), 129 (11), 121 (9), 120 (11), 104 (7), 102 (13), 91 (5), 77 (7), 51 (6), 45 (13), 44 (23), 42 (11), 39 (6), 36 (27), 34 (11).
Compounds: 4-(5-heptyl-1,3,4-thiadiazol-2-yl)benzene-1,3-diol (2), 4,4′-{[5,5′-(benzene-1,4-diyl)]bis(1,3,4-thiadiazol-2-yl)}di(benzene-1,3-diol) (7) and 4-(2,4-dichlorophenoxymethyl)benzene-1,3-diol (9) were prepared according to the procedure already describedCitation19,Citation20.
Measurement of ChEs activities
Acetylcholinesterase (AChE, E.C. 3.1.1.7, from the electric eel), butylcholinesterase (BuChE, E.C. 3.1.1.8, from equine serum), acetylthiocholine iodide (ATCh), butylthiocholine iodide (BTCh), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), neostigmine bromide and donepezil hydrochloride monohydrate were purchased from Sigma-Aldrich (Steinheim, Germany). The inhibitory activities against AChE and BuChE of the prepared compounds were performed by means of the method previously developed by Ellman et al., using donepezil and neostigmine as the reference compoundsCitation21. This is based on the reaction of released thiocholine to give a coloured product with a chromogenic reagent. Seven different concentrations of the synthesized compounds in the range 10−3–10−9 M were measured at 412 nm. All the assays were under 0.1 M KH2PO4/K2HPO4 buffer (pH = 8) using a Varian Cary 50 Spectrophotometer. Enzyme solutions were prepared to give 2 units/mL in 2 mL aliquots.
The assay medium contained phosphate buffer, pH 8.0 (1 mL), 50 μL of 0.01 M DTNB, 10 μL of enzyme, 50 μL of acetylthiocholine iodide (ATCh) and 50 μL of the test compound solution. ATCh was added to the assay medium after 10 min of incubation time. The activity was determined by measuring the increase in absorbance at 412 nm for 1 min interval at 37 ± 0.2°C. For determining the blank value, additionally 50 μL buffer replaced the enzyme solution. In vitro the BuChE assay uses the similar method to that described above.
Each concentration was analyzed in triplicate. The 50% inhibitory concentration (IC50) was calculated from a dose–response curve obtained by plotting the percentage of inhibition versus the log concentration with the use of GraFit 4.09 softwareCitation22. The results were expressed as the mean ± standard deviation (SD).
Results and discussion
5-Substituted (1,3,4-thiadiazol-2-yl)benzene-1,3-diols were obtained by the reaction of the commercially available hydrazides or thiosemicarbazides with sulfinylbis[(2,4-dihydroxyphenyl)methanethione] (STB) or with its analogue sulfinylbis[(5-chloro-2,4-dihydroxyphenyl)methanethione] (SClTB) in methanol under reflux (1.5–4 h) in moderate to good yields (60–89%) as outlined in . The key intermediates were obtained from 2,4-dihydroxybenzenecarbodithioic acids and SOCl2 in diethyl etherCitation23. The substituents panel of compounds is shown in . Purity of compounds was monitored by the reversed-phase (RP-18) HPLC chromatography (methanol–water).
All new compounds showed analytical and spectroscopic data in good accordance with the proposed structure. In the 1H NMR spectra the OH protons are usually detected as broad bands in the range ca. 11 and 10 ppm. The resonance signals of aromatic protons of the β-resorcinol moiety appear as two characteristic dublets at ca. 8 ppm (J = 8.7 Hz) and 6.5 ppm (J = 2.3 Hz) corresponding to H-C(5) and H-C(2), respectively and as doublet of doublets at ca. 6.45 ppm (J = 8.7 and 2.3 Hz) of H-C(6). In the case of additional substitution in position 5 by chlorine atom protons of that ring appear as two characteristic singlets at ca. 6.7 and 8 ppm corresponding to H-C(2) and H-C(5), respectivelyCitation13–16. Singlet is registered for H-C(isoxazole) at 7.88 ppm in the case of compound 10. Protons of 2,5-disubstituted thiophene appeared as two doublets at ca. 7.77 and 7.63 ppm with J = 3.9 HzCitation(11,Citation12). In the 13C NMR spectrum characteristic signals of substituted 1,3,4-thiadiazole ring carbon atoms appear in the range 169-162 ppm.
In the IR spectrum there are strong bands in the region about 3400–3100 corresponding to ν (O-H) and additionally ν (N-H) in the case of compounds 8, 14, 15. Band of ν (C=N) appears in the region 1635–1610 cm−1.
