Abstract
Caspase inhibitors are usually administered intracranially. There’s very limited evidence showing that they can be used intraperitoneally, and still have a beneficial effect. We tested the hypothesis that, during focal cerebral ischemia, caspase inhibitors when used in combination with an anesthetic agent results in a significantly reduction in the neuronal damage. Male Sprague Dawley rats were randomly divided into six different groups: control, Isoflurane, Propofol, Isoflurane and Caspase-3 inhibitor intraperitoneally (IP), propofol and Caspase-3 inhibitor IP and only caspase-3 inhibitor, during post-ischemia. Neurological evaluation and histochemical analysis was assessed post-ischemia. The treatment proposed, resulted in a significant decrease in the cerebral infarction volume. Combination of treatments, and caspase-3 inhibitor alone significantly decreased the number of TUNEL and cleaved caspase-3 positive cells in the boundary area of cortical infarction. IP administration appears to reach cerebral targets similarly to intracerebral model. This combination reduces the neurological damage caused by focal cerebral ischemia.
Introduction
Stroke is the world’s second leading cause of mortalityCitation1 and 85% of all strokes are due to vessel occlusions resulting in ischemia. The brain is especially sensitive to ischemia, such that even a brief cerebral ischemic period can initiate a complex sequence of events such as receptor-mediated death signals, and altered expression of pro- and anti-apoptotic proteins ultimately resulting in either necrosis or apoptosisCitation2,Citation3. Post-ischemic neuronal death is a dynamic process in which neurons continue to die over a long period of time after the initiating ischemic injury resulting in two injury zones: the core and the penumbraCitation4.
Recent studies have demonstrated the ability of anaesthetic agents, such as isoflurane or propofol, to reduce neuronal injury following cerebral ischemiaCitation5,Citation8. Isoflurane has been shown to suppress glutamate-mediated excitotoxicity and ischemic depolarizationsCitation8,Citation11 suggesting a neuroprotective role. Isoflurane has also been shown to modulate glutamate-receptor-mediated calcium influx preventing a deleterious increase in calcium influxCitation10. Moreover, propofol has been demonstrated to reduce neuronal damage produced by focal cerebral ischemiaCitation12 by reducing glutamate toxicityCitation13,Citation14 and inhibiting oxidative injuryCitation15. However, the beneficial effects of anaesthetic appear to be only transientCitation16, and combination with other therapeutic agents could possibly improve the outcome following focal cerebral ischemia. Following cerebral ischemia, cells in the surrounding area of the core, known as the penumbra, remain metabolic active with limited blood supply resulting in delayed apoptotic cell death and continuing expansion of the cerebral infarct. Apoptosis or program cell death plays an important role in the pathogenesis of cerebral ischemiaCitation17 and an important mechanism by which apoptosis occurs is via caspase activationCitation18. Caspase activation results in the proteolytic cleavage of a number of vital cellular components, ultimately leading to neuronal apoptosis. Consequently, caspase activity appears to be an important mediator of neuronal cell death after cerebral ischemia. In fact, administration of a caspase inhibitor following cerebral ischemia has been reported to significantly reduce infarct volumeCitation19,Citation22. Moreover, it has been reported that caspase-3 inhibitors increased cell survival after ischemia, which strongly supports a cell death effector role for caspase-3 in ischemic brain injuryCitation23. Similarly, knocking out the caspase-3 gene is associated with resistance to brain ischemiaCitation24. Consequently, the inhibition of caspase-3-like protease activity is of great interest and may have therapeutic significance for the treatment of cerebral ischemia in which caspase-mediated cell death plays an important pathophysiological roleCitation18.
The present study was therefore conducted to investigate the behavioural and histological effects of the combination of isoflurane or propofol anaesthesia and intraperitoneal administration of caspase-3 inhibitor in rats subject to permanent focal cerebral ischemia.
