Abstract
Cord blood has numerous facilities for life and used in many different areas. Cord blood contains many different catalytic proteins including antioxidant enzymes. Here we purified human cord blood glutathione reductase (hcbGR), glutathione S-transferase (hcbGST) and human cord blood glutathione peroxidase (hcbGPx) from human cord blood erythrocytes and analyzed the inhibition effects of the antibiotics incorporating cefuroxime, ceftriaxone, ceftizoxime and cefoperazone, on these enzymes. KI values for the drugs ranged from 10.42 to 28.72 µM for hcbGR, 32.7 to 244.8 µM for hcbGPx, and 32.39 to 267.3 µM for hcbGST. Cefuroxime caused the highest inhibition on all enzymes with KI values of 10.42, 32.39, 32.7 µM for hcbGR, hcbGST, and hcbGPx, respectively. All drugs displayed non-competitive inhibition regardless of their structures. Since these drugs are often used during pregnancy, identification of possible undesired impacts on various parameters has a great importance for pharmacological and medical applications.
Keywords::
Introduction
After a baby is born and the umbilical cord is cut, some blood remains in the blood vessels of the placenta and the portion of the umbilical cord that remains attached to it. After birth, the baby no longer needs this extra blood. This blood is called placental blood or umbilical cord blood: “cord blood” for shortCitation1.
Cord blood contains red blood cells, white blood cells, platelets, and plasma, like blood. In addition, cord blood is a rich source of stem cells that may have potentially lifesaving benefits for baby and familyCitation1. It is collected because of its stem cell contents, including hematopoietic cells, which can be used to treat hematopoietic and genetic disordersCitation2.
The tripeptide reduced glutathione (GSH: l-γ-glutamyl-cysteinyl-glycine) is the most important intracellular nonprotein thiol in mammalian cells. It is found at concentrations of 1–3 mM in human brain cellsCitation3, and it possesses reducing and nucleophilic properties. It can exist in either a reduced (GSH) or oxidized (GSSG) form. Glutathione reductase (EC 1.8.1.7, GR) is an ubiquitous flavoenzyme that catalyzes the NAD(P)H-dependent reduction of GSSG to GSH. This enzyme maintains adequate levels of the cellular GSH pool, which is critical not only for maintaining the cellular redox status by keeping sulfhydryl groups of cytosolic proteins in their reduced formCitation4,Citation5 but also because numerous toxic or potentially toxic compounds, including some metals, are either taken up by or removed from the cells by GSH-mediated pathwaysCitation6. Glutathione peroxidase (EC 1.11.1.9, GPx) catalyzes the reduction of hydrogen peroxide by GSH. Different groups demonstrated the presence of glutathione peroxidase in erythrocytesCitation7. Glutathione S-transferases (EC 2.5.1.18, GSTs), are members of a multigene family of isoenzymes ubiquitously expressed in most living organisms. It was subsequently shown that these enzymes catalyze the conjugation of GSH to a variety of electrophilic compounds, thus establishing the now widely accepted role of GSTs as cell housekeepers involved in the detoxification of endogenous as well as exogenous substancesCitation8.
Antibiotics are powerful drugs that fight against bacterial infections and save lives when used properly. They either kill bacteria or keep them from reproducing. Antibiotics are widely used to deal with various disorders, but there are few reports of their effects on enzyme activities. Some studies found either increases or decreases in mammalian enzyme activities, and the inhibitor or activator effects of some antibiotics have been rarely investigated. Nevertheless, a few recent investigations regarding the inhibitory actions of different type of drugs and chemicals on GR have been reported by our groupCitation9–15.
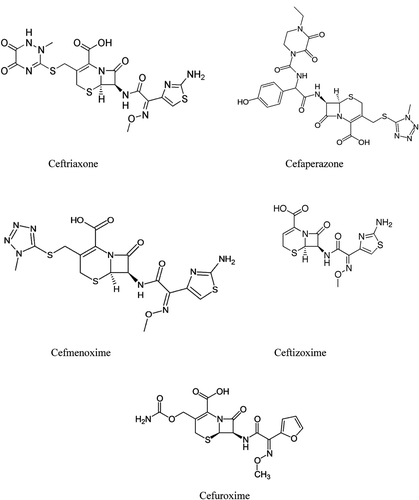
Cefuroxime is a second-generation cephalosporin antibiotic whereas ceftriaxone, ceftizoxime and cefoperazone are third-generation cephalosporin antibiotics. Third-generation cephalosporin antibiotics have broad spectrum activity against gram-positive and gram-negative bacteriaCitation16. As known, all of these drugs are commonly used during pregnancy.
