Abstract
A series of some 4-(aza substituted) methylene substituted dihydroxy coumarines were evaluated for their antioxidant and antielastase activities. Different in vitro methodologies such as total reducing power, 1,1-diphenyl-2-picryl-hydrazil (DPPH·) free radical scavenging, ABTS radical scavenging activity were used as antioxidant activity. All the tested compounds exhibited potent free radical scavenging ability and antielastase activites.
Introduction
Antioxidants in biological systems have multiple functions which include protection from oxidative damage and in the major signaling pathways of cells. The major action of antioxidant in cell is to prevent damage caused by the action of reactive oxygen species (ROS), such as superoxide, hydroxyl, peroxide and nitric acid radicals are generated in living organisms during excessive metabolismCitation1. ROS cause extensive oxidative damage to cells leading to age related degenerative disease, cancer, and a wide range of other human diseaseCitation2. Several synthetic antioxidants, such as butylated hydroxy anisole (BHA), butylated hydroxytoluene (BHT), tert-butylhydroquinone (TBHQ) as well as propyl gallate are currently in use. However, their uses have been limited for the fact that they may be responsible for liver damage and carcinogenesisCitation3. For this reason, this problem has been overcome by new synthetic or natural compounds.
Elastase (EC 3.4.21.37), an important granule enzyme, is a serine proteases of the chymotrypsin family that hydrolytically degrades extracellular matrix components such as elastin, proteoglycans, fibronectin and collagen types I-IVCitation4. Elastases is particularly abundant in the lung, skin and arteriesCitation5. Excessive neutrofil elastase activity can lead to severe pathology through the degradation of elastin and collagen in the airways, resulting in microvascular injury and interstitial edemaCitation6. The human neutrophil elastase (HNE) is believed to play an important role in the pathophysiology of an array of inflammatory diseases, including chronic obstructive pulmonary disease (COPD)Citation7, cystic fibrosisCitation8, acute respiratory distress syndromeCitation9, and ischemia/reperfusion injuryCitation10.
Coumarins and their derivatives have been found to exhibit a variety of biological and pharmacological activities and have raised considerable interest because of their potential beneficial effects on human healthCitation11. They have been reported to possess among others: antibioticCitation12, antibacterialsCitation13, antitumorCitation14, antiviral agentsCitation15-17, anticoagulantsCitation18,Citation19, against psoriasisCitation20, antioxidantCitation21, anticancerCitation22,Citation23, anti-inflammatoryCitation24-26, analgesicCitation27 and diuretic propertiesCitation28. Apart from the medicinal applications coumarins are also used as sweetener, fixative of perfumesCitation29, enhancer of natural oils such as lavender, a food additive in combination with vanillin, a flavour/odour stabilizer in tobaccoCitation29, an odour masker in paints and rubber. Owning to the widespread applications, synthetic and biological activity evaluation of coumarins and their derivatives has been a subject of intense investigations.
In this study, we have synthesized some new 4-(aza substituted) methylene substituted dihydroxy coumarines. In the literature there is no data on the antielastase, and antioxidant activities of 4-(aza substituted) methylene substituted dihydroxy coumarines. In this study, antielastase and antioxidant activities of 4-(aza substituted) methylene substituted dihydroxy coumarines were determined for the first time. Their antioxidant activity were assessed by various in vitro assays and compared to the activities of synthetic standard antioxidant compounds.
Materials and methods
Chemicals
All chemical and solvents were of analytical grade.
General
All chemicals and solvents are commercially available and were used after distillation or treatment with drying agents. Mp: cap. melting-point apparatus (Barnstead-Electrothermal 9200, Iowa USA ); uncorrected. IR Spectra: solutions in KBr pellets with a Perkin-Elmer 100.
FTIR spectrometer (Cambridge, England). 1H- and 13C-NMR spectra (in DMSO): 200 (50) MHz Varian spectrometer (Danbury, CT); δ in ppm; Me4Si as the internal standard. Mass spectra: Agilent 6230 TOF (ESI-MS) (CA, USA).
