Abstract
The condensation reaction of 3,5-diamino-1,2,4-triazole with methoxy-, chloro-, bromo-, iodo- and nitro-substituted 2-hydroxybenzaldehydes formed triazole Schiff bases (L1)–(L6). The synthesized ligands have been characterized through physical, spectral and analytical data. Furthermore, the reaction of synthesized Schiff bases with the oxovanadium(IV) sulphate in (1:2) (metal:ligand) molar ratio afforded the oxovanadium(IV) complexes (1)–(6). All the complexes were non-electrolytic and showed a square-pyramidal geometry. The synthesized compounds have been screened for in-vitro antibacterial, antifungal and brine shrimp bioassay. The bioactivity data showed the complexes to be more active than the original Schiff bases.
Introduction
Triazole derivatives have attracted a wide spread attentionCitation1 due to their diverse coordination behaviour and biological activities. 1,2,4-Triazoles have been known for a potential use in a wide variety of therapeutically interesting drugs including CNS stimulant, anti-anxietyCitation2 and also antimycotic compounds such as fluconazole, intraconazole and voriconazoleCitation3. In view of the significant therapeutic uses, the chemistry of 1,2,4-triazoles represents an important class of compounds owing to their synthetic and effective biological properties like antibacterialCitation4, antifungalCitation5, anticonvulsantCitation6, antiproliferativeCitation7, antitumorCitation8, antitubercularCitation9, anticancerCitation10, anti HIVCitation11 and antiviralCitation12.
Vanadium chemistry has established its strong role as antimicrobialCitation13, anti-tumorCitation14, anti-leukemicCitation15, spermicidalCitation16, anti-amoebicCitation17, antioxidantCitation18 and osteogenicCitation19 activity in bioinorganic chemistry. Oxovanadium(IV) complexes are known as potential inhibitors of various enzymes. Recent advances in catalytic and medicinal properties of vanadium complexes have motivated their designing, synthesis and potential bioactive use in drug chemistry. Another important thrust of vanadium chemistry in the context of medicinal application has arisen from their ability to promote the insulin mimeticCitation20 activity in patho-physiological state of diabetes mellitus in humans. This biological and catalytic relevance of vanadium has promoted the synthesis of model vanadium compounds containing O, N donor ligands.
In view of all the above facts and as a continuation of our research work on the synthesis and biological properties of triazole derived Schiff base ligands, we wish to report a series of oxovanadium(IV) complexes with newly synthesized biologically active ligands prepared by the condensation reaction of 3,5-diamino-1,2,4-triazole with methoxy-, chloro-, bromo-, iodo- and nitro-substituted 2-hydroxybenzaldehydes. These triazole ligands and their oxovanadium(IV) complexes have been subjected to in vitro antibacterial activity against bacterial strains such as, Escherichia coli, Shigella flexneri, Pseudomonas aeruginosa, Salmonella typhi, Staphylococcus aureus and Bacillus subtilis and for antifungal activity against fungal strains, Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani and Candida glabrata. These compounds were also studied for in vitro cytotoxic activity. The reported series of compounds could be an appreciable extension in the chemistry of vanadium as vanadium based new class of antibacterial and antifungal agents.
Experimental
All the chemicals used were of analytical grade. 1,2,4-Triazole was purchased from Sigma Aldrich. Fisher Johns melting point apparatus was used for recording melting points. IR spectra were recorded on SHIMADZU FT-IR spectrophotometer. Elemental analysis was carried out on Perkin Elmer (USA model). 1H and 13C NMR spectra were recorded on a Bruker Spectrospin Avance DPX-400 spectrometer using TMS as internal standard and d6-DMSO as a solvent. Electron impact mass spectra (EIMS) were recorded on JEOL MS Route instrument. In vitro antibacterial, antifungal and cytotoxic properties were studied at HEJ Research Institute of Chemistry, International Centre for Chemical Sciences, University of Karachi, Pakistan.
Synthesis of (2-{(E)-[(5-amino-1H-1,2,4-triazol-3-yl)imino]methyl}phenol (L1)
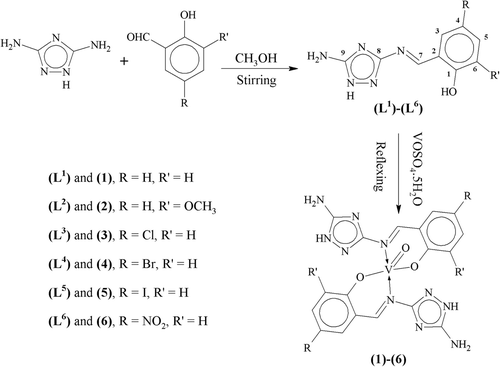
An equimolar solution of 3,5-diamino-1,2,4-triazole (0.99g, 10 mmol) and 2-hydroxybenzaldehyde (1.07 mL, 10 mmol) in methanol (50 mL) was vigorously stirred for 3 h. A precipitated product was formed during stirring which was cooled to room temperature, filtered, washed with methanol (3 × 5 mL), then with diethyl ether (2 × 5 mL) and dried under vacuum. Crystallization was carried out in a mixture of methanol:dioxane (1:4) to obtain TLC pure product (L1) in 75% yield. The same method was applied for the preparation of all other ligands (L2)–(L6) ().