A number of investigations focus on the property of umbilical cord. For instance, the immunologic properties of cord blood were reported to differ from mature bone marrow or peripheral blood stem cells. Also, cord blood cells were shown to produce increased amounts of anti-inflammatory cytokine interleukin-10, which may downmodulate graft-versushost diseaseCitation17. However, there are not sufficient data on the inhibition of cordblood enzymes by various factors such as drugs, nutritients or etc. It is important to identify the enzyme activities during pregnancy and characterize the factors increasing or decreasing those activities. Therefore, in an effort to provide beneficial data for further investigations, the present study aimed to evaluate the in vitro effects of commonly used antibiotics on hcbGR, hcbGPx, and hcbGST enzymes.
Materials and methods
Chemicals
2,′ 5′-ADP Sepharose-4B was obtained from Pharmacia. Glutathione-sepharose 4B, Sephadex G-200, l-chloro-2,4-dinitrobenzene (CDNB), t-butyl hydroperoxide, protein assay reagents, GSH, GSSG, NADPH were obtained from Sigma-Aldrich Co. All other chemicals were analytical grade and obtained from Merck.
Glutathione reductase activity
Enzymatic activity was measured by Beutler’s methodCitation18 with a Shimadzu Spectrophotometer UV-(1208), at 25°C. The assay system contained 100 mM Tris-HCl buffer pH 8.0, including 0.5 mM EDTA, 3.3 mM GSSG and 0.1 mM NADPH. One enzyme unit is defined as the oxidation of 1 µmol NADPH per min under the assay condition at 25°C.
Glutathione peroxidase activity
Glutathione peroxidase was assayed in a l-ml system containing 0.1 M potassium phosphate buffer, pH 7.0, 0.2 mM NADPH, 1 i.u. glutathione reductase, 4 mM GSH, 4 mM EDTA, 4 mM sodium azide. The reaction mixture was incubated at 37°C for 10 min after which 10 μl of 10 mM t-butyl hydroperoxide were added to start the reaction. No t-butyl hydroperoxide was added to the blank cuvette. The rate of reaction was measured at 37°C by following the decrease in the absorbance at 340 nm using a spectrophotometerCitation7.
Glutathione S-transferase activity
Enzymatic activity was determined spectrophotometrically by measuring the conjugation of CDNB with GSHCitation8. The 1 ml assay mixture contained 0.5 mM CDNB, 1 mM GSH, and 100 mM potassium phosphate buffer, pH 6.5. The rate of increase in absorbance at 340 nm was measured for 5 mm at 37°C against a blank containing the reaction mixture without enzyme.
Preparation of the hemolysate
Erythrocytes were purified from fresh human cord blood obtained from the Blood Centre of Erzincan Hospital. From normal pregnant women ad term, following the birth by caesarian section or normal delivery, 2 mL of umbilical cord blood was withdrawn by syringe just before the separation placenta and transferred into K3EDTA anticoagulated blood tubes and mixed. The blood samples were stored at −40°C until analysis. The blood samples were centrifuged at 2250g for 15 min and the plasma and buffy coat were removed. The packed red cells were washed three times with KCl (0.16 M) and hemolyzed with 5 volume of ice-cold water and then centrifuged (4°C, 10,000g, for 30 min) to remove the ghosts and intact cellsCitation5,Citation11
Ammonium sulphate precipitation
The hemolysate was subjected to precipitation with ammonium sulphate at different intervals depending on the enzymes. Enzyme activity was determined both in the supernatant and in the precipitate for each respective precipitation. The precipitate was dissolved in phosphate buffer (50 mM; pH = 7.0). The resultant solution was clear, and contained partially purified enzyme. Then it was dialysed at 4°C in 1 mM EDTA + 10 mM K-phosphate buffer (pH 7.5) for 2 h with two changes of bufferCitation11. Partially purified enzyme solution was kept at 4°C.
Partial purification of hcbGPx
The purification steps are given in . The hemolysate was centrifuged for 30 min at 10,000g to remove the ghosts. Solid ammonium sulfate was added to the supernatant with constant stirring and the precipitate formed between 25 and 50% saturation of (NH4)2SO4 was separated by centrifugation and suspended in 10 mM potassium phosphate buffer, pH 7.0, with 0.7 mM β-mercaptoethanol and dialyzed against the same buffer to remove the (NH4)2SO4Citation7.
Table 1. Purification scheme of hcbGR.
