Abstract
In this work, we synthesized fourteen different compounds which contain hydrazone bridged thiazole and pyrrole rings. For this purpose, pyrrole-2-carboxaldehydes were reacted directly with thiosemicarbazide in ethanol and then obtained thiosemicarbazones were condensed with α-bromoacetophenone derivatives (Hantzsch reaction) to give 1-substituted pyrrole-2-carboxaldehyde [4-(4-substituted phenyl)-1,3-thiazol-2-yl] hydrazones. The structures of the obtained compounds were elucidated by using IR, 1H-NMR and FAB+-MS spectral data and elemental analyses results. All of the compounds were screened for their antibacterial and antifungal activities against twelve different microorganisms by using microbroth dilution method. Ketoconazole and chloramphenicol were used as standard drugs. All of the compounds showed good activity against Staphylococcus aureus and Enterococcus faecalis.
Introduction
Throughout history, there has been a continual battle between humans and the multitude of microorganisms that causes infections and diseasesCitation1. The high incidence of microbial disorders due to the difficulties in antimicrobial prophylaxis and treatment, the rapid emergence of new infections, the threat of multidrug-resistant microorganisms against current antibiotics, and many induced side effects due to widespread use of antimicrobial agents are major problems to overcome the common pathogens. Thus, there is a need to search for new and efficacious antimicrobial agents for the treatment of resistant infectionsCitation2–7.
Among various heterocycles that have been explored for developing pharmaceutically important molecules, thiazoles, fused thiazoles and thiazoles linked to various heterocylic rings through different linkages have recently attracted great attentionCitation8. Thiazoles and their derivatives have concerned continuing interest over the years because of their varied biological activities such as antiallergic, antihypertensive, antiinflammatory, antischizophrenic, antibacterial, anti-HIV, and FabH inhibitorsCitation9. It has also been reported in the literature that phenyl-thiazole analogues possess efficient antifungal activityCitation10. Moreover, thiazolylhydrazinesCitation11 and (4-aryl-thiazol-2-yl)hydrazinesCitation12 have been shown to exhibit significant antimicrobial activity. These studies confirmed that thiazole ring is a good pharmacophore group for the design of bioactive moleculesCitation13 which is also act as a bioisoster of the imidazole ringCitation14,Citation15.
Hydrazone is a versatile moietyCitation16,Citation17 that has an obvious role especially in antimicrobial activityCitation18. Additionally, the pyrrole containing heterocyclic compounds have attracted attention particularly as antimicrobial agentsCitation19,Citation20.
For these reasons, in this paper we report synthesis and biological evaluation of a new series of 2,4-disubstituted-1,3-thiazoles bearing pyrrole ring on double bond C=N and a 4′-substituted phenyl in position C4 of thiazole nucleus, respectively.
Experimental
Chemistry
All chemicals were purchased from Sigma-Aldrich Chemical Co. All melting points (m.p.) were determined by Electrothermal 9100 digital melting point apparatus and were uncorrected. Spectroscopic data were recorded with the following instruments: 1H-NMR, Bruker 400 MHz spectrometer; MS-FAB, VG Quattro Mass spectrometer and Elemental analyses were performed on a Perkin Elmer EAL 240 elemental analyser (Perkin-Elmer, Norwalk, CT, USA).
Preparation of 1-substituted pyrrol-2-carbaldehyde thiosemicarbazones (1a, 1b)
A mixture of thiosemicarbazide (0.27 g, 3 mmol) and 2-formylpyrrole or N-methyl-2-formylpyrrole (3 mmol) in ethanol (20 mL) was refluxed for 2 h. The progress of the reaction was monitored by TLC. The resulting mixture was cooled, poured into ice water, filtered and then recrystallized from ethanol to afford thiosemicarbazones (1a, 1b)Citation21.