2-{(E)-[(5-Amino-1H-1,2,4-triazol-3-yl)imino]methyl}phenol (L1)
Yield: 75% (1.52 g); colour (yellow); m.p. 181–183°C; 1H NMR (DMSO-d6, 400 MHz): δ 6.01 (s, 2H, NH2), 6.98 (t, 1H, J = 8.4 Hz, C4-H), 7.12 (d, 1H, J = 7.8 Hz, C6-H), 7.41 (t, 1H, J = 7.4 Hz, C5-H), 7.63 (d, 1H, J = 7.9 Hz, C3-H), 8.8 (s, 1H, C7-H), 10.31 (s, 1H, OH), 12.22 (s, 1H, NH); 13C NMR (DMSO-d6): δ 117.67 (C6), 118.97 (C2), 121.23 (C4), 130.35 (C3), 133.18 (C5), 158.12 (C1), 159.75 (C9), 160.63 (C7), 163.28 (C8); IR (KBr, cm−1): 3420 (OH), 3350 (NH2), 3190 (NH), 1631 (HC=N), 1594 (C=N, triazole), 1025 (N-N); EIMS (70eV) m/z (%): 203 ([M]+, 100), 186 (85), 172 (48), 161 (36), 147 (81), 132 (72), 120 (47), 104 (70), 91 (31), 77 (84); Anal. Calcd. for C9H9N5O (203.20): C 53.20, H 4.46, N 34.47; Found C 53.16, H 4.43, N 34.45.
2-{(E)-[(5-Amino-1H-1,2,4-triazol-3-yl)imino]methyl}-3-methoxyphenol (L2)
Yield: 83% (1.93 g); colour (light yellow); m.p. 252–253°C; 1H NMR (DMSO-d6, 400 MHz): δ 2.86 (s, 3H, OCH3), 6.02 (s, 2H, NH2), 6.98 (dd, 1H, J = 8.8, 8.9 Hz, C4-H), 7.12 (d, 1H, J = 8.8 Hz, C5-H), 7.53 (d, 1H, J = 8.9 Hz, C3-H), 8.78 (s, 1H, C7-H), 10.22 (s, 1H, OH), 12.18 (s, 1H, NH); 13C NMR (DMSO-d6): δ 55.39 (OCH3), 119.72 (C2), 121.86 (C4), 124.23 (C5), 129.55 (C3), 149.95 (C1), 151.2 (C6), 156.65 (C9), 157.19 (C7), 162.28 (C8); IR (KBr, cm−1): 3420 (OH), 3346 (NH2), 3187 (NH), 2910 (OCH3), 1628 (HC=N), 1595 (C=N, triazole), 1022 (N-N); EIMS (70eV) m/z (%): 233 ([M]+, 13), 218 (100), 202 (32), 177 (9), 171 (19), 164 (7), 150 (12), 134 (27), 123 (14), 104 (20), 77 (22); Anal. Calcd. for C10H11N5O2 (233.23): C 51.50, H 4.75, N 30.03, O 13.72; Found C 51.47, H 4.72, N, 29.98.
2-{(E)-[(5-Amino-1H-1,2,4-triazol-3-yl)imino]methyl}-5-chlorophenol (L3)
Yield: 73% (1.74 g); colour (light yellow); m.p. 222–224°C; 1H NMR (DMSO-d6, 400 MHz): δ 6.04 (s, 2H, NH2), 6.97 (d, 1H, J = 7.8 Hz, C6-H), 7.48 (dd, 1H, J = 7.8, 2.4 Hz, C5-H), 7.84 (d, 1H, J = 2.4 Hz, C3-H), 8.87 (s, 1H, C7-H), 10.26 (s, 1H, OH), 12.27 (s, 1H, NH);13C NMR (DMSO-d6): δ 119.05 (C6), 120.27 (C2), 125.52 (C4), 131.72 (C3), 133.13 (C5), 156.12 (C9), 159.63 (C7), 160.33 (C1), 163.35 (C8); IR (KBr, cm−1): 3428 (OH), 3345 (NH2), 3192 (NH), 1636 (HC=N), 1606 (C=N, triazole), 1032 (N-N), 819 (C-Cl); EIMS (70eV) m/z (%): 237 ([M]+, 100), 221 (5), 205 (5), 181 (18), 166 (6), 154 (11), 131 (14), 127 (10), 111 (8), 75 (7); Anal. Calcd. for C9H8ClN5O (237.65): C 45.49, H 3.39, N 29.47; Found C 45.46, H 3.36, N 29.45.