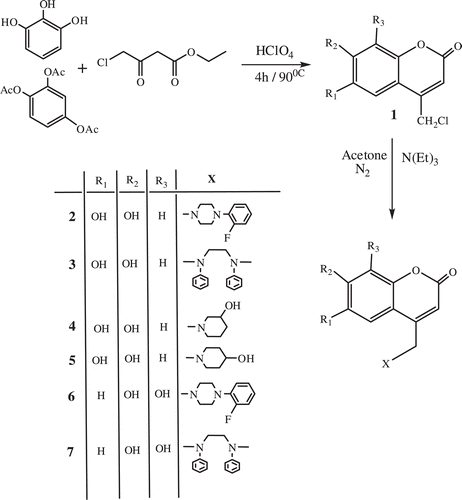
Synthesis of 4-chloromethyl-7,8-dihydroxy-2H-chromen-2-one (1)
A mixture of appropriate phenol & phenylacetate (0.03 mol), ethyl 4-chloroacetoacetate (0.06 mol) and HClO4 (10 mL, 70%) was heated to 90°C for 4 h. The resulting mixture was cooled, diluted with water and the precipitates were collected by filtration. The dried crude product was purified by recrystallization from ethanolCitation30.
Synthesis of coumarin compounds
A mixture of 4-chloromethyl-dihydroxy coumarin (0.010 mol), amino compound (0.010 mol) and acetone (300 mL) was stirred to room temperature under N2 for 24 h. Triethyl amine (0.010 mol) added and stirred for 1 h and then the mixture was refluxed for 3 h. After the removal of solution by evaporation, the resulting mixture was triturated with water, and the precipitates were collected by filtration. The crude product was dried under vacuumCitation30.
The DPPH radical scavenging activity of the dihydroxy coumarin derivatives was measured according to the procedure described by Brand-Williams et al. (1995)Citation31. The ABTS radical scavenging activities of the coumarin derivatives were measured according to the procedure described by Arnao et al. (2001)Citation32. The reducing powers of the dihydroxy coumarin derivatives were determined according to the method described by Oyaizu (1986)Citation33. Cuprac reducing antioxidant capacity of the dihydroxy coumarin derivatives was determined according to the method described by Apak et al. (2006)Citation34. The antioxidant and radical scavenging activities of the compounds were compared with standards. The elastase inhibitor activity was examined using N-succinyl-Ala-Ala-Ala-p-nitroanilide (STANA) as the substrate and by the measuring of the release of p-nitroaniline at 410 nmCitation35.
Result and discussion
The 4-azasubstituted methylene 6,7-dihydroxy and 7,8-dihydroxy coumarin compounds were synthesized with reaction of 4-chloromethyl-6,7-dihydroxy coumarin or 4-chloromethyl-7,8-dihydroxy coumarin and various amino compounds. Both 4-azasubstituted methylene 6,7-dihydroxy coumarin and 7,8-dihydroxy coumarin derivatives were prepared in good yields. The synthesized original products were identified with 1H-NMR, 13C-NMR and mass spectrometry as displayed on .
4-((4-(2-Fluorophenyl)piperazin-1-yl)methyl)-7,8-dihydroxy-2H-chromen-2-one (2): Yield (79% ); m.p. 188-190°C; IR: 1053.83 (C-O), 1320.83 (C-N), 1695.21(C=O), 3322.83(Ar-OH); 1H-NMR (DMSO) δ (ppm): 10.34 (s), 9.42 (s), 7.15 (d), 6.82 (d), 6.39 (s), 4.91 (s), 3.41 (s); 13C-NMR (DMSO) δ (ppm): 160.81, 157.95, 152.08, 150.42, 149.93, 144.33, 133.13, 132.96, 116.14, 114.94, 113.02, 111.61, 110.78, 107.05, 59.75, 42.17, 41.37; ESI-MS(TOF) (M+H)+:371.0623 Anal. Calc. For (C20H19FN2O4): 370.13.