Preparation of 1-substituted pyrrole-2-carboxaldehyde [4-(4-substituted phenyl)-1,3-thiazol-2-yl]) hydrazones (2a–2n)
An anhydrous ethanolic solution (10 mL) of 1a or 1b (0.80 mmol) and α-bromoacetophenone (0.16 g, 0.80 mmol) was stirred at room temperature, until all the thiosemicarbazone disappeared in TLC. Then the deposit was filtered and recrystallized from ethanol to give target compounds (2a–2n).
Pyrrole-2-carboxaldehyde (4- phenyl-1,3-thiazol-2-yl) hydrazone (2a):
Yield: 90%. M.p. 125°C. IR (KBr) νmax(cm−1): 3380-3122 (N-H), 3056 (aromatic C-H), 1619-1483 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 6.16 (brs, 1H, pyrrole-H), 6.50 (brs, 1H, pyrrole-H), 6.97 (brs, 1H, pyrrole-H), 7.33 (s, 1H, C5-H thiazole), 7.35–7.47 (m, 3H, Ar-H), 7.81–7.83 (m, 2H, Ar-H), 8.07 (s, 1H, -CH=N-N), 11.35 (s, 1H, pyrrole N-H), 11.80 (brs, 1H, C=N-NH, D2O exch.).For C14H12N4S calculated: 62.66 % C, 4.51 % H, 20.88 % N; found: 62.67 % C, 4.53 % H, 20.86 % N. MS (FAB) [M+1]+: m/z 267.
Pyrrole-2-carboxaldehyde [4-(4-methylphenyl)-1,3-thiazol-2-yl] hydrazone (2b):
Yield: 82%. M.p. 156°C. IR (KBr) νmax(cm−1): 3385-3150 (N-H), 3026 (aromatic C-H), 1610-1477 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 2.32 (s, 3H, C-CH3), 6.12 (brs, 1H, pyrrole-H), 6.40 (brs, 1H, pyrrole-H), 6.89 (brs, 1H, pyrrole-H), 7.17 (s, 1H, C5-H thiazole), 7.21 (d, 2H, Ar-H, J = 8.4 Hz), 7.72 (d, 2H, Ar-H, J = 8 Hz), 7.91 (s, 1H, -CH=N-N), 11.21 (s, 1H, pyrrole N-H), 11.79 (brs, 1H, C=N-NH, D2O exch.). For C15H14N4S calculated: 63.80 % C, 5.00 % H, 19.84 % N; found: 63.82 % C, 5.02 % H, 19.81 % N. MS (FAB) [M+1]+: m/z 283.
Pyrrole-2-carboxaldehyde [4-(4-methoxyphenyl)-1,3-thiazol-2-yl] hydrazone (2c):
Yield: 85%. M.p. 155 °C. IR (KBr) νmax(cm−1): 3369-3135 (N-H), 3021 (aromatic C-H), 1598-1487 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 3.78 (s, 3H, -OCH3), 6.13 (brs, 1H, pyrrole-H), 6.41 (brs, 1H, pyrrole-H), 6.90 (brs, 1H, pyrrole-H), 6.97 (d, 2H, Ar-H, J = 8.5 Hz), 7.08 (s, 1H, C5-H thiazole), 7.77 (d, 2H, Ar-H, J = 8 Hz), 7.93 (s, 1H, -CH=N-N), 11.22 (s, 1H, pyrrole N-H), 11.75 (brs, 1H, C=N-NH, D2O exch.). For C15H14N4OS calculated: 60.38 % C, 4.73 % H, 18.78 % N; found: 60.34 % C, 4.70 % H, 18.75 % N. MS (FAB) [M+1]+: m/z 299.
Pyrrole-2-carboxaldehyde [4-(4-bromophenyl)-1,3-thiazol-2-yl) hydrazone (2d):
Yield: 80%. M.p. 170°C. IR (KBr) νmax(cm−1): 3349-3126 (N-H), 3016 (aromatic C-H), 1592-1479 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 6.14 (brs, 1H, pyrrole-H), 6.46 (brs, 1H, pyrrole-H), 6.94 (brs, 1H, pyrrole-H), 7.38 (s, 1H, C5-H thiazole), 7.62 (d, 2H, Ar-H, J = 8.8 Hz), 7.78 (d, 2H, Ar-H, J = 8.8 Hz), 8.01 (s, 1H, -CH=N-N), 11.31 (s, 1H, pyrrole N-H), 11.82 (brs, 1H, C=N-NH, D2O exch.). For C14H11BrN4S calculated: 48.43 % C, 3.19 % H, 16.14 % N; found: 48.41 % C, 3.22 % H, 16.15 % N. MS (FAB) [M+1]+: m/z 348.