Table 2. Partial purification scheme of hcbGPx.
Table 3. Purification scheme of hcbGST.
Purification of hcbGR
Preparation of 2′, 5′-ADP Sepharose-4B affinity column was previously describedCitation9,Citation11,Citation13. The dialyzed sample obtained previously was loaded onto the 2′, 5′-ADP Sepharose-4B affinity column and the column was washed with 25 ml of 0.1 M K-acetate + 0.1 M K-phosphate, pH 6, and 25 ml of 0.1 M K-acetate + 0.1 M K-phosphate, pH 7.85. Washing was continued with 50 mM K-phosphate buffer including 1 mM EDTA, pH 7.0, until the final absorbance difference became 0.05 at 280 nm. The enzyme was eluted with a gradient mixture of 0 to 0.5 mM GSH + 1 mM NADPH in 50 mM K-phosphate, containing 1 mM EDTA (pH 7.0). Active fractions were collected and dialyzed with equilibration buffer. All of the procedures were performed at 4°CCitation5,Citation13.
After dried Sephadex G-200 (2 g) was used for a 165 ml column (2 × 50 cm) bed volume. The gel was incubated in distilled water at 90°C for 5 h. After removal of the air in the gel, it was loaded onto the column. Flow rate was adjusted to 15 ml/h by means of a peristaltic pump. Then the column was equilibrated with 50 mM Tris-HCl + 50 mM KCl buffer, pH 7.0, until the final absorbance difference became 0 at 280 nm. The dialyzed sample was mixed with 5% glycerol. The final sample was loaded onto the column and elutions were collected in 2 ml amounts. In each fraction, enzyme activity was determined at 340 nm. Active fractions were collected and stored at −20°C for testing the enzyme purity by electrophoresisCitation5,Citation9,Citation11,Citation15.
Purification of hcbGST
The hemolysate supernate was passed through a 1 × 10 cm GSH sepharose 4B column at a flow rate of approximately 10 ml/h. The column was washed for 1 h with 20 mM potassium phosphate buffer (pH 7.2), followed by elution of 2 mM GSH with cold 50 mM Tris buffer (pH 9.5 at 4°C)Citation19,Citation20. Fractions containing GST activity were pooled and dialyzed overnight at 4°C against 2 liters of 20 mM potassium phosphate buffer (pH 7.0).
Protein determination
Protein during the purification steps was determined spectrophotometrically at 595 nm according to the Bradford method using bovine serum albumin as the standardCitation21.
SDS polyacrylamide gel electrophoresis
SDS polyacrylamide gel electrophoresis was performed after purification of the cord blood izo enzymes. It was carried out according to Laemmli procedureCitation22.
In vitro drug effects
In order to determine the effects of some drugs on hcbGR and hcbGST, different concentrations of corresponding drugs were tested. These enzyme activities were measured and an experiment in the absence of drug was used as control (100% activity). The IC50 values were obtained from activity (%) vs. drug concentration plots. The GSSG concentrations ranged from 0.015 to 0.15 mM for the determination of kinetic parameters with hcbGR. The GSH concentrations ranged from 0.25 to 2.0 mM for the determination of kinetic parameters with hcbGPx. The CDNB concentrations ranged from 0.01 to 0.075 mM for the determination of kinetic parameters with hcbGST. Inhibitors solutions were added to the reaction medium, resulting in 3 different fixed concentrations of inhibitors in 1 ml of total reaction volume. Lineweaver-Burk graphsCitation23 were drawn by using 1/V vs. 1/[S] values and KI constant were calculated from these graphs. Regression analysis graphs were drawn for IC50 using inhibition % values by a statistical package (SPSS-for windows; version 10.0) on a computer.
Results and discussion
GR from human cord blood erythrocytes was purified in this study by ammonium sulphate precipitation, 2′, 5′-ADP Sepharose 4B affinity chromatography and Sephadex G 200 gel filtration chromatography. The purified enzyme was characterized with a specific activity of 12.333 EU/mg proteins, a yield of 32.89% and a purification coefficient of 1312 (). GPx was partially purified by hemolysate preparation, ammonium sulphate precipitation and dialys. The purified preparation was characterized with a specific activity of 0.0958 EU/mg proteins, a yield of 43.9% and a purification coefficient of 13.5 (). GST was purified by GSH Sepharose 4B affinity chromatography. The purified enzyme had a specific activity of 11.80 EU/mg proteins, a yield of 92.55% and a purification coefficient of 4916 (). Inhibition effects of the drugs were tested under in vitro conditions; IC50 values were calculated by Activity (%)-[Inhibitor] graphs and are given in . KI values were calculated from Lineweaver-Burk graphsCitation23 and are given in .