2-{(E)-[(5-Amino-1H-1,2,4-triazol-3-yl)imino]methyl}-5-bromophenol (L4)
Yield: 77% (2.17 g); colour (light yellow); m.p. 227–229°C; 1H NMR (DMSO-d6, 400 MHz): δ 6.03 (s, 2H, NH2), 6.97 (d, 1H, J = 8.8 Hz, C6-H), 7.58 (dd, 1H, J = 8.8, 2.4 Hz, C5-H), 8.04 (d, 1H, J = 2.4 Hz, C3-H), 8.83 (s, 1H, C7-H), 10.24 (s, 1H, OH), 12.25 (s, 1H, NH); 13C NMR (DMSO-d6): δ 116.02 (C4), 119.78 (C6), 121.34 (C2), 133.09 (C3), 136.53 (C5), 156.12 (C9), 159.92 (C1), 158.78 (C7), 164.89 (C8); IR (KBr, cm−1): 3424 (OH), 3343 (NH2), 3192 (NH), 1634 (HC=N), 1603 (C=N, triazole), 1027 (N-N), 564 (C-Br); EIMS (70eV) m/z (%): 282 ([M]+, 100), 266 (13), 250 (7), 225 (20), 212 (8), 199 (26), 171 (5), 157 (9), 103 (14), 76 (11); Anal. Calcd. for C9H8BrN5O (282.09): C 38.32, H 2.86, N 24.83; Found C 38.28, H 2.84, N 24.81.
2-{(E)-[(5-Amino-1H-1,2,4-triazol-3-yl)imino]methyl}-5-iodophenol (L5)
Yield: 80% (2.63 g); colour (deep yellow); m.p. 231–232°C; 1H NMR (DMSO-d6, 400 MHz): δ 6.03 (s, 2H, NH2), 6.85 (d, 2H, J = 8.6 Hz, C6-H), 7.72 (dd, 2H, J = 8.6, 2.2 Hz, C5-H), 8.19 (d, 1H, J = 2.2 Hz, C3-H), 8.80 (s, 1H, C7-H), 10.21 (s, 1H, OH), 12.21 (s, 1H, NH); 13C NMR (DMSO-d6): δ 114.97 (C4), 118.65 (C6), 120.76 (C2), 140.17 (C3), 142.24 (C5), 156.12 (C9), 158.69 (C7), 159.42 (C1), 163.26 (C8); IR (KBr, cm−1): 3426 (OH), 3344 (NH2), 3188 (NH), 1630 (HC=N), 1599 (C=N, triazole), 1027 (N-N); EIMS (70eV) m/z (%): 329 ([M]+, 100), 312 (36), 298 (5), 273 (15), 245 (9), 203 (10), 146 (14), 131 (16), 119 (5), 103 (5), 76 (12); Anal. Calcd. for C9H8IN5O (329.09): C 32.85, H 2.54, N 21.28; Found C 32.81, H 2.51, N 21.25.
2-{(E)-[(5-Amino-1H-1,2,4-triazol-3-yl)imino]methyl}-5-nitrophenol (L6)
Yield: 67% (1.66 g); colour (dark yellow); m.p. 242–244°C; 1H NMR (DMSO-d6, 400 MHz): δ 6.07 (s, 2H, NH2), 7.19 (d, 1H, J = 8.7 Hz, C6-H), 8.24 (dd, 1H, J = 8.7, 2.3 Hz, C5-H), 8.75 (d, 1H, J = 2.3 Hz, C3-H), 8.93 (s, 1H, C7-H), 10.31 (s, 1H, OH), 12.30 (s, 1H, NH); 13C NMR (DMSO-d6): δ 118.17 (C6), 120.85 (C2), 126.93 (C3), 135.62 (C5), 140.75 (C4), 156.12 (C9), 160.16 (C7), 162.52 (C1), 166.05 (C8); IR (KBr, cm−1): 3431 (OH), 3347 (NH2), 3199 (NH), 1638 (HC=N), 1607 (C=N, triazole), 1350 (NO2), 1034 (N-N); EIMS (70eV) m/z (%): 248 ([M]+, 22), 232 (100), 216 (20), 192 (31), 172 (5), 166 (19), 149 (22), 138 (11), 122 (14), 76 (10); Anal. Calcd. for C9H8N6O3 (248.19): C 43.55, H 3.25, N 33.86; Found C 43.52, H 3.22, N 33.83.
Synthesis of oxovanadium(IV) complex (1)
To a hot magnetically stirred dioxane (50 mL) solution of 2-{(E)-[(5-amino-1H-1,2,4-triazol-3-yl)imino]methyl}phenol (0.41g, 2 mmol), a methanol solution (30 mL) of VOSO4.5H2O (0.163g, 1 mmol) was added. The mixture was refluxed for 3 h during which a precipitated product was formed. It was then cooled to room temperature. The precipitates thus formed were filtered, washed with methanol, dioxane and then with diethyl ether and dried under vacuum. It was crystallized in a mixture of water:dioxane (1:3) to obtain TLC pure complex (1). All other complexes (2)–(6) were prepared following the same method ().