4,4'-(Ethane-1,2-diylbis(phenylazanediyl))bis(methylene)bis(7,8-dihydroxy-2H-chromen-2-one) (3): Yield (79%); m.p. 125°C; IR: 1040.43 (C-O), 1603.86 (aromatic ring), 1671.44 (C=O), 3270.15 (Ar-OH); 1H-NMR (DMSO) δ (ppm): 9.31 (t), 9.06 (d), 8.8 (dd), 8.58 (s), 7.09 (s), 5.43 (s), 4.23 (s); 13C-NMR (DMSO) δ (ppm): 160.97, 160.83, 153.92, 153.65, 152.07, 150.42, 150.27, 149.93, 147.84, 147.59, 144.33, 144.27, 143.08, 133.15, 130.33, 130.01, 130.15, 123.04, 118.04, 117.52, 116.12, 115.32, 113.05, 112.54, 111.61, 111.47, 111.38, 110.78, 59.76, 51.30, 44.90, ESI-MS(TOF) (M-H)+:591.3021 Anal. Calc. For (C34H28N2O8): 592.18.
7,8-Dihydroxy-4-((3-hydroxypiperidin-1-yl)methyl)-2H-chromen-2-one (4): Yield (71% ); m.p. 122°C; IR: 1043.12 (C-O), 1673.76 (C=O), 3252.00 (Ar-OH); 1H-NMR (DMSO) δ (ppm): 10.25 (s), 9.42 (s),7.15 (d), 6.82 (d),6.40 (s),4.92 (s), 2.48 (s), 2.33 (s), 1.99 (s), 1.20 (s), 1.16 (s); 13C-NMR (DMSO-d6) δ (ppm): 161.30, 160.83, 157.98, 152.11, 150.42, 144.33, 133.14, 132.96, 116.17, 113.03, 111.61, 110.78, 59.73, 43.66, 43.01, 42.18, 41.67, 41.37; ESI-MS(TOF) (M+H)+: 292.0467 Anal. Calc. For (C15H17NO5): 291.11.
7,8-Dihydroxy-4-((4-hydroxypiperidin-1-yl)methyl)-2H-chromen-2-one (5): Yield (72% ); m.p. 117°C; IR: 1044.42 (C-O), 1702.66 (C=O) 3328.46 (Ar-OH),; 1H-NMR (DMSO) δ (ppm): 10.17 (s), 9.40 (s), 7.20 (t), 6.81 (t), 6.39 (s), 6.24 (s), 4.91 (s), 4.66 (s), 3.64 (s), 2.76 (s), 2.48 (s), 2.24(s), 1.70 (s), 1.40 (d), 1.15 (t); 13C-NMR (DMSO) δ (ppm): 161.33, 160.84, 160.69, 157.99, 152.10, 150.42, 149.93, 144.27, 133.15, 133.06, 116.36, 116.14, 114.94, 113.05, 112.91, 112.12, 110.78, 107.02, 59.74, 42.18, 41.35, 31.14; ESI-MS(TOF) (M+H)+:292.0560 Anal. Calc. For (C15H17NO5): 291.11.
4-((4-(2-Fluorophenyl)piperazin-1-yl)methyl)-6,7-dihydroxy-2H-chromen-2-one (6): Yield (75%); m.p. 212-213.4°C; IR: 1274.98 (C-O), 1664.07 (C=O), 3304.78 (Ar-OH); 1H-NMR (DMSO) δ (ppm): 10.28 (s); 9.39 (s), 7.25 (s), 7.10 (m), 6.76 (d), 6.37 (s), 6.23 (s), 4.87 (s), 3.62 (s), 3.41 (s), 3.32 (s), 3.03 (s), 2.63 (s), 2.49 (s), 2.06(s), 1.81 (s); 13C-NMR (DMSO) δ (ppm): 161.72, 161.28, 158.05, 157.15, 153.20, 151.37, 151.31, 151.04, 150.74, 149.00, 148.92, 148.48, 143.62, 143.43, 140.05, 125.58, 123.31, 120.03, 116.86, 116.45, 111.85, 111.00, 110.55, 110.09, 109.56, 109.07, 103.63, 103.41, 59.82, 53.31, 47.79; ESI-MS(TOF) (M-H)+:369.1980 Anal. Calc. For (C20H19FN2O4): 370.13.