Table 4. Ki and IC50 values obtained from regression analysis graphs for hcbGR, hcbGST and hcbGPx in the presence of different drugs.
The undesirable biologic effects of oxidative agents, such as free radical and reactive oxygen species (ROS), are eliminated by enzymatic and nonenzymatic antioxidant defense systems. Enzymatic defense is provided by many enzyme systems such as glutathione reductase, glutathione peroxidase, glutathione S-transferase, superoxide dismutase, catalase, aldoketoreductase and DNA repair enzymesCitation24–28. The products of oxidative damage initiated by hydroxyl radicals like 4-hydroxyalkenals (membrane lipid peroxides) and base propenals (products of oxidative DNA degradation) are highly cytotoxic. Glutathione S-transferases (GSTs) detoxify such endogenously produced electrophiles by conjugation with glutathione (GSH) as well as by acting as glutathione peroxidases to detoxify toxic base propenals like thymidine hydroperoxideCitation29. Particularly, GR is essential for the maintenance of cellular glutathione in its reduced form, which is highly nucleophilic for many reactive electrophilsCitation30,Citation31.
Numerous studies have demonstrated that oxidative stress is a key pathogenic factor in the development of illness complications. Antioxidant enzymes constitute a supportive team of enzymes which provide defense against the reactive intermediates of dioxygen reduction. These enzymes are cooperative in several aspectsCitation32,Citation33. Our groups recently investigated the interaction of human erythrocyte GR enzyme with several types of analgesic, anaesthetic drugs and antibiotics as well as metal ionsCitation5,Citation9–15.
It is well-known that many chemicals and drugs affect the metabolism via enzyme activities and the results may be hazardous. Thus, drug enzyme interactions should be well-characterized. Although there is a lack of studies regarding drug-enzyme interactions, the investigations on this issue is increasing day by day. Here we purified the GR and GST enzymes with simple chromatographic methods and GPx partially; and determined inhibitory potentials of commonly used drugs. As seen in , all drugs showed inhibitory action on these enzymes. Cefuroxime was the most powerful inhibitor for these enzymes. The second most potent inhibitor was ceftriaxone with the KI values of 17.34, 34.1, and 35.41 µM for hcbGR, hcbGPx and hcbGST, respectively. Ceftizoxime caused relatively lower inhibition on hcbGR but much lower on hcbGST and hcb GPx. Cefoperazone displayed very low inhibition with KI values of 28.72 µM for hcbGR, 267.3 µM for hcbGST and 244.8 µM for hcbGPx, respectively. Overall data indicates that cefuroxime and ceftriaxone showed relatively higher inhibitory effect as compared with others and that these drugs inhibit the enzymes in non-competitive manner. This result is not surprising because molecular structures of the drugs are not similar to physiological substrates of the enzymes (). The drugs are most likely interacting with the regions other than the active site, probably with the sulphydril groups as they are quite bulky. As mentioned above, small molecule (especially drug)-enzyme interaction studies have gained a great interest over the recent yearsCitation34. Our study is thus a contribution to the literature data on the inhibiton of GR, GPx and GST. Our study also constitutes a starting point for the enzymatic evaluation of cord blood and provides useful information for further kinetic investigations. Moreover, findings of this investigation help to identify possible undesired impacts of these drugs, in the case of uncontrolled usage, for pharmacological and medical applications.
Declaration of interest
The authors report no declarations of interest
References
- Chen YB, Hochberg EP, Feng Y, Neuberg D, Rawal B, Motyckova G et al. Characteristics and outcomes after autologous stem cell transplant for patients with relapsed or refractory diffuse large B-cell lymphoma who failed initial rituximab, cyclophosphamide, adriamycin, vincristine, and prednisone therapy compared to patients who failed cyclophosphamide, adriamycin, vincristine, and prednisone. Leuk Lymphoma 2010;51:789–796.
- Cairo MS, Wagner JE. Placental and/or umbilical cord blood: an alternative source of hematopoietic stem cells for transplantation. Blood 1997;90:4665–4678.
- Slivka A, Mytilineou C, Cohen G. Histochemical evaluation of glutathione in brain. Brain Res 1987;409:275–284.
- Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 2000;267:4912–4916.