Pyrrole-2-carboxaldehyde [4-(4-chlorophenyl)-1,3-thiazol-2-yl] hydrazone (2e):
Yield: 81%. M.p. 200°C. IR (KBr) νmax(cm−1): 3386-3220 (N-H), 3027 (aromatic C-H), 1609-1475 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 6.15 (brs, 1H, pyrrole-H), 6.49 (brs, 1H, pyrrole-H), 6.97 (brs, 1H, pyrrole-H), 7.38 (s, 1H, C5-H thiazole), 7.51 (d, 2H, Ar-H, J = 8.8 Hz), 7.85 (d, 2H, Ar-H, J = 8.4 Hz), 8.08 (s, 1H, -CH=N-N), 11.36 (s, 1H, pyrrole N-H), 11.72 (brs, 1H, C=N-NH, D2O exch.). For C14H11ClN4S calculated: 55.53 % C, 3.66 % H, 18.50 % N; found: 55.52 % C, 3.65 % H, 18.52 % N. MS (FAB) [M+1]+: m/z 303.5.
Pyrrole-2-carboxaldehyde [4-(4-fluorophenyl)-1,3-thiazol-2-yl] hydrazone (2f):
Yield: 88%. M.p. 166°C. IR (KBr) νmax(cm−1): 3376-3145 (N-H), 3015 (aromatic C-H), 1611-1483 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 6.12 (brs, 1H, pyrrole-H), 6.41 (brs, 1H, pyrrole-H), 6.90 (brs, 1H, pyrrole-H), 7.21–7.25 (m, 3H, Ar-H and C5-H thiazole), 7.87 (d, 2H, Ar-H, J = 8.1 Hz), 7.92 (s, 1H, -CH=N-N), 11.21 (s, 1H, pyrrole N-H), 11.79 (brs, 1H, C=N-NH, D2O exch.).For C14H11FN4S calculated: 58.73 % C, 3.87 % H, 19.57 % N; found: 58.72 % C, 3.85 % H, 19.55 % N. MS (FAB) [M+1]+: m/z 287.
Pyrrole-2-carboxaldehyde [4-(4-nitrophenyl)-1,3-thiazol-2-yl] hydrazone (2g):
Yield: 86%. M.p. 200°C. IR (KBr) νmax(cm−1): 3368-3190 (N-H), 3023 (aromatic C-H), 1612-1449 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 6.15 (brs, 1H, pyrrole-H), 6.44 (brs, 1H, pyrrole-H), 6.93 (brs, 1H, pyrrole-H), 7.67 (s, 1H, C5-H thiazole), 7.98 (s, 1H, -CH=N-N), 8.30 (d, 2H, Ar-H, J = 8.4 Hz), 8.33 (d, 2H, Ar-H, J = 9.2 Hz), 11.28 (s, 1H, pyrrole N-H), 11.82 (brs, 1H, C=N-NH, D2O exch.). For C14H11N5O2S calculated: 53.66 % C, 3.54 % H, 22.35 % N; found: 53.62 % C, 3.55 % H, 22.37 % N. MS (FAB) [M+1]+: m/z 314.