Estimation of vanadium metal
The vanadium content of complex (1) was determined volumetrically by using 0.1N KMnO4 solution as an oxidizing agent in the presence of dil. H2SO4. A 0.1 g sample of the vanadyl complex (1) was placed in a silica crucible, decomposed by gentle heating and then by adding 1–2 mL of conc. HNO3, two to three times. An orange coloured mass (V2O5) was obtained after decomposition and complete drying. It was dissolved in minimum amount of dil. H2SO4 and the solution so obtained was diluted with distilled water to 100 mL in a measuring flask. The amount of vanadium in the tested sample was calculatedCitation21 by using the standard relationship of 1mL of 0.1N KMnO4 considered as 0.005094 g of vanadium. All other vanadyl complexes (2)–(6) were estimated following the same method.
Pharmacology
The test procedures for in-vitro antibacterial, antifungal, minimum inhibitory concentration (MIC) and cytotoxicity bioassay have already been reportedCitation22.
Results and discussion
The triazole Schiff base ligands (L1)–(L6) were prepared by the condensation reaction of 3,5-diamino-1,2,4 triazole with a series of methoxy-, chloro-, bromo-, iodo- and nitro-substituted 2-hydroxybenzaldehydes under stirring (). All the synthesized ligands were air, moisture stable. These were all amorphous solids which melted at 181–252°C. All the ligands were only soluble in dioxane, DMF and DMSO. The oxovanadium(IV) complexes (1–6) of the prepared ligands were synthesized in a molar ratio 1:2 (metal:ligand). All the oxovanadium(IV) complexes were light green to dark green coloured amorphous solids. All the complexes decomposed on heating rather than melting. They were all only soluble in DMSO and DMF. The elemental analysis and solubility data strongly recommended that these complexes were monomers and showed 1:2 stoichiometry of type ML2 where M is metal and L is ligand. The analytical data given in (), also agreed well with the proposed structure of the complexes ().
Table 1. Physical and analytical data of the oxovanadium(IV) complexes.
IR spectra
Some of the distinctive IR spectral bands of the triazole ligands and their oxovanadium (IV) complexes are reported in experimental part and (). The triazole ligands have potentially active donor sites for coordination such as, azomethine-N), hydroxyl-O which have the capability of coordinating with the vanadium metal atom. All the ligands showed bands at 3420–3431, 3343–3350, 3187–3199, 1594–1607 and 1022–1034 cm−1 respectively due to v(OH), v(NH2), v(N-H), v(C=N) and v(N-N) vibrations. Two bandsCitation23 at 3310 and 3350 cm−1 were observed due to the presence of two amino groups of the original 3,5-diamino-1,2,4-triazole moiety. All triazole ligands showed an absence of one of the amino group band at 3310 cm−1 emerging into a new band of azomethine v(HC=N) linkageCitation24 at 1628–1638 cm−1 giving thus a clue for condensation of one amino group of the triazole moiety; however, another band of amino group at 3350 cm−1 remained unchanged giving a clue of its non-participation in condensation reaction. The ligand (L2) exhibited band at 2910 cm−1 due to v(OCH3) group. Moreover, the ligands (L3) and (L6) showed vibrations at 810 and 1355 cm−1 due to Cl and NO2 groups, respectively. A weaker band at 564 cm−1 was observed by the ligand (L4) which was assigned to v(C-Br) vibration.
Table 2. Physical and spectral data of oxovanadium(IV) complexes.
On comparison of IR spectra of oxovanadium (IV) complexes with the spectra of ligands indicated that the triazole ligands were principally coordinated with the vanadium(IV) metal atom bidentately. The coordination modes of bonding are discussed as under:
(i) All the ligands showing IR spectral band at 1628–1638 cm−1 due to azomethine, ν(HC=N) shiftedCitation25 to lower frequency (12–15 cm−1) at 1616–1626 cm−1 indicating the coordination of the azomethine-N with the vanadium(IV) metal atom.
(ii) Absence of band at 3420–3431 cm−1 due to ν(OH) and in turn, appearance of a new band at 1376–1386 cm−1 assignedCitation26 to ν(C-O) in the oxovanadium(IV) complexes, confirmed deprotonation and coordination of hydroxyl-O to the oxovanadium(IV) metal.
(iii) Te appearance of a new lower frequency bands at 519–529 and 423–438 cm−1 were assigned to ν(V-O) and ν(V-N) respectively indicated the coordination of these ligands with the oxovanadium(IV) metal ion through hydroxyl-O and azomethine-N.
(iv) A new band which was not observed in the spectra of the ligands but appeared in the spectra of complexes at 959–970 cm−1 was assigned to the presence (V=O)Citation27.
(v) The bands at 3343–3350, 3187–3199, 1594–1607 and 1022–1034 cm−1 respectively due v(NH2), v(N-H), v(C=N) and v(N-N) vibrations in the spectra of triazole moiety remained unchanged indicating their non-involvement in the coordination.
All the above mentioned evidences supported the coordination of the ligands with the vanadium (IV) metal via the azomethine-N and deprotonation of hyroxyl-OH groups.