The mass spectra (EI) of the compounds show molecular ion peaks, however, with various intensities. The major fragmentation pathway of 4-(1,3,4-thiadiazol-2-yl)benzene-1,3-diols involves the cleavage of the C(2)-N(3) and S-C(5) bonds 1,3,4-thiadiazole ring with the formation of (OH)2C6H3CS+ (m/z 153) ion. The cleavage of S-C(2) and N-N bonds which directs to (OH)2C6H3CN+ (m/z 135) fragmentation is also observed. Characteristic relatively strong band corresponding to tropylic cation C7H7+ (m/z 91) is observed for benzyl derivativeCitation13. It is the effect of α and β atoms disconnection in relation to the phenyl ring.
All compounds have been evaluated as AChE and BuChE inhibitors. Their inhibitory potency was expressed as the half of maximal inhibitory concentration, IC50. It was determined by the modified Ellman’s methodCitation21. Donepezil and neostigmine were used as the reference drugs. shows that most of the studied compounds were able to inhibit in vitro both enzymes – AChE and BuChE but in a very broad range of concentrations. The IC50 values for AChE are ranged from > 500 to 0.053 μM and for BuChE from > 500 to 0.105 μM. At the same it proves that all synthesized particles are significantly more active towards AChE than BuChE, with the exception of compounds 5 and 14. Compound 9 of the highest activity against AChE shows inhibition effect similar to that of neostigmine. Simultaneously it is 947-fold more active against AChE compared to BuChE. That compound can serve as a selective inhibition agent for AChE over BuChE.
Table 1. In vitro inhibition (IC50, μM) and selectivity of the studied compounds on AChE and BuChE.
To find the influence of the type of 1,3,4-thiadiazole ring substitution on potency of compounds the structure-activity analyses SAR have been performed. To obtain better results some previously described compounds were included in the biological screening. SAR shows that alkyl derivatives (compounds 1 and 2) are good inhibitors of both enzymes. Compound 2 with the heptyl substituent is one of the most active derivatives. From the group of compounds with two 1,3,4-thiadiazole ringsCitation(5,Citation6,7) compound 7 with benzene as a link shows the highest affinity for AChE. Derivative 9 with the 2,4-dichlorophenoxymethyl substituent is the most active against AChE of all compounds of 1,3,4-thiadiazole series. The presence of the additional heterocyclic ring (oxazolyl, thiophenyl) does not improve activities of compounds. Placement of chlorine atom in the benzenodiole moiety does not change substantially affinity of compounds for both enzymesCitation(13–16). The analogs possessing naphthalenylCitation(3) or biphenylylCitation(12) substituent are inactive.
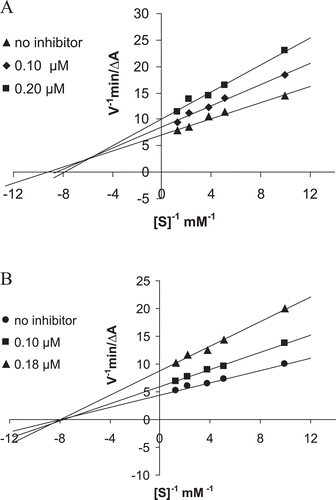
The linear Lineweaver-Burk equation which is a double reciprocal form of the Michaelis-Menten equation was used to evaluate the type of inhibition. The graphical analysis of steady-state inhibition data for representative compounds 2 and 7 is shown in and , respectively. In the graph shows that the mechanism of AChE inhibition of compound 2 is the mixed-type. In the lines crossing the x axis in the same point indicate unchanged KM and decreased Vmax with the increasing inhibitor concentrations. This is a typical trend of non-competitive inhibition which is similar to that of donepezilCitation4.
Conclusion
In conclusion, we synthesized new (1,3,4-thiadiazol-2-yl)benzene-1,3-diol derivatives and evaluated their anticholinesterase activities. The results indicate that compound 9 is the most potent inhibitor of AChE being 947-fold more active against AChE than BuChE. That compound can serve as a selective inhibition agent. Whereas the heptyl derivative shows comparable high affinity for both enzymes in the low nM range. The kinetic studies suggest that in a series of the investigated compounds, inhibition mechanisms can be various. The obtained results could be useful for designing of new AChE and BuChE inhibitors, resulting in greater selectivity as well as the increasing inhibitory potency.
Declaration of interest
The authors declared no conflict of interest.