- Senturk M, Irfan Kufrevioglu O, Ciftci M. Effects of some analgesic anaesthetic drugs on human erythrocyte glutathione reductase: an in vitro study. J Enzyme Inhib Med Chem 2009;24:420–424.
- Chouchane S, Snow ET. In vitro effect of arsenical compounds on glutathione-related enzymes. Chem Res Toxicol 2001;14:517–522.
- Awasthi YC, Beutler E, Srivastava SK. Purification and properties of human erythrocyte glutathione peroxidase. J Biol Chem 1975;250:5144–5149.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–7139.
- Coban TA, Senturk M, Ciftci M, Kufrevioglu OI. Effects of some metal ions on human erythrocyte glutathione reductase: an in vitro study. Protein Pept Lett 2007;14:1027–1030.
- Sentürk M, Gülçin I, Ciftci M, Küfrevioglu OI. Dantrolene inhibits human erythrocyte glutathione reductase. Biol Pharm Bull 2008;31:2036–2039.
- Senturk M, Kufrevioglu OI, Ciftci M. Effects of some antibiotics on human erythrocyte glutathione reductase: an in vitro study. J Enzyme Inhib Med Chem 2008;23:144–148.
- Tekman B, Ozdemir H, Senturk M, Ciftci M. Purification and characterization of glutathione reductase from rainbow trout (Oncorhynchus mykiss) liver and inhibition effects of metal ions on enzyme activity. Comp Biochem Physiol C Toxicol Pharmacol 2008;148:117–121.
- Sentürk M, Talaz O, Ekinci D, Cavdar H, Küfrevioglu OI. In vitro inhibition of human erythrocyte glutathione reductase by some new organic nitrates. Bioorg Med Chem Lett 2009;19:3661–3663.
- Ceyhun SB, Sentürk M, Ekinci D, Erdogan O, Ciltas A, Kocaman EM. Deltamethrin attenuates antioxidant defense system and induces the expression of heat shock protein 70 in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:215–223.
- Akkemik E, Senturk M, Ozgeris FB, Taser P, Ciftci M. In vitro effects of some drugs on human erythrocyte glutathione reductase. Turk J Med Sci 2011;41;235–241.
- Pichichero ME. Cephalosporins can be prescribed safely for penicillin-allergic patients. J Fam Pract 2006;55:106–112.
- Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J et al. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med 1994;179:493–502.
- Beutler E. Red Cell Metabolism. A Manual of Biochemical Methods. Orlando: Grune and Stratton Inc 1984;134.
- Simons PC, Vander Jagt DL. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal Biochem 1977;82:334–341.
- Güvercin S, Erat M, Sakiroglu H. Determination of some kinetic and characteristic properties of glutathione S-transferase from bovine erythrocytes. Protein Pept Lett 2008;15:6–12.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Laemmli DK. Clevage of structual proteins during in assembly of the head of bacteriophage T. Nature 1970;227:680–683.
- Lineweaver H, Burk DJ. The Determination of Enzyme Dissociation Constants. Am Chem Soc 1934;56:658–666.
- Ekinci D, Sentürk M, Beydemir S, Küfrevioglu OI, Supuran CT. An alternative purification method for human serum paraoxonase 1 and its interactions with sulfonamides. Chem Biol Drug Des 2010;76:552–558.
- Ekinci D, Ceyhun SB, Sentürk M, Erdem D, Küfrevioglu OI, Supuran CT. Characterization and anions inhibition studies of an a-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–748.
- Cankaya M, Aktas M, Kuzucu M, Gül I, Coban TA. Effects of some drugs on human cord blood erythrocyte carbonic anhydrases I and II: an in vitro study. J Enzyme Inhib Med Chem 2012. doi:10.3109/14756366.2011.604852.
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem 1983;52:711–760.
- Çakmak R, Durdagi S, Ekinci D, Sentürk M, Topal G. Design, synthesis and biological evaluation of novel nitroaromatic compounds as potent glutathione reductase inhibitors. Bioorg Med Chem Lett 2011;21:5398–5402.
- Marrs KA. The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 1996;47:127–158.
- Carlberg I, Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Enzyme Inhib Med Chem 2012. doi:10.3109/14756366.2011.615745.
- Ekinci D, Sentürk M. Assesment of metal inhibition of antioxidant enzyme glutathione reductase from rainbow trout liver. J Enzyme Inhib Med Chem 2011.
- Kaplowitz N, Aw TY, Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol 1985;25:715–744.
- Black SM, Wolf CR. The role of glutathione-dependent enzymes in drug resistance. Pharmacol Ther 1991;51:139–154.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.