1H NMR spectra
All the ligands (L1)–(L6) displayedCitation28 distinguishing protons of azomethine (CH=N) and NH group at 8.78–8.93 and 12.18–12.3 ppm, respectively as a singlet. Also, distinctive OH proton of all the ligands was observed at 10.20–10.31 ppm as a singlet, which disappeared on exchange with D2O. The ligand (L1) showed peaks of protons C4-H and C5-H at 6.98 and 7.41 ppm, respectively as a triplet and, C3-H and C6-H protons were observed at 7.12 and 7.63 ppm, respectively as a doublet. The methoxy (OCH3) protons of ligand (L2) were observed at 3.86 ppm as a singlet. The C5-H proton was observed at 6.98 ppm as a double doublet. Remaining protons, C4-H and C6-H appeared at 7.12 and 7.53 ppm, correspondingly as doublet. The ligands, (L3-L5) showed C3-H protons as a doublet at 6.97, 6.97 and 6.85 ppm, and C4-H protons appeared as double doublet at 7.48, 7.58 and 7.72 ppm, respectively. Due to the inductive effect of azomethine (CH=N) functional group, the C6-H protons were deshielded at 7.84, 8.04 and 8.19 ppm, as a doublet. However, the ligand (L6) exhibited C3-H proton at 7.19 ppm as a doublet. It was found that due to electron withdrawing effect of the NO2 group, C4-H proton appeared as double doublet at 8.24 ppm. All the ligands demonstratedCitation31 characteristic amino (NH2) proton at 6.01–6.07 ppm as a singlet which provided an evidence for condensation of only one amino group of triazole moiety. The conclusions drawn from this study provided further support to the modes of bonding discussed earlier in IR spectra. All the ligands having protons due to heteroaromatic/aromatic groups were found as to be in their expected region. However, the number of protons calculated from the1H NMR integration curves and those obtained from the values of the expected analytical data (CHN) results confirmed the proposed structures of the Schiff base ligands.
13C NMR spectra
The ligands (L1)–(L6) showed the triazole carbons C8 and C9 at 156.12–166.05 ppm. In addition, the characteristic azomethine carbons C7 of the ligands were observed in the region at 157.19–160.63 ppm. The downfield shifting of C1 was observed at 158.12–160.12 ppm due to the attachment of hydroxyl (OH) group at C1 position except the ligand (L2) which possessed same peak at 149.95 ppm due to electron donating effect of methoxy-C attached to adjacent carbon at C6 position showing its peak at 151.2 ppm. Similarly, the carbons C2 of all the triazole ligands were found in the region at 118.97–121.34 ppm due to the inductive effect of azomethine carbon. However, the peaks for the carbons C3-C6 were observed at 114.97–142.26 ppm. Also, the peaks for C4 carbon of ligands (L3) and (L6) appeared downfield at 125.52 and 140.75 ppm, respectively due to the electronegative nature of chloro and nitro groups at this position. The same peaks for C4 carbon of (L4) and (L5) were observed upfield at 114.97 and 116.02 ppm respectively, due to lesser electronegative nature of bromo and iodo groups. Moreover, the methoxy-C in (L2) appeared in the region at 55.39 ppm. Furthermore, all these studies indicated that the expected values of carbon atoms compromised well with the number of carbon atoms present in the proposed structures of the compounds.
Conductance and magnetic susceptibility measurements
The molar conductance values of the oxovanadium(IV) complexes (1)–(6) were taken in DMF and showed their conductance values in the range 35.9–42.9 Ω−1 cm2 mol−1 (), which is an indicative of non-electrolyticCitation29 nature. The effective magnetic moments of the oxovanadium(IV) complexes at room temperature were found in the range at 1.70–1.82 B.M. indicative of presence of a single electron consistent with half-spin (S = 1/2) orbital contribution. The oxovanadium(IV) complexes possessed +4 oxidation state for vanadium which is an indicative of square pyramidal geometry. These values were compatible to the reported values for their square-pyramidal geometryCitation30. Moreover, the data suggested that the prepared complexes were all mononuclear.
UV/visible spectra
The UV/visible spectra of all the oxovanadium(IV) complexes in DMF displayed three () distinctive low to high intensity bands (v1, v2 and v3) which were assigned to b2 (dxy) → eπ(dxz, dyz), b2 (dxy) → b1 (dx2 − y2) and b2 (dxy) → a1 (dz2) transitions, respectively. The first band observed at 13435–13567 cm−1 was assigned to b2 → eπ d-d transitions. The second band was observed at 18239–18728 cm−1 which can be attributed to b2→ b1 and the presence of third band at 26189–26378 cm−1 can be assigned to the transitions b2→ a1. The fourth band of high intensity observed at 37155–37409 cm−1 was due to metal → ligand charge transfer (MLCT). All these observations provided a strong evidence for the complexes to show a square-pyramidal geometryCitation30.