4,4'-(Ethane-1,2-diylbis(phenylazanediyl))bis(methylene)bis(6,7-dihydroxy-2H-chromen-2-one) (7): Yield (77%); m.p. 169°C; IR: 1237.69 (C-O), 1665.31 (C=O), 3273.62 (Ar-OH), 1H-NMR (DMSO) δ (ppm): 10.46 (s), 9.45 (s), 7.20 (dd), 6.70 (m), 6.38 (s), 5.73 (s), 4.87 (s), 4.74 (s), 3.70 (s), 3.32 (s), 3.04 (s), 2.48 (s), 1.16 (t), 13C-NMR (DMSO) δ (ppm): 161.44, 161.37, 153.23, 152.95, 151.33, 151.05, 150.75, 148.78, 147.84, 147.50, 143.54, 143.46, 130.48, 130.23, 130.05, 118.70, 112.65, 112.38, 110.23, 110.18, 103.47, 46.11, 42.30; ESI-MS(TOF) (M-H)+:591.2861 Anal. Calc. For (C34H28N2O8): 592.18.
DPPH has been widely used to evaluate the free radical scavenging effectiveness of various antioxidant substancesCitation33. DPPH is a stable free radical and accept an electrone and hydrogen radical to become a stable diamagnetic moleculeCitation36. The DPPH radical scavenging activity of dihydroxy coumarin derivatives are presented in . Trolox, rutin, BHA and BHT were used as references. DPPH free radical scavenging activity of these compounds and standards also increased with increasing concentrations. All the tested compounds showed higher free radical scavenging activities when compared BHA, BHT, Trolox and rutin. Dihydroxy coumarin derivatives (2–7) and standards comparable scavenging activities were also expressed in IC50 (the effective concentration at which the DPPH radicals were scavenged by 50% ) value (). Compound (7) had the highest scavenging activity among all the compounds tested and standards (IC50 = 10.09 µM). The high DPPH scavenging power of compound 7 suggests that it could be able to directly remove free radicals important for lipid peroxidation. It is known that phenolic compounds play a key role as antioxidants due to the presence of hydroxyl substituents in their aromatic structure, which enables them to scavenge free radicalsCitation37. Coumarin derivatives could be involved in transferring labile electrons to the DPPH radical. It is important to point out that the DPPH scavenging activity of compound 7, 3, 2, 6, 5 and 4 was stronger than that of all standards. Scavenging effects of dihydroxy coumarin derivatives and standards on the DPPH radical activity decreased in the order of 7 >3> 2 > 6 > 5> 4 > rutin > Trolox> BHA>BHT.
Table 1. DPPH and ABTS radical scavenging activities and elastase inhibitor activity of dihydroxy coumarin derivatives.
ABTS radical assay is a conventional and excellent model for assessing the antioxidant activities of hydrogen donating and chain breaking antioxidantCitation38. The role of antioxidant is to remove free radicals. This radical can directly react with antioxidants. showed the ABTS radical scavenging activity of dihydroxy coumarin derivatives compared with BHT, BHA, Trolox and rutin. All tested compounds showed between 99.17–92.80% inhibition at 100 µg/mL. Besides, compounds 2, 3, 4, 5, 7 and displayed better radical scavenging activities than BHA, BHT and Trolox in this assay. Compound 7 exhibited better ABTS radical scavenging standard activities than the antioxidants BHT, BHA, Trolox and rutin. The scavenging activity of dihydroxy coumarin compounds and standards on ABTS decreased in the order: 7> 3> rutin > 2 > 5 > 6> 4 > BHT>BHA>Trolox (). The presence of –OH, –N, and –F– groups on the heterocyclic ring system seem to increase the activity of compounds. For reason, it could be concluded that -NH and -OH groups were important contributors to their ABTS radical scavenging activities.
Antioxidants can be reductants, and inactiviation of oxidants by reductants can be described as redox reactions in which one reactions species is reduced at the expense of the oxidation of the other. As it is known transition ions, such as ferrous and cupric, accelerate lipid oxidation by breaking down hydrogen and lipid peroxides to reactive free radicals via the Fenton reactionCitation39. Therefore, chelating agents known as secondary antioxidants are important to retard the radicalic degradation. There are several methods for the determination of antioxidant activities. In this study, cupric reducing antioxidant capacity and reducing power was used.