Materials and methods
Animals
All experiments were conducted in accordance with the National Institute of Health Guide and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use committee of the University of South Florida, College of Medicine, Tampa, Fl. To evaluate the effects of isoflurane or propofol combination with a caspase 3 inhibitor, male Sprague Dawley rats (Harlan Sprague Dawley, Indianapolis, IN, USA) weighing 250–300 g were used for this study. All rats were acclimated for at least 7 days in a standard solid-bottom rodent cage under a 12 h light-dark cycle. Rats were allowed access to food and water ad lib and randomly assigned to one of the six experimental groups ():
Figure 1. Experimental design. (A) Timing of events. (B) Procedures performed on each group. Animals in all groups received middle cerebral artery occlusion (MCAO) and common carotid artery occlusion (CCAO) surgery under 1.5% isoflurane anesthesia. Immediately following focal cerebral ischemia, and depending on the assigned group, animals received 90 min of anaesthetic treatment or no treatment, and three intraperitoneal (IP) injections of either caspase-3 inhibitor (C3I) or dimethyl sulfoxide (DMSO) at day 0, 1, and 7 post-ischemia. Neurological evaluation was assessed in all groups at day 0, 4, and 14 post-ischemia. Animals in all groups were euthanized 14 days after focal cerebral ischemia, and brains were collected for analysis.
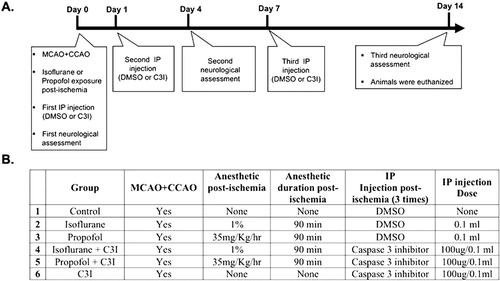
Control group (n = 10): animals received middle cerebral artery occlusion (MCAO).
Isoflurane group (n = 7): animals received MCAO followed by 90 min of 1% isoflurane in 100% Oxygen in a sealed chamber.
Propofol group (n = 7): animals received MCAO followed by 90 min of 35 mg/kg/h propofol, via tail vein catheter.
Isoflurane + caspase-3 inhibitor group (n = 7): animals received MCAO followed by 90 min of 1% isoflurane in 100% oxygen in a sealed chamber and three intraperitoneal (IP) injections of caspase-3 inhibitor (C3I).
Propofol + caspase-3 inhibitor group (n = 7): animals received MCAO followed by 90 min of 35 mg/kg/h propofol, via tail vein catheter, and three IP injections of C3I.
C3I group (n = 7): animals received MCAO followed by three IP injections of C3I.
Permanent focal cerebral ischemia
Rats were placed in the prone position on a stereotaxic frame, and anaesthesia was maintained with 1.5% isoflurane in an 80/20% air/O2 mixture using a nose cone. The rat head was fixed in a stereotactic head holder with the rat in a prone position and one hole was drilled 1 mm posterior and 4 mm lateral to the bregma. An intracerebral guide cannula (Harvard Biosciences Inc, Holliston, MA, USA) was inserted into the hole and sealed in place with dental cement. A laser Doppler optic rigid probe (500 μm) was inserted into the guide cannula and signals were continuously monitored using a moorVMS-LDF Laser Doppler Monitor (Moor Instruments Inc, Wilmington, DE, USA). Rats were then turned in a supine position and a left-sided focal cerebral ischemia was induced by permanent middle cerebral artery and common carotid artery occlusion (MCAO) using a modification of the MCAO technique described by Longa et al.Citation25. The common carotid artery (CCA) was exposed and permanently ligated using a 4-0-monofilament prolene suture. Then, a 4.0-cm length of 4-0-monofilament prolene suture was introduced into the internal carotid through a puncture. The prolene suture was gently advanced into the middle cerebral artery until slight resistance was felt and a reduction in the regional cerebral blood flow (monitored as described above) was observed. The thread was then secured in place with a 4-0 silk. Doppler measurements allowed to detect a reduction in cerebral blood flow at least 60% of control value. If not so, the rat was excluded from the experiments.
Physiological measurements before and after focal cerebral ischemia
Body temperature of the rats was maintained at 37 ± 0.5°C using a thermostatically controlled heating pad. The tail artery was cannulated to monitor mean arterial blood pressure (MAP), heart rate (HR), hemoglobin saturation (Hb Sat) and hematocrit (Hct) during the MCAO procedure, using a SurgiVet monitor (SurgiVet Advisor Monitor, Model number 92V303100). Hematocrit values were collected using a Damon IEC MB Micro Hematocrit Desk-Top Lab Centrifuge. Measurements were taken before and after the ischemic event.