1-Methylpyrrole-2-carboxaldehyde (4- phenyl-1,3-thiazol-2-yl) hydrazone (2h):
Yield: 76%. M.p. 224°C. IR (KBr) νmax(cm−1): 3368-3156 (N-H), 3013 (aromatic C-H), 2969 (aliphatic C-H), 1589-1457 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 3.87 (s, 3H, N-CH3), 6.17 (brs, 1H, pyrrole-H), 6.51 (brs, 1H, pyrrole-H), 7.01 (brs, 1H, pyrrole-H), 7.33 (s, 1H, C5-H thiazole), 7.36–7.47 (m, 3H, Ar-H), 7.82 (d, 2H, Ar-H, J = 8 Hz), 8.18 (s, 1H, -CH=N-N), 11.91 (brs, 1H, C=N-NH, D2O exch.).For C15H14N4S calculated: 63.80 % C, 5.00 % H, 19.84 % N; found: 63.82 % C, 5.04 % H, 19.83 % N. MS (FAB) [M+1]+: m/z 283.
1-Methylpyrrole-2-carboxaldehyde [4-(4-methylphenyl)-1,3-thiazol-2-yl] hydrazone (2i):
Yield: 81%. M.p. 182°C. IR (KBr) νmax(cm−1): 3389-3179 (N-H), 3024 (aromatic C-H), 2987 (aliphatic C-H), 1585-1446 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 2.33 (s, 3H, C-CH3), 3.87 (s, 3H, N-CH3), 6.10 (brs, 1H, pyrrole-H), 6.48 (brs, 1H, pyrrole-H), 6.98 (brs, 1H, pyrrole-H), 7.23 (s, 1H, C5-H thiazole), 7.24 (d, 2H, Ar-H, J = 8 Hz), 7.71 (d, 2H, Ar-H, J = 8 Hz), 8.12 (s, 1H, -CH=N-N), 11.92 (brs, 1H, C=N-NH, D2O exch.). For C16H16N4S calculated: 64.84 % C, 5.44 % H, 18.90 % N; found: 64.82 % C, 5.41 % H, 18.93 % N. MS (FAB) [M+1]+: m/z 297.
1-Methylpyrrole-2-carboxaldehyde [4-(4-methoxyphenyl)-1,3-thiazol-2-yl] hydrazone (2j):
Yield: 82%. M.p. 209°C. IR (KBr) νmax(cm−1): 3388-3169 (N-H), 3009 (aromatic C-H), 2974 (aliphatic C-H), 1579-1477 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 3.80 (s, 3H, O-CH3), 3.87 (s, 3H, N-CH3), 6.12 (brs, 1H, pyrrole-H), 6.52 (brs, 1H, pyrrole-H), 7.00-7.02 (m, 3H, Ar-H and pyrrole-H), 7.16 (s, 1H, C5-H thiazole), 7.75 (d, 2H, Ar-H, J = 8 Hz), 8.18 (s, 1H, -CH=N-N), 11.89 (brs, 1H, C=N-NH, D2O exch.). For C16H16N4OS calculated: 61.52 % C, 5.16 % H, 17.93 % N; found: 61.53 % C, 5.18 % H, 17.96 % N. MS (FAB) [M+1]+: m/z 313.
1-Methylpyrrole-2-carboxaldehyde [4-(4-bromophenyl)-1,3-thiazol-2-yl] hydrazone (2k):
Yield: 76%. M.p. 214°C. IR (KBr) νmax(cm−1): 3392-3189 (N-H), 3015 (aromatic C-H), 2986 (aliphatic C-H), 1579-1454 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 3.87 (s, 3H, N-CH3), 6.09 (brs, 1H, pyrrole-H), 6.45 (brs, 1H, pyrrole-H), 6.97 (brs, 1H, pyrrole-H), 7.36 (s, 1H, C5-H thiazole), 7.61 (d, 2H, Ar-H, J = 8.4 Hz), 7.78 (d, 2H, Ar-H, J = 8.8 Hz), 8.08 (s, 1H, -CH=N-N), 11.88 (brs, 1H, C=N-NH, D2O exch.). For C15H13BrN4S calculated: 49.87 % C, 3.63 % H, 15.51 % N; found: 49.86 % C, 3.65 % H, 15.53 % N. MS (FAB) [M+1]+: m/z 362.