Biological screening
Antibacterial bioassay (in vitro)
The results obtained from in-vitro antibacterial studies are summarised in . The antibacterial studies revealed that all the Schiff base ligands and their oxovanadium(IV) complexes contributed significantly towards enhancing the biological activity. It is evident that coordination makes the ligands strongly antibacterial and inhibits the growth of bacteria more than the parent ligand. All compounds were tested against four Gram-negative (E. coli, S. flexneri, P. aeruginosa, S. typhi) and two Gram-positive (S. aureus, B. subtilis) bacterial strains. The results obtained were compared with that of the standard drug imipenum. The percentage of activity was compared with the activity of the standard drug considering its activity as 100% (Figures as Supplementary data). All ligands and their oxovanadium(IV) complexes possessed wide-ranging biological activity against all Gram-negative and Gram-positive bacterial strains. The obtained results showed that the ligand (L1) exhibited a significant (57–61%) activity against (c) and (f) strain and rest of all the strains showed moderate (35–52%) activity. The ligand (L2) displayed a significant (39–50%) activity against all the strains except (c) strain which demonstrated weaker (30%) activity. Similarly, the ligand (L3) showed significant (58–69%) activity against (a), (b), (e) and (f) bacterial strains and moderate (41–50%) activity was observed against (c) and (d). However, the ligand (L4) exhibited significant (55–57%) activity against (a), (c) and (d) strain and moderate (39–46%) was observed against (b), (e) and (f). Moreover, the ligands (L5) and (L6) showed overall significant (57–71%) activity against all the bacterial strains. In addition, all the oxovanadium(IV) complexes (1)–(6) possessed overall significant (55–84%) activity against all the bacterial strains. It is confirmed that antibacterial activity was overall enhancedCitation31 upon coordination.
Table 3. Antibacterial activity (concentration used 1 mg/mL of DMSO) of triazole Schiff bases and oxovanadium(IV) complexes.
Antifungal bioassay (in vitro)
In vitro antifungal studies of all the compounds were carried out against T. longifusus, C. albican, A. flavus, M. canis, F. solani and C. glabrata fungal strains (). All ligands and their oxovanadium(IV) complexes possessed good antifungal activity against one or more fungal strains. The results of inhibition obtained from these studies were compared with those of the standard drugs, miconazole and amphotericin B (Figures as Supplementary data). From the antifungal results, it was shown that the ligand (L1) exhibited significant (58%) activity against (b), moderate (37–48%) against (a), (c), (e) and (f) but showed no activity against (d) fungal strain. However, (L2) observed significant (56%) activity against (b), moderate (35–46%) against (a) and (d)–(f) but inactive against (c) fungal strain. Similarly, the ligand (L3) showed significant (55–62%) activity against (a), (c) and (f), and moderate (37–46%) against (b), (d) and (e) fungal strains. Also, significant (54–56%) activity was observed by the ligand (L4) against (b) and (d), and moderate (42–44%) against (a), (c), (e) and (f) fungal strains. Beside this, (L5) showed significant (55%) activity against (d), moderate (38–42%) against (b), (c) and (f). Also, weaker (22–26%) activity was shown against (a) and (e) fungal strains. Ligand (L6) showed significant (54–61%) activity against (a-d) and moderate (45–48%) was observed against (e) and (f) fungal strains. The oxovanadium(IV) complex (1) showed significant (54–67%) activity against (a), (b), (e) and moderate (36–40%) against (c), (d) and (f) strains. In addition, the complex (2) possessed significant (55–70%) activity against (b), (d) and (f) but moderate (34–49%) activity was displayed against (a), (c) and (e) strains. The complex (4) possessed significant (59–77%) activity against (a), (b) and (f) but moderate (38–43%) against (c), (e) strains. The complex (5) possessed moderate (38–45%) activity against (a) and (e) strains and rest of all strains showed significant (54–69%) activity. The complexes (3) and (6) showed overall significant (54–81%) activity against all strains.
Table 4. Antifungal activity (concentration used 200 µg/mL) of triazole Schiff bases and oxovanadium(IV) complexes.
Minimum inhibitory concentration
All the synthesized triazole ligands and their oxovanadium(IV) complexes possessing promising antibacterial activity (above 80%) were selected for minimum inhibitory concentration (MIC) studies and obtained results are reported in (). The antibacterial results indicated that only compounds (3)–(6) were found to display activity greater than 80%, therefore these compounds were further selected for their MIC screening. The compound (3) possessed activity against E. coli, S. flexneri and S. aureus and no activity was observed against P. aeruginosa, S. typhi and B. subtilis. The compound (4) only showed activity against P. aeruginosa and compound (5) against E. coli and S. aureus. Similarly, the compound (6) possessed activity against all bacterial strains except S. flexneri. The MIC values of these compounds fall in the range 2.199 × 10−7 to 2.832 × 10−3 M. The compound (6), however, was found to be the most active showing maximum inhibition 2.199 × 10−7 M against S. aureus.
Table 5. Minimum inhibitory concentration (M/mL) of the selected compounds (3), (4), (5) and (6) against selected bacteria.