Anaesthetic treatment following focal cerebral ischemia
Isoflurane
Rats in groups 2 and 4 received 1% isoflurane in 100% O2 in a chamber for 90 min following the MCAO procedure. Isoflurane (Forane, Ohmeda Caribe, NJ, USA) was delivered via a standard anesthesia vaporizer.
Propofol
Rats in groups 3 and 5 received 35 mg/kg/h of propofol (Diprivan; AstraZeneca) via tail vein catheter for 90 min following the MCAO procedure.
Caspase-3 inhibitor administration
The caspase-3 inhibitor (Z-Asp-Glu-Val-Fluoromethyl Ketone (Z-DEVD-FMK) BD PharMingen, San Jose, CA, USA) was injected intraperitoneally (IP) at a dose of 100 µg dissolved in 0.1 mL Dimethyl sulfoxide. Rats in groups 4, 5 and 6 received three IP injections at day 0, 1 and 7 following permanent focal cerebral ischemia ().
Neurological assessment
Rats in all groups underwent neurological evaluation at day 0, 4 and 14 after the ischemic event. Each rat was assigned a score according to a eight-point behavioural rating modified from a scale previously describedCitation26: 0 = no deficit; 1 = failure to extent contralateral forepaw fully; 2 = decreased grip of contralateral forelimb while tail pulled; 3 = spontaneous movement in all directions, contralateral circling; 4 = circling or walking to left or right; 5 = walking only if stimulated; 6 = unresponsiveness to stimulation, with a depressed level of consciousness; and 7 = dead. Neurological testing was performed by an observer who was blinded as to group assignments.
Brain collection and processing
Rats were allowed to survive for 14 days following permanent focal cerebral ischemia, and then euthanized with a CO2 overdose, then transcardially perfused with 0.9% saline solution, followed by 4% paraformaldehyde in 0.1M PBS, pH 7.4. Rats were then decapitated, and their brains removed, immersed in 4% paraformaldehyde, refrigerated for 24 h, and changed to 10, 20 and 30% sucrose every 24 h. A cryostat was used to collect serial coronal sections (50 m) starting at the infarct area at a 250 m interval, resulting in approximately 12 sections per animal. Sections were mounted onto slides and used for histochemistry analysis.
Hematoxylin and Eosin staining
The volume of cerebral infarction, at 14 days following MCAO, in each animal was assessed using hematoxylin and Eosin (H and E) stain. Prepared slides were stained in hematoxylin for 5 min, rinsed in tap water, and differentiated in acid alcohol for two to three quick dips. Slides were then washed in tap water for 5 min, followed by immersion in eosin for 5 min before being dipped in 95% ethyl alcohol (three to five dips), washed in absolute ethanol for 5 min, washed in xylene (twice for 2 min each) and mounted with permount and coverslips.
Terminal deoxynucleotidyl transferase dUTP nick end labelling staining
DNA fragmentation, at 14 days following MCAO, was examined using the in situ cell death detection kit, Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) ApopTag Peroxidase In Situ Apoptosis Detection Kit (cat no. S7100, Chemicon, Temecula, CA, USA) according to the manufacturer’s instructions. Sections were incubated with 3% bovine serum albumin in 0.1 mol/L Tris-HCL (pH 7.5) for 30 min, followed by 50 μL of TUNEL reaction mixture on each sample for 60 min at 37°C. After washing, slides were incubated with 0.3% H2O2 in methanol for 10 min, then followed by incubation with 3% bovine serum albumin in 0.1 mol/L Tris-HCL (pH 7.5) for 30 min at room temperature. Subsequently, incubation with peroxidase-conjugated anti-fluorescein (diluted 1:5) at 37°C for 30 min was performed and the number of apoptotic cells at the margin of the area of infarction was quantified.