The reducing power of prepared dihydroxy coumarin derivatives, which may serve as a significant reflection of the antioxidant activity, was determined using the iron (III) to iron (II) reduction assay. The presence of reductants in the solution causes the reduction of the Fe+3/ferricyanide complex to the ferrous form. Therefore, the Fe+2 ion can be monitored by measurement of the formation of Perl’s Prussian blue at 700 nm. The higher activity was found at compound 5. Tested compounds (7, 4, 3 and 2) showed lower activity than BHA at 100 µg/mL concentration. Trolox and rutin showed lower activity than BHA and BHT. There was a correlation found between reducing capabilities and substituents. The reason for the higher reducing power capacity of the compounds can be explained by looking into the structure of compounds. The presence of carbonyl group, alkene and alkyne ring system seems to increase the reductive capacity of the compoundsCitation40. All tested compounds showed some degree of reducing power. The reducing power of dihydroxy coumarin derivatives exhibited the following order; 5 > BHA > 6 > BHT > 3 > 2 > 7 > rutin > Trolox > 4 (Supplementary Table).
The cuprac method is simultaneously cost effective, rapid, stable, selective and suitable for a variety of antioxidants regardless of chemical type or hydrophilicityCitation41. Cupric ions (Cu2+) reducing ability of dihydroxy coumarin derivatives is shown in Supplementary Table. Cupric ion (Cu2+) reducing antioxidant capacity increased with increasing concentration of dihydroxy coumarin derivatives (2.5–10 µg/mL). The highest reducing capacity was found for compound 2. All of the dihydroxy coumarin derivatives have the highest effect on cupric ions (Cu2+) reducing ability when compared to BHT (Supplementary Table).
The inhibition effect of elastase activity is shown in . In this study, elastase inhibitor activity of dihydroxy coumarin derivatives was found to increase dose dependently. The inhibition was increased with increasing dihydroxy coumarin concentration (). All of the dihydroxy coumarin derivatives exhibited good elatase inhibitor activity. Lower IC50 values indicate higher enzyme inhibitor activity. Compound 7 proved to be the most potent showing an enzyme inhibitory activity with an IC50 = 0.125 µM. Ursolic acid showed lower activity than compound 2, 3, 5, 6 and 7. A series of esters and amides of coumarin derivatives was evaluated as inhibitors of serin proteasesCitation42,Citation43. Bissonnette et al. have demonstrated the potency of synthetic coumarinic derivatives to inhibit human leukocyte elastaseCitation44. Previous studies have shown that the attachment of various leaving groups (halogen, carboxylate, heterocyclic sulfide, sulfone, methoxy) to the triazole compound yields highly potent inhibitors of human leukocyte elastaseCitation45. We have demonstrated the potency of the dihydroxy coumarin derivatives to inhibit elastase.
In this study, synthesized dihydroxy coumarin derivatives showed a good antioxidant activity in all tests. According to the our results, there is a correlation between radical scavenging and antioxidant activities of compounds and substituents. The results showed that the synthesized dihydroxy coumarin derivatives had antioxidant and antielastase activities. For reason, 4-(aza substituted) methylene substituted dihydroxy coumarines may be considered as a main elastase inhibitory. These dihydroxy coumarin derivatives can be used in pharmacy and cosmetic industries due to their excellent antielastase and antioxidant activities.
Declaration of interest
This study was supported by Giresun University Scientific Research Projects Unit No: FEN-BAP-08/025.
Supplementary Material
Download PDF (25.7 KB)References
- Aruoma IO, Cuppette SL. Antioxidant methodology: in vivo and in vitro concepts. Illinois: AOAS Press 1997.
- Prakash D, Upadhyay G, Singh BN, Sing HB. Antioxidant and free radical-scavenging activities of seeds anda gri-wastes of some varieties of soybean (Glycine Max). Food Chem 2007; 104:783–790.
- Hussain HH, Babic G, Durst T, Wright JS, Flueraru M, Chichirau A et al. Development of novel antioxidants: design, synthesis, and reactivity. J Org Chem 2003;68:7023–7032.