1-Methylpyrrole-2-carboxaldehyde [4-(4-chlorophenyl)-1,3-thiazol-2-yl] hydrazone (2l):
Yield: 85%. M.p. 202°C. IR (KBr) νmax(cm−1): 3369-3156 (N-H), 3033 (aromatic C-H), 2967 (aliphatic C-H), 1589-1457 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 3.87 (s, 3H, N-CH3), 6.09 (brs, 1H, pyrrole-H), 6.45 (brs, 1H, pyrrole-H), 6.96 (brs, 1H, pyrrole-H), 7.35 (s, 1H, C5-H thiazole), 7.47 (d, 2H, Ar-H, J = 8.4 Hz), 7.86 (d, 2H, Ar-H, J=8.8 Hz), 8.11 (s, 1H, -CH=N-N), 11.89 (brs, 1H, C=N-NH, D2O exch.). For C15H13ClN4S calculated: 56.87 % C, 4.14 % H, 17.68 % N; found: 56.89 % C, 4.15 % H, 17.69 % N. MS (FAB) [M+1]+: m/z 317.5.
1-Methylpyrrole-2-carboxaldehyde [4-(4-fluorophenyl)-1,3-thiazol-2-yl] hydrazone (2m):
Yield: 73%. M.p. 222°C. IR (KBr) νmax(cm−1): 3318-3196 (N-H), 3024 (aromatic C-H), 2979 (aliphatic C-H), 1593-1446 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 3.87 (s, 3H, N-CH3), 6.11 (brs, 1H, pyrrole-H), 6.50 (brs, 1H, pyrrole-H), 7.00 (brs, 1H, pyrrole-H), 7.26-7.30 (m, 3H, Ar-H and C5-H thiazole), 7.88 (d, 2H, Ar-H, J = 8 Hz), 8.16 (s, 1H, -CH=N-N), 11.82 (brs, 1H, C=N-NH, D2O exch.). For C15H13FN4S calculated: 59.98 % C, 4.36 % H, 18.65 % N; found: 59.97 % C, 4.38 % H, 18.69 % N. MS (FAB) [M+1]+: m/z 301.
1-Methylpyrrole-2-carboxaldehyde [4-(4-nitrophenyl)-1,3-thiazol-2-yl] hydrazone (2n):
Yield: 72%. M.p. 199°C. IR (KBr) νmax(cm−1): 3368-3186 (N-H), 3028 (aromatic C-H), 2989 (aliphatic C-H), 1610-1446 (C=C and C=N).1H NMR (DMSO-d6) δ ppm: 3.87 (s, 3H, N-CH3), 6.09 (brs, 1H, pyrrole-H), 6.43 (brs, 1H, pyrrole-H), 6.95 (brs, 1H, pyrrole-H), 7.64 (s, 1H, C5-H thiazole), 8.08 (s, 1H, -CH=N-N), 8.09 (d, 2H, Ar-H, J = 8.8 Hz), 8.26 (d, 2H, Ar-H, J = 8.8 Hz), 11.92 (brs, 1H, C=N-NH, D2O exch.). For C15H13N5O2S calculated: 55.03 % C, 4.00 % H, 21.39 % N; found: 55.02 % C, 3.95 % H, 21.35 % N. MS (FAB) [M+1]+: m/z 328.
Microbiology
The study was designed to compare MICs obtained by the CLSI reference M7–A7 broth microdilution methodCitation22,Citation23. MIC readings were performed twice for each chemical agent. Final products were tested for their in-vitro growth inhibitory activity against human pathogenic as Gram-positive bacteria; Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29212) and Listeria monocytogenes (obtained from Faculty of Pharmacy Anadolu University, Eskisehir, Turkey), as Gram-negative bacteria; Pseudomonas aeruginosa (ATCC 27853), Klebsiella pneumoniae (ATCC 13883), Escherichia coli (ATCC 35218), Salmonella typhimurium (NRRL B- 4420) and Yersinia entercolitica (ATCC 35669) and yeast as Candida albicans, Candida glabrata (ATCC 36583), Candida krusei (obtained from Faculty of Medicine Osmangazi University, Eskisehir, Turkey) and Candida parapsilosis (ATCC 22019). Chloramphenicol and ketoconazole were used as control drugs.