Cleaved caspase-3 immunohistochemistry
Free-floating sections were rinsed three times in phosphate-buffered saline (PBS, pH 7.4) for 5 min per rinse between each step. Sections were then permeabilized in 1.0% Triton X-100 for 10 min, and treated with 0.1 M citrate buffer (pH 6.0) at 37°C for 30 min. Subsequently, endogenous peroxidase activity was quenched with 0.6% H2O2 solution in PBS for 20 min. Sections were blocked for 1 h in 2% normal goat serum (cat. No S-1000, Vector Laboratories, Burlingame, CA, USA) and 0.25% Triton X-100 in PBS (PBS-TS). Following incubation in primary antibody solution (rabbit anti-cleaved caspase-3 (Asp 175), 1:200 in PBS; Cell Signaling Technology, Beverly, MA, USA) overnight at 4°C, sections were incubated in secondary antibody solution (biotinylated goat anti-rabbit IgG 1:1000; cat no. BA-1000, Vector Laboratories, Burlingame, CA, USA) in PBS-TS, and washed in PBS before incubation for 1 h in avidin-biotin substrate (ABC kit cat. No. PK-6100, Vector laboratories; Burlingame, CA, USA). Sections were then washed in PBS for 10 min and reacted with 3, 3′-diaminobenzidine tetrahydrochloride (DAB) solution (cat. No. 1856090, Thermo Scientific, Rockford, IL, USA), mounted, dehydrated and coverslipped with mounting medium (Cytoseal 60, Stephens Scientific, Riverdale).
Microscopy and cell quantification
A Nikon Eclipse 600 microscope was used and quantifications were performed using unbiased stereology methodsCitation27,Citation28 by the Stereo Investigator software, version 6 (MicroBrightField, Colchester, VT, USA). Each counting frame (75 μm × 75 μm) was placed at an intersection of the lines forming a virtual grid (100 μm × 100 μm), which was randomly generated and placed by the software within the outline structure. Tunel and cleaved caspase-3 positive cells were counted using the optical fractionator method of unbiased stereologic cell counting techniques. Optical dissectors were 100 μm × 100 μm. Each counting frame was placed at an intersection of the lines forming a virtual grid (200 μm × 200 μm) that was randomly generated and randomly placed by the software within the outline structure. Tunel and cleaved caspase-3 positive cells were counted using a 60× oil lens and were included in the measurement only when they came into focus within the dissector, dissector height of 20 μm and the average thickness of mounted sections was 50 μm; thickness was measured at random intervals through every section and estimated by the software program. Infarct volume as assessed by H&E histochemistry was estimated using the Cavalieri methodCitation27,Citation29, based on the summed cross sectional area estimates from the parallel coronal H&E stained sections with a known distance of 600 µm at a random start.
Statistical analysis
The data are presented as mean ± SEM. A One way Analysis of variance (ANOVA) followed by the Bonferroni post-hoc test were used to compare the differences from the control group. p values less than 0.05 were considered statistically significant. GraphPad Prism version 5.0 for Mac (GraphPad software, San Diego, CA, USA, www.graphpad.com) was used to analyze the data.
Results
Physiological values
Physiological values before and after focal cerebral ischemia are summarized in . There were no significant differences in Mean arterial blood pressure (MAP), heart rate (HR), hemoglobin saturation (Hb), and hematocrit (Ht) among the groups.
Table 1. Physiological Variables before and after Common Carotid Occlusion and Middle Cerebral Occlusion.
Neurological assessment
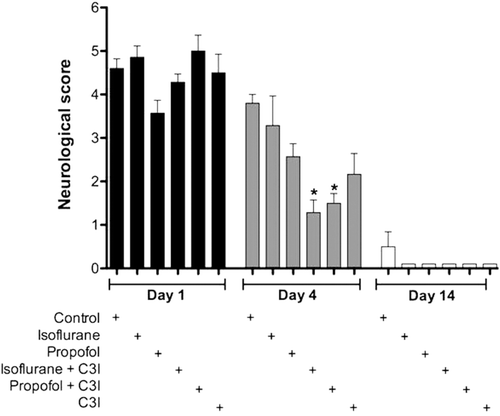
The results of neurological assessment are shown in . At 4 days after permanent focal cerebral ischemia, rats receiving the combination of isoflurane with C3I and propofol with C3I post-ischemia (F5, 37 = 7.32; p < 0.05) showed a significantly better neurological outcome as compared to the control group which received no post-ischemic treatment.