- Siedle LG, Gustavsoon L, Johansson S, Murillo R, Castro V, Bohlin L, Merfot I. The effect of sesquiterpene lactones on the release of human neutrophil elastase. Biochem Pharmacol 2003; 65: 897–903.
- Siedle B, Hrenn A, Merfort I. Natural compounds as inhibitors of human neutrophil elastase. Planta Med 2007;73:401–420.
- Khlebnikov AI, Schepetkin IA, Quinn MT. Structure-activity relationship analysis of N-benzoylpyrazoles for elastase inhibitory activity: a simplified approach using atom pair descriptors. Bioorg Med Chem 2008;16:2791–2802.
- Abboud RT, Vimalanathan S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int J Tuberc Lung Dis 2008;12:361–367.
- Voynow JA, Fischer BM, Zheng S. Proteases and cystic fibrosis. Int J Biochem Cell Biol 2008;40:1238–1245.
- Hashimoto S, Okayama Y, Shime N, Kimura A, Funakoshi Y, Kawabata K et al. Neutrophil elastase activity in acute lung injury and respiratory distress syndrome. Respirology 2008;13:581–584.
- Uchida Y, Freitas MC, Zhao D, Busuttil RW, Kupiec-Weglinski JW. The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury. Transplantation 2010;89:1050–1056.
- Melagraki G, Afantitis A, Igglessi-Markopoulou O, Detsi A, Koufaki M, Kontogiorgis C et al. Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their α-lipoic acid adducts. Eur J Med Chem 2009;44:3020–3026.
- Maxwell A. The interaction between coumarin drugs and DNA gyrase. Mol Microbiol 1993;9:681–686.
- Musicki B, Periers AM, Laurin P, Ferroud D, Benedetti Y, Lachaud S et al. Improved antibacterial activities of coumarin antibiotics bearing 5′,5′-dialkylnoviose: biological activity of RU79115. Bioorg Med Chem Lett 2000;10:1695–1699.
- Chimichi S, Boccalini M, Cosimelli B, Viola G, Vedaldi D, Dall’Acqua F. New geiparvarin analogues from 7-(2-oxoethoxy)coumarins as efficient in vitro antitumoral agents. Tetrahedron Lett 2002; 43: 7473–7476.
- Yoakim C, Bonneau PR, Déziel R, Doyon L, Duan J, Guse I et al. Novel nevirapine-like inhibitors with improved activity against NNRTI-resistant HIV: 8-heteroarylthiomethyldipyridodiazepinone derivatives. Bioorg Med Chem Lett 2004;14:739–742.
- Uchiumi F, Hatano T, Ito H, Yoshida T, Tanuma S. Transcriptional suppression of the HIV promoter by natural compounds. Antiviral Res 2003;58:89–98.
- Yu D, Suzuki M, Xie L, Morris-Natschke SL, Lee KH. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med Res Rev 2003;23:322–345.
- Jeddy AS, Gleason BL. Aspirin and warfarin versus aspirin monotherapy after myocardial infarction. Ann Pharmacother 2003;37:1502–1505.
- Pineo G, Hull RD. Coumarin therapy in thrombosis. Hematol Oncol Clin North Am 2003;17:201–16, viii.
- Guiotto A, Chilin A, Manzini P, Dall’Acqua F, Bordin F, Rodighiero P. Synthesis and antiproliferative activity of furocoumarin isosters. Farmaco 1995;50:479–488.
- Kontogiorgis CA, Hadjipavlou-Litina DJ. Synthesis and biological evaluation of novel coumarin derivatives with a 7-azomethine linkage. Bioorg Med Chem Lett 2004;14:611–614.
- Kamal A, Adil SF, Tamboli JR, Siddardha B, Murthy USN. Synthesis of cyclodextrin derivatives of amino-(thiazolyl) coumarins as potential antimicrobial agents. Lett Drug Des Discov 2009; 6: 201–209.
- Ito C, Itoigawa M, Mishina Y, Filho VC, Enjo F, Tokuda H et al. Chemical constituents of Calophyllum brasiliense. 2. Structure of three new coumarins and cancer chemopreventive activity of 4-substituted coumarins. J Nat Prod 2003;66:368–371.