In order to ensure that the solvent per se had no effect on bacteria or yeast growth, a control test was also performed containing inoculated broth supplemented with only DMSO at the same dilutions used in our experiments and found inactive in culture medium.
Antimicrobial assay
The cultures were obtained from Mueller–Hinton broth (Difco) for the bacterial strains after overnight incubation at 35 ± 1°C. The yeasts were maintained in Sabouroud dextrose broth (Difco) after overnight incubation 35 ± 1°C. The inocula of test microorganisms adjusted to match the turbidity of a Mac Farland 0.5 standard tube as determined with a spectrophotometer and the final inoculum size was 0.5–2.5 × 105 CFU/mL for antibacterial and antifungal assays. Testing was carried out in Mueller–Hinton broth and Sabouroud dextrose broth (Difco) at pH 7 and the two-fold serial dilutions technique was applied. The last well on the microplates containing only inoculated broth was kept as controls and the last well with no growth of microorganism was recorded to represent the MIC expressed in μg/mL. For both the antibacterial and antifungal assays the compounds were dissolved in DMSO. Further dilutions of the compounds and standard drugs in test medium were prepared at the required quantities of 800, 400, 200, 100, 50, 25, 12.5, 6.25, 3.125, 1.5625 μg/mL concentrations with Mueller–Hinton broth and Sabouroud dextrose broth. Each experiment in the antimicrobial assays was replicated twice in order to define the MIC values. Chloramphenicol and ketoconazole were used as control drugs.
Results and discussions
Chemistry
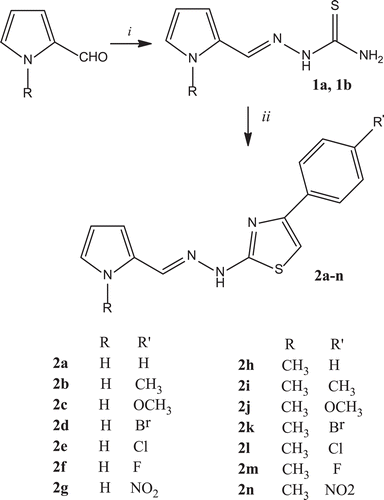
In this work, we synthesized fourteen different compounds, which contain hydrazone bridged thiazole and pyrrole rings. Pyrrole-2-carboxaldehyde and N-methylpyrrole-2-carboxaldehyde were reacted directly with thiosemicarbazide in ethanol by refluxing and the obtained thiosemicarbazones (1a, 1b) subsequently were condensed with α-bromoacetophenone derivatives (Hantzsch reaction) to give 1-substituted pyrrole-2-carboxaldehyde [4-(4-substituted phenyl)-1,3-thiazol-2-yl] hydrazones (2a-n) as shown in . All synthesized compounds were fully characterized by analytical and spectral data.
The FT-IR spectra of the final products showed characteristic absorption bands at 3120–3330 cm−1 for –NH– and at 1590–1670 cm−1 for azomethine group (–CH=N–). The 1H-NMR spectra of compounds showed signals at δ 7.91–8.18 and δ 11.72–11.92 corresponding to azomethine (–CH=N–) proton and hydrazide (NH) proton, respectively. The broad singlet peak seen at δ 11.21–11.36 indicated the pyrrole N-H proton for the compounds 2a-g. The C5-H proton of the thiazole was observed as a singlet at δ 7.08–7.67. The appearance of a pair of doublets and/or multiplets at δ 6.97–8.33 was due to the aromatic protons of phenyl ring. The C-H protons of the pyrrole ring were resonated at δ 6.09–7.01 region as broad singlets as expected. M+1 peaks in FAB-MS spectra were in agreement with the calculated molecular weight of the target compounds (2a–2n). Elemental analysis results for C, H, and N elements were satisfactory with calculated values of the compounds.