Figure 2. Neurological assessment using the eight-point behavioural rating scale. Behaviour measurements on post-MCAO day 4, showed a significantly better neurological outcome in both groups receiving a combination of an anaesthetic and a caspase 3 inhibitor (C3I). This trend is voided by post-day 14, when all animals approached baseline scores. Data are presented as mean ± SEM. *p < 0.05 versus control group at day 4.
Infarct volume
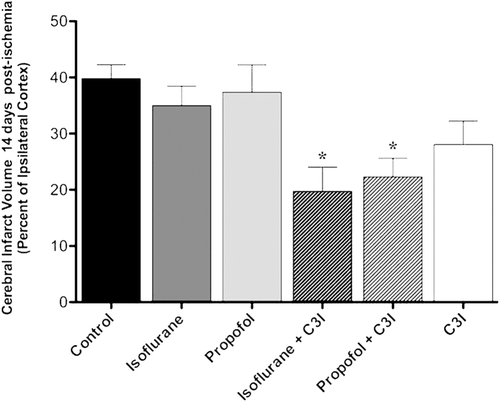
Cerebral infarction volumes are presented in . Post-ischemic administration of anesthetic and C3I combination significantly reduced MCAO- induced infarct volume evaluated on day 14. The infarct volume was significantly less in the isoflurane plus C3I group and the propofol plus C3I treated group as compared to the control group (F5, 30 = 5.361; *p < 0.05).
Figure 3. Cerebral infarction volume in each group measured 14 days following permanent focal ischemia. The graph shows the quantification of the average volume of the infarct 14 days after permanent focal cerebral ischemia as determined by stereological analysis of hematoxylin and eosin histochemistry. Data are presented as mean ± SEM. *p < 0.05 versus control group. C3I: Caspase 3 inhibitor.
Apoptotic DNA fragmentation, TUNEL analysis
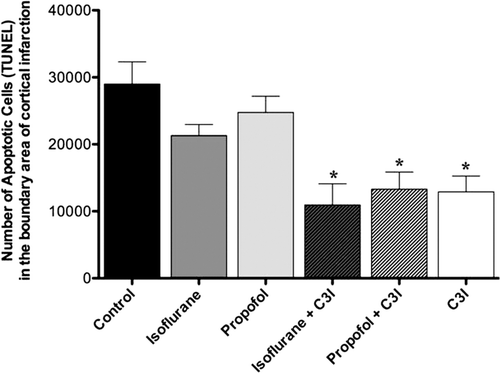
shows the number of apoptotic cells at the margin of the infarction area, assessed on day 14 post-ischemia in all groups. The number of apoptotic cells was significantly decreased in the isoflurane plus C3I group, the propofol plus C3I group, and C3I alone group as compared to the control group (F5, 37 = 7.39, *p < 0.05).
Figure 4. Estimated total number of TUNEL positive cells in the boundary area of cortical infarction 14 days post-ischemia. The number of TUNEL positive cells was significantly decreased in the isoflurane plus C3I, propofol plus C3I, and C3I alone as compared to the control group. Data are presented as mean ± SEM. *p < 0.05 versus control group. C3I: Caspase-3 inhibitor.
Cleaved caspase-3 analysis
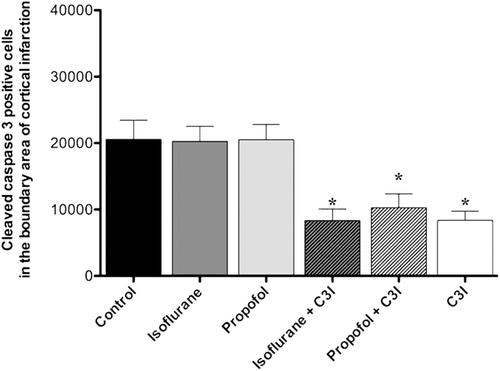
The number of activated caspase-3 positive cells in the boundary area of cortical infarction at 14 days post-ischemia is shown in . Quantitative analysis revealed a significant decrease in the number activated caspase-3 positive cells in the isoflurane plus C3I group, propofol plus C3I group, and C3I group alone as compared to the control group (F5, 37 = 7.01, *p < 0.05). Showing a similar pattern of TUNEL staining.