References
- Carreiras MC, Marco JL. Recent approaches to novel anti-Alzheimer therapy. Curr Pharm Des 2004;10:3167–3175.
- O’Neill MF. Difficult times for Alzheimer’s treatments. Drug Discov Today 2005;10:1333–1335.
- Davis KL, Powchik P. Tacrine. Lancet 1995;345:625–630.
- Tsuno N. Donepezil in the treatment of patients with Alzheimer’s disease. Expert Rev Neurother 2009;9:591–598.
- Kavanagh S, Gaudig M, Van Baelen B, Adami M, Delgado A, Guzman C et al. Galantamine and behavior in Alzheimer disease: analysis of four trials. Acta Neurol Scand 2011;124:302–308.
- Farlow MR, Grossberg GT, Meng X, Olin J, Somogyi M. Rivastigmine transdermal patch and capsule in Alzheimer’s disease: influence of disease stage on response to therapy. Int J Geriatr Psychiatry 2011;26:1236–1243.
- Grossberg GT. The ABC of Alzheimer’s disease: behavioral symptoms and their treatment. Int Psychogeriatr 2002;14 Suppl 1:27–49.
- Michaelis ML. Drugs targeting Alzheimer’s disease: some things old and some things new. J Pharmacol Exp Ther 2003;304:897–904.
- Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci 2003;4:131–138.
- Ciapetti P, Giethlen B. Molecular Variations Based on Isosteric Replacements. In: Wermuth CM. The Practice of Medicinal Chemistry, USA: Academic Press Burlington, 2008:290–342.
- Sengupta AK, Garg M. New 5-arylamino-2 N-(2′-mercaptoacetylamino-4′-arylthiazolo)-thiadiazoles(III) - as AChE inhibitors. J Indian Chem Soc 1980;57:1241–1242.
- Misra HK. Synthesis of some new substituted 1,3,4-oxadiazoles as potential insecticidal, antibacterial and anti-acetylcholine esterase agents. Arch Pharm (Weinheim) 1983;316:487–493.
- Martinez A, Alonso D, Castro A, Arán VJ, Cardelús I, Baños JE et al. Synthesis and potential muscarinic receptor binding and antioxidant properties of 3-(thiadiazolyl)pyridine 1-oxide compounds. Arch Pharm (Weinheim) 1999;332:191–194.
- Sarkandi DN, Firoozpour L, Asadipour A, Sheibani V, Asli MAM, Davood A, Shafiee A, Foroumadi A. Synthesis of 1-benzyl-4-2-(5-phenyl-1,3,4-thiadiazole-2-yl)aminoethyl piperidine as potential Alzheimer’s disease modifying agent. Asian J Chem 2011;23:2503–2505.
- Alonso M, Martinez A. GSK-3 inhibitors: discoveries and developments. Curr Med Chem 2004;11:755–763.
- Gupta AK, Misra HK. Synthesis and evaluation of substituted quinazolone derivatives for antibacterial, antifungal, and antiacetylcholinesterase activities. J Pharm Sci 1980;69:1313–1317.
- Altintop MD, Kaplancikli ZA, Ozdemir A, Turan-Zitouni G, Temel HE, Akalin G. Synthesis and anticholinesterase activity and cytotoxicity of novel amide derivatives. Arch Pharm (Weinheim) 2012;345:112–116.
- Skrzypek A. Zastosowanie spektrofotometrycznej metody Ellmana w ocenie właściwości inhibicyjnych nowych 2,5 – dipodstawionych 1,3,4-tiadiazoli w stosunku do acetylocholinoesterazy. III Sympozjum Naukowego: Nauka i Przemysł – metody spektroskopowe w praktyce, nowe wyzwania i możliwości. 2010, p 229–234.
- Matysiak J, Skrzypek A, Niewiadomy A. Synthesis and antifungal activity of novel 5-substituted 4-(1,3,4-thiadiazol-2-yl) benzene-1,3-diols. Heteroat Chem 2010;7:533–540.
- Matysiak J, Nasulewicz A, Pelczynska M, Switalska M, Jaroszewicz I, Opolski A. Synthesis and antiproliferative activity of some 5-substituted 2-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles. Eur J Med Chem 2006;41:475–482.
- Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
- Leatherbarrow RJ, Stains, UK: Erithacus Software Ltd; 1999.
- Matysiak J, Niewiadomy A. Application of sulfinyl bis(2,4-dihydroxythiobenzoyl) in the synthesis of N-substituted 2-amino-5-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles. Synth Commun 2006;36:1621–1630.