Antimicrobial activity
Antimicrobial activity was investigated by finding minimum inhibitory concentration (MIC) of the synthesized compounds and reference agents against S. aureus, L. monocytogenes, E. coli, P. aeruginosa, Y. enterocolitica, E. faecalis, K. pneumoniae S. typhimurium, C. albicans, C. glabrata, C. krusei, C. parapsilosis. Resuts are summarized in .
Table 1. Antimicrobial activities of the compounds (2a-n) (µg/mL).
In general evaluating, it was observed that all of the compounds had higher antimicrobial activity against gram-positive bacteria than gram negative bacteria and fungi.
Most of the tested compounds revealed higher selectivity toward E. faecalis and S. aureus, whereas all of the compounds lacked antibacterial activity against L. monocytogenes among gram positives. Comparing with chloramphenicol, compounds 2d-l had similar MIC value of 12.5 µg/mL against S. aureus. Among all of the strains, E. faecalis was the most susceptible species. While compounds 2a, 2d, 2e, 2i, 2k and 2m showed higher antimicrobial activity, compounds 2f, 2g, 2h, 2j and 2l showed equipotent activity to standard drug against E. faecalis.
With regard to the activity against K. pneumoniae, potential activity was displayed by compound 2m (MIC 50 µg/mL), which were twice as active as chloramphenicol (MIC 100 µg /mL). The compounds 2b, 2c, 2d, 2e, 2h, 2i, 2j, 2k and 2n (MIC 200 µg/mL) displayed half the potency of chloramphenicol, meanwhile compound 2a (MIC 100 µg/mL) showed equal potency to chloramphenicole (MIC 100 µg/mL) against the same organism.
Compound 2m was determined as the most active compound against P. aeruginosa and E. coli. Among other gram negative bacterial strains, Y. enterocolitica and S. typhimurium exhibited resistance all of the tested compounds.
The antifungal activity of the compounds was studied against four Candida species, the most sensitive Candida was established as C. glabrata toward 2a and 2m compounds, which had the same MIC value (50 µg/mL) with ketoconazole. However, there was not any obvious inhibitory effect against other Candida strains.
An insight into the structures of the active compounds revealed that the tested compounds belong two main structure series; pyrrole-2-carboxaldehyde [4-(4-substituted phenyl)-1,3-thiazol-2-yl] hydrazone (2a-g) and 1-methylpyrrole-2-carboxaldehyde [4-(4- substituted phenyl)-1,3-thiazol-2-yl] hydrazone (2h-n). These series differ from each other due to methyl substituent at N-H position of the pyrrole ring. It was observed that methyl substitution (N-CH3) on pyrrole ring influenced the activity essentially. It seemed that most of the compounds bearing methyl substituent on pyrrole moiety had higher activity according to the compounds, which do not contain methyl substitution.
On the other hand, it was determined that substitution of the phenyl moiety at para position also caused changes in activity. In antibacterial activity evaluating, the most active compound was 2e, which includes fluoro substituent on phenyl ring and non-substituted pyrrole. Among the N-methylpyrrole moiety containing compounds (2h-n), the compound 2m, which carries fluoro substituent on phenyl ring displayed notable improvement in the spectrum of both antibacterial and antifungal activity. Furthermore, compound 2m seemed to be much more active than chloramphenicol against E. faecalis and K. pneumoniae bacterial strains.
In antifungal activity evaluating, it was observed that 2a and 2m were the most active compounds. In compounds 2g and 2n, nitro substitution on phenyl moiety at para position caused a reduction in both antibacterial and antifungal activity. Thus, it can be claimed that substitution of phenyl moiety at para position with an electron withdrawing group reduces the antimicrobial activity. Among all of the compounds, there were not any clear inhibitory activity against L. monocytogenes, Y. enterocolitica, S. typhimurium and C. krusei.
Declaration of interest
The authors report no declarations of interest.
References
- Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Med 2006;119:S3–10; discussion S62.
- Moudgal V, Little T, Boikov D, Vazquez JA. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob Agents Chemother 2005;49:767–769.
- Hernandez S, López-Ribot JL, Najvar LK, McCarthy DI, Bocanegra R, Graybill JR. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob Agents Chemother 2004;48:1382–1383.