Figure 5. Estimated total number of cleaved caspase-3 positive cells in the boundary area of cortical infarction. The number of cleaved caspase-3 positive cells was significantly decreased in the animals that received isoflurane+ C3I, propofol + C3I and C3I alone as compared to the control group. Data are presented as mean ± SEM. *p < 0.05 versus control group. C3I: Caspase-3 inhibitor.
Discussion
In our study, combination of isoflurane or propofol anesthesia and caspase-3 inhibitor significantly decreased the volume of the infarct caused by permanent focal cerebral ischemia, when the injury was assessed 14 days following the insult. Both combinations demonstrated greater efficacy than the caspase-3 inhibitor administration alone. On the other hand, either isoflurane or propofol administered alone did not significantly reduce the neuronal damage suggesting that caspase-mediated neuronal apoptosis contributes to the development of ischemic injury so that caspase inhibition results in a sustained neuroprotection.
Sakai et al. (see Ref. Citation5) demonstrated that isoflurane reduces the damage caused by ischemia. They found that infarct volume and neurological score were better in animals treated with isoflurane after 14 days in a model of temporary ischemia. In our experiment with isoflurane alone, we did not find differences in neurological scores after 2 weeks. We believe this is due to differences in the tools used to measure neurologic outcome and in the differences in ischemia models. We used a 8 point neurological score scale that focus only in gross motor deficits while Sakai et al. used a 48 point scale combining assessments of the motor and sensory system. It is possible that our scale did not pick differences that could be present at day 14. Temporary models of ischemia although widely used only reflects 5% or less of the patients that get to the hospital and get thrombolytic treatment. We decided to use a permanent model of ischemia that represents 95% of the patient’s population. In the neurological score we found that the combination of anesthetics with caspase inhibitors substantially improve neurological deficit 4 days after the insult. We believe that the reason is because at day 4 the infarct is still under development and although this is a permanent model of ischemia the caspase inhibitor and the anesthetics work together to diminish cell damage. This is evident by an early improvement in a motor only assessment. It is very important to notice the correlation of findings in our study; the caspase inhibitor in combination with anesthetic was able to improve neurologic outcome at early time points (4 days) but not the caspase inhibitor by it self or the anesthetic alone. The caspase inhibitor it self was able to decrease the number of TUNEL positive cells but was not able to reduce the infarct size. This finding suggest that although there is DNA damage that is been controlled by the caspase inhibitor, it is necessary to block some other mechanisms leading to cell dead in order to have a true positive effect.
Caspase inhibitor administration has been done intracraniallyCitation34,Citation35, mostly due to the blood-brain barrier (BBB) protection. The BBB, formed by endothelial cells lining cerebral microvessels, plays a vital in protecting the neuronal environment from blood-borne agents and under physiologic conditions, this brain capillary endothelium allows selective entry of key nutrients, but prevents a majority of circulating compounds. However, following cerebral ischemia, a disruption of the BBB has been shownCitation30–32. Dynamic temporal changes in the BBB permeability have been described in experimental models of cerebral ischemia. For instance, following transient global cerebral ischemia, BBB permeability has been shown to peak at 1 and 24 h and receding at 6 h after reperfusionCitation33. Moreover, studies looking at BBB disruption following a transient mild MCAO showed a significantly elevated permeability at 24 h, plateaued at 3 days and secondary increased 7 days after MCAOCitation33. Consequently, BBB disruption allows the IP administration of caspase-3 inhibitor in order to assess perinfarct tissues, circumventing the need for invasive intracranial administration.