- Kontogiorgis CA, Hadjipavlou-Litina DJ. Synthesis and antiinflammatory activity of coumarin derivatives. J Med Chem 2005;48:6400–6408.
- Ghate M, Manohar D, Kulkarni V, Shobha R, Kattimani SY. Synthesis of vanillin ethers from 4-(bromomethyl) coumarins as anti-inflammatory agents. Eur J Med Chem 2003;38:297–302.
- Silván AM, Abad MJ, Bermejo P, Sollhuber M, Villar A. Antiinflammatory activity of coumarins from Santolina oblongifolia. J Nat Prod 1996;59:1183–1185.
- Kulkarni GM, Kulkarni MV, Patil VD. ChemInform Abstract: Synthesis and antiinflammatory activity of some new triheterocyclic thiazoles. Rev Roum Chim. 1990; 35: 549–554.
- Deana AA, Stokker GE, Schultz EM, Smith RL, Cragoe EJ Jr, Russo HF et al. 2-(Aminomethyl)phenols, a new class of saluretic agents. 5. Fused-ring analogues. J Med Chem 1983;26:580–585.
- Tyagi YK, Kumar A, Raj HG, Vohra P, Gupta G, Kumari R, Kumar P, Gupta RK. Synthesis of novel amino and acetyl amino-4-methyl coumarins and evaluation of their antioxidant activity. Eur J Med Chem 2005;40:413–420.
- Gumus A, Karadeniz S, Ugras HI, Bulut M, Cakir U, Goren AC. Synthesis, complexation, and biological activity studies of 4-aminomethyl-7,8-dihydroxy coumarines and their crown ether derivatives. J Heterocyclic Chem 2010; 47: 1127–1133.
- Brands-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm-Wiss Technol 1995; 28: 25–30.
- Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 2001; 3: 239–244.
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jap J Nutr 1986; 44: 307–315.
- Apak R, Güçlü K, Ozyürek M, Esin Karademir S, Erçag E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int J Food Sci Nutr 2006;57:292–304.
- James AE, Timothy DW, Gordon L. Inhibition of human leukocyte and pancreatic elastase by homologues of bovine pancreatic trypsin inhibitors. Biochemistry 1996; 35: 9090–9096.
- Soares JR, Dinis TC, Cunha AP, Almeida LM. Antioxidant activities of some extracts of Thymus zygis. Free Radic Res 1997;26:469–478.
- Villaño D, Fernández-Pachón MS, Moyá ML, Troncoso AM, García-Parrilla MC. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007;71:230–235.
- Leong LP, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem 2002; 76: 69–75.
- Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 1984;219:1–14.
- Peksel A, Celik C, Ocal N, Yanardag R. Antioxidant and radical scavenging activities of some norcantharidin and bridged perhydroisoindole derivatives. J Serbian Chem Soc 2012;77. DOI: 10.2298/JSC120123036P.
- Köksal E, Gülçin I, Beyza S, Sarikaya O, Bursal E. In vitro antioxidant activity of silymarin. J Enzyme Inhib Med Chem 2009;24:395–405.
- Pochet L, Frédérick R, Masereel B. Coumarin and isocoumarin as serine protease inhibitors. Curr Pharm Des 2004;10:3781–3796.
- Pochet L, Doucet C, Dive G, Wouters J, Masereel B, Reboud-Ravaux M et al. Coumarinic derivatives as mechanism-based inhibitors of α-chymotrypsin and human leukocyte elastase. Bioorg Med Chem 2000;8:1489–1501.
- Bissonnette EY, Tremblay GM, Turmel V, Pirotte B, Reboud-Ravaux M. Coumarinic derivatives show anti-inflammatory effects on alveolar macrophages, but their anti-elastase activity is essential to reduce lung inflammation in vivo. Int Immunopharmacol 2009;9:49–54.
- Sokmen BB, Gumrukcuoglu N, Ugras S, Ugras HI, Yanardag R. Synthesis, antibacterial, antielastase, antiurease and antioxidant activities of new methoxy substitued bis-1,2,4-triazole derivatives. J Enzyme Inhib Med Chem doi:10.3109/14756366.2011.631536