- Krogh-Madsen M, Arendrup MC, Heslet L, Knudsen JD. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin Infect Dis 2006;42:938–944.
- Spellberg B, Powers JH, Brass EP, Miller LG, Edwards JE Jr.. Trends in antimicrobial drug development: implications for the future. Clin Infect Dis 2004;38:1279–1286.
- Coates A, Hu Y, Bax R, Page C. The future challenges facing the development of new antimicrobial drugs. Nat Rev Drug Discov 2002;1:895–910.
- Shah PM. The need for new therapeutic agents: what is the pipeline? Clin Microbiol Infect 2005;11 Suppl 3:36–42.
- Al-Saadi MS, Faidallah HM, Rostom SA. Synthesis and biological evaluation of some 2,4,5-trisubstituted thiazole derivatives as potential antimicrobial and anticancer agents. Arch Pharm 2008;341:424–434.
- Lv PC, Wang KR, Yang Y, Mao WJ, Chen J, Xiong J et al. Design, synthesis and biological evaluation of novel thiazole derivatives as potent FabH inhibitors. Bioorg Med Chem Lett 2009;19:6750–6754.
- Chimenti F, Bizzarri B, Maccioni E, Secci D, Bolasco A, Fioravanti R et al. Synthesis and in vitro activity of 2-thiazolylhydrazone derivatives compared with the activity of clotrimazole against clinical isolates of Candida spp. Bioorg Med Chem Lett 2007;17:4635–4640.
- Cukurovali A, Yilmaz I, Gur S, Kazaz C. Synthesis, antibacterial and antifungal activity of some new thiazolylhydrazone derivatives containing 3-substituted cyclobutane ring. Eur J Med Chem 2006;41:201–207.
- Bharti SK, Nath G, Tilak R, Singh SK. Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. Eur J Med Chem 2010;45:651–660.
- Nora De Souza MV. Synthesis and biological activity of natural thiazoles: An important class of heterocyclic compounds. J Sulfur Chem 2005;26:429–449.
- De Logu A, Saddi M, Cardia MC, Borgna R, Sanna C, Saddi B et al. In vitro activity of 2-cyclohexylidenhydrazo-4-phenyl-thiazole compared with those of amphotericin B and fluconazole against clinical isolates of Candida spp. and fluconazole-resistant Candida albicans. J Antimicrob Chemother 2005;55:692–698.
- Chimenti F, Bizzarri B, Bolasco A, Secci D, Chimenti P, Granese A et al. Synthesis and biological evaluation of novel 2,4-disubstituted-1,3-thiazoles as anti-Candida spp. agents. Eur J Med Chem 2011;46:378–382.
- Uppal G, Bala S, Kamboj S, Saini M. Therapeutic review exploring antimicrobial potential of hydrazones as promising lead. Der Pharm Chem 2011;31:250–268.
- Rollas S, Küçükgüzel SG. Biological activities of hydrazone derivatives. Molecules 2007;12:1910–1939.
- Vicini P, Zani F, Cozzini P, Doytchinova I. Hydrazones of 1,2-benzisothiazole hydrazides: synthesis, antimicrobial activity and QSAR investigations. Eur J Med Chem 2002;37:553–564.
- Mohamed MS, El-Domany RA, Abd El-Hameed RH. Synthesis of certain pyrrole derivatives as antimicrobial agents. Acta Pharm 2009;59:145–158.
- Idhayadhulla A, Kumar RS, Nasser AJA, Manilal A. Synthesis of some new pyrrole derivatives and their antimicrobial activity. Der Pharm Chem 2011;3:210–218.
- Anderson FE, Duca CJ, Scudi JV. Some heterocyclic thiosemicarbazones. J Am Chem Soc 1951;73:4967–4968.
- CLSI methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically approved standard, CLSI Document M7–A7 (seventh ed.), 1-56238-587-9 (2006)
- CLSI Performance Standards for Antimicrobial Susceptibility Testing 16th Informational Supplement, CLSI document M100-S16, 1-56238-588-7 (2006)