Cytotoxic bioassay (in vitro)
All the synthesized Schiff base ligands (L1)–(L6) and their oxovanadium(IV) complexes (1)–(6) were subjected to cytotoxic activity (brine shrimp bioassay). It is evident from the data reported in (), that all ligands were found to be inactive for their bioassay showing LD50 values in the range of 2.835 × 10−4 to 3.926 × 10−2 M/mL. Only, the compounds (3) and (6) showed activity at (LD50 = 4.116 × 10−5) and (LD50 = 5.615 × 10−4), respectively. The data clearly showed that only the oxovanadium complexes exhibited effective cytotoxic nature rather than the parent uncomplexed ligands (L1)–(L6). It was interesting to note that only bromo- and nitro-substituted complexes possessed potent cytotoxic activity against Artemia salina. This activity relationship may help development of certain cytotoxic agents for clinical application.
Table 6. Brine shrimp bioassay data of triazole Schiff bases (L1)–(L6) and oxovanadium(IV) complexes (1)–(6).
Conclusion
The prepared triazole ligands act as bidentate ligands for coordination with the vanadium metal via azomethine-N and benzilidene-O. Antimicrobial results indicated that all the oxovanadium (IV) complexes possessed enhanced biological activity against one or more bacterial/fungal strains as compared to their uncomplexed parent triazole ligands. In general, it is believed that the functional groups present in the compounds such as azomethine-N and other heteroatoms like oxygen and nitrogen are responsible for improved biological activity. Our present studies are reported with the conclusion that those compounds which were biologically inactive became active and less biologically active became more upon complexation/coordination with the vanadium metal atom. It has been suggested that the chelation process reduces the polarity of the metal ion by coordinating with ligands, which in turn increases the lipophilic nature of the vanadium metal. This lipophilic nature of metal thus enhances its penetration through the lipoid layer of cell membrane of the microorganism and thus potentially killing more of them.
Declaration of interest
The authors declare no conflicts of interest.
Supplementary Material
Download PDF (177.7 KB)Acknowledgments
The authors are thankful to Higher Education Commission (HEC), Government of Pakistan for the award to carry out this research work. We are also indebted to HEJ research Institute of Chemistry, University of Karachi, Pakistan, for providing their help in taking NMR and mass spectra and, for the help in carrying out antibacterial, antifungal and brine shrimp bioassays.
References
- Klingele MH, Brooker S. The coordination chemistry of 4-substituted 3,5-di(2-pyridyl)-4H-1,2,4-triazoles and related ligands. Coord Chem Rev 2003;241:119–132.
- Tarzia G, Occelli E, Toja E, Barone D, Corsico N, Gallico L et al. 6-(Alkylamino)-3-aryl-1,2,4-triazolo[3,4-a]phthalazines. A new class of benzodiazepine receptor ligands. J Med Chem 1988;31:1115–1123.
- Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007;356:348–359.
- Bagihalli GB, Avaji PG, Patil SA, Badami PS. Synthesis, spectral characterization, in vitro antibacterial, antifungal and cytotoxic activities of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. Eur J Med Chem 2008;43:2639–2649.
- Rezaei Z, Khabnadideh S, Pakshir K, Hossaini Z, Amiri F, Assadpour E. Design, synthesis, and antifungal activity of triazole and benzotriazole derivatives. Eur J Med Chem 2009;44:3064–3067.
- Cui XS, Jing C, Chai KY, Lee JS, Quan ZS. Synthesis and anticonvulsant evaluation of 3-substituted-4-(4-hexyloxyphenyl)-4H-1,2,4-triazoles. Med Chem Res 2009;18:49–58.
- Manfredini S, Vicentini CB, Manfrini M, Bianchi N, Rutigliano C, Mischiati C et al. Pyrazolo-triazoles as light activable DNA cleaving agents. Bioorg Med Chem 2000;8:2343–2346.
- Romagnoli R, Baraldi PG, Cruz-Lopez O, Lopez Cara C, Carrion MD, Brancale A et al. Synthesis and antitumor activity of 1,5-disubstituted 1,2,4-triazoles as cis-restricted combretastatin analogues. J Med Chem 2010;53:4248–4258.
- Kaplancikli ZA, Turan-Zitouni G, Chevallet P. Synthesis and antituberculosis activity of new 3-alkylsulfanyl-1,2,4-triazole derivatives. J Enzyme Inhib Med Chem 2005;20:179–182.
- Shivarama Holla B, Veerendra B, Shivananda MK, Poojary B. Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur J Med Chem 2003;38:759–767.
- Akhtar T, Hameed S, Al-Masoudi NA, Khan KM. Synthesis, and anti-HIV activity of new chiral 1,2,4-triazoles and 1,3,4-thiadiazoles. Heteroatom Chem 2007;18:316–322.
- Holla BS, Akberali PM, Shivananda MK. Studies on nitrophenylfuran derivatives part Xii. synthesis, characterization, antibacterial and antiviral activities of some nitrophenylfurfurylidene-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines. Farmaco 2001;56:919–927.
- Muhammad N, Ali S, Shahzadi S, Khan AN. Oxovanadium(IV) complexes of non-steroidal anti-inflammatory drugs: Synthesis, spectroscopy, and antimicrobial activity. Russ J Coord Chem 2008;34:448–453.
- Noblía P, Vieites M, Parajón-Costa BS, Baran EJ, Cerecetto H, Draper P et al. Vanadium(V) complexes with salicylaldehyde semicarbazone derivatives bearing in vitro anti-tumor activity toward kidney tumor cells (TK-10): Crystal structure of [VVO2(5-bromosalicylaldehyde semicarbazone)]. J Inorg Biochem 2005;99:443–451.
- Dong Y, Narla RK, Sudbeck E, Uckun FM. Synthesis, X-ray structure, and anti-leukemic activity of oxovanadium(IV) complexes. J Inorg Biochem 2000;78:321–330.
- D’Cruz OJ, Dong Y, Uckun FM. Spermicidal activity of oxovanadium(IV) complexes of 1, 10-phenanthroline, 2,2′-bipyridyl, 5′-bromo-2′-hydroxyacetophenone and derivatives in humans. Biol Reprod 1999;60:435–444.
- Maurya MR, Agarwal S, Abid M, Azam A, Bader C, Ebel M et al. Synthesis, characterisation, reactivity and in vitro antiamoebic activity of hydrazone based oxovanadium(IV), oxovanadium(V) and mu-bis(oxo)bis{oxovanadium(V)} complexes. Dalton Trans 2006;937–947.
- Etcheverry SB, Ferrer EG, Naso L, Rivadeneira J, Salinas V, Williams PA. Antioxidant effects of the VO(IV) hesperidin complex and its role in cancer chemoprevention. J Biol Inorg Chem 2008;13:435–447.
- Evelina GF, Salinas MV, Correa MJ, Luciana N, Barrio DA, Etcheverry SB et al. Synthesis, characterization, antitumoral and osteogenic activities of quercetin vanadyl(IV) complexes. J Biol Inorg Chem 2006;11:791–801.
- Sakurai H, Kojitane Y, Yoshikawa Y, Kawabe K, Yasui H. Antidiabetic vanadium(IV) and zinc(II) complexes. Coord Chem Rev 2002;226:187–189.
- Maurya RC, Mishra DD, Pillai V. Synthesis and characterization of some mixed-ligand thiocyanato complexes of cadmium(ii), mercury(ii), zinc(II) with 2-or 3-pyrazoline-5-one derivatives. Synth React Inorg Met-Org Chem 1995;25:139–150.
- Chohan ZH, Sumrra SH, Youssoufi MH, Hadda TB. Metal based biologically active compounds: Design, synthesis, and antibacterial/antifungal/cytotoxic properties of triazole-derived Schiff bases and their oxovanadium(IV) complexes. Eur J Med Chem 2010;45:2739–2747.
- Guennouna L, Eljastimi J, Guediraa F, Marakchi K, Kabbaj OK, El Hajji A et al. Molecular geometry and vibrational studies of 3,5-diamino-1,2,4-triazole using quantum chemical calculations and FT-IR and FT-Raman spectroscopies. Spectrochimica Acta Part A 2011;78:347–353.
- Bagihalli GB, Badami PS, Patil SA. Synthesis, spectral characterization and in vitro biological studies of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. J Enzyme Inhib Med Chem 2009;24:381–394.
- Chohan ZH, Hanif M. Design, synthesis, and biological properties of triazole derived compounds and their transition metal complexes. J Enzyme Inhib Med Chem 2010;25:737–749.
- Maurya RC, Singh H, Pandey A. Oxovanadium(IV) complexes of bioinorganic and industrial relevance: Synthesis and characterization of some oxovanadium(IV) complexes involving schiff bases derived from biologically active 4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one and certain aromatic amines. Synth React Inorg Met-Org Chem 2002;32:231–246.
- Xie M, Gao L, Li L, Liu W, Yan S. A new orally active antidiabetic vanadyl complex–bis(alpha-furancarboxylato)oxovanadium(IV). J Inorg Biochem 2005;99:546–551.
- Chernyshev VM, Rakitov VA, Astakhov AV, Sokolov AN, Zemlyakov ND, Taranushich VA. Regioselective synthesis of alkyl derivatives of 3,5-diamino-1,2,4-triazole. Russ J Appl Chem 2006;79:624–630.
- Agarwal RK, Singh L, Sharma DK, Singh R. Synthesis, spectral and thermal investigations of some oxovanadium(iv) complexes of hydrazones of isonicotinic acid hydrazide. Turk J Chem 2005;29:309–316.
- Raman N, Raja JD, Sakthivel A. Design, synthesis, spectroscopic characterization, biological screening, and DNA nuclease activity of transition metal complexes derived from a tridentate Schiff base. Russ J Coord Chem 2008;34:400–406.
- Chohan ZH, Sumrra SH. Synthesis, characterization and biological properties of thienyl derived triazole Schiff bases and their oxovanadium(IV) complexes. J Enzyme Inhib Med Chem 2012;27:187–193.