Our study showed that at day 4, neurological score significantly improved in the groups that received the combination of anesthetic and caspase-3 inhibitor, but not the caspase inhibitor alone, suggesting a neuroprotective effect of the combination. Since the purpose of this experiment was to elucidate the effect of anesthetics (isoflurane or Propofol) and caspase inhibitors, we intentionally anesthetized the animals with Isoflurane during the surgical procedure. Anaesthetics have a preconditioning effect against neuronal damage; the goal was to treat the animals with isoflurane so no other variables of confusion were included in the study. From our study we concluded that even with the possible preconditioning effect added by the isoflurane prior and during the insult, in a model of permanent middle cerebral artery occlusion plus common carotid artery occlusion this effect is not enough to prevent the damage, it is still necessary to add the caspase blocker. Our results also suggest that intraperitoneal injection of caspase-3 inhibitor in combination with isoflurane or propofol has a significant effect in reducing the volume of the infarct, when the injury was assessed at 14 days following the insult. However, tunel-positive cells and cleaved caspase-3-positive cells numbers were significantly decreased by the anaesthetic-caspase inhibitor combination or the caspase-inhibitor alone, but not by the anaesthetics alone.
In conclusion, this study supports the view that improved neurological scores can be achieved after MCAO by a combination of neuroprotective strategies, namely immediate exposure for 90′ to a general anesthetic (either isoflurane or propofol), plus a caspace inhibitor administered intraperitoneally after the ischemic event. The optimal combination of these protective strategies must be ascertained more carefully by future experiments, but the identification of the intraperitoneal route is a useful adjunt for prolonged systemic interventions.
References
- Mackay J, Mensah GA. (2011). The Atlas of Heart Disease and Stroke. Atlanta, GA: World Health Organization; Centers for Disease Control and Prevention.
- Neumar RW. Molecular mechanisms of ischemic neuronal injury. Ann Emerg Med 2000;36:483–506.
- Graham SH, Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab 2001;21:99–109.
- Ginsberg MD. Adventures in the pathophysiology of brain ischemia: penumbra, gene expression, neuroprotection: the 2002 Thomas Willis Lecture. Stroke 2003;34:214–223.
- Sakai H, Sheng H, Yates RB, Ishida K, Pearlstein RD, Warner DS. Isoflurane provides long-term protection against focal cerebral ischemia in the rat. Anesthesiology 2007;106:92–9; discussion 8.
- Kitano H, Kirsch JR, Hurn PD, Murphy SJ. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab 2007;27:1108–1128.
- Matchett GA, Allard MW, Martin RD, Zhang JH. Neuroprotective effect of volatile anesthetic agents: molecular mechanisms. Neurol Res 2009;31:128–134.
- Patel PM, Drummond JC, Cole DJ, Kelly PJ, Watson M. Isoflurane and pentobarbital reduce the frequency of transient ischemic depolarizations during focal ischemia in rats. Anesth Analg 1998;86:773–780.
- Patel PM, Drummond JC, Cole DJ, Goskowicz RL. Isoflurane reduces ischemia-induced glutamate release in rats subjected to forebrain ischemia. Anesthesiology 1995;82:996–1003.
- Bickler PE, Buck LT, Hansen BM. Effects of isoflurane and hypothermia on glutamate receptor-mediated calcium influx in brain slices. Anesthesiology 1994;81:1461–1469.
- Harada H, Kelly PJ, Cole DJ, Drummond JC, Patel PM. Isoflurane reduces N-methyl-D-aspartate toxicity in vivo in the rat cerebral cortex. Anesth Analg 1999;89:1442–1447.
- Adembri C, Venturi L, Tani A, Chiarugi A, Gramigni E, Cozzi A et al. Neuroprotective effects of propofol in models of cerebral ischemia: inhibition of mitochondrial swelling as a possible mechanism. Anesthesiology 2006;104:80–89.
- Wang H, Luo M, Li C, Wang G. Propofol post-conditioning induced long-term neuroprotection and reduced internalization of AMPAR GluR2 subunit in a rat model of focal cerebral ischemia/reperfusion. J Neurochem 2011;119:210–219.
- Velly LJ, Guillet BA, Masmejean FM, Nieoullon AL, Bruder NJ, Gouin FM et al. Neuroprotective effects of propofol in a model of ischemic cortical cell cultures: role of glutamate and its transporters. Anesthesiology 2003;99:368–375.
- Yamaguchi S, Hamaguchi S, Mishio M, Okuda Y, Kitajima T. Propofol prevents lipid peroxidation following transient forebrain ischemia in gerbils. Can J Anaesth 2000;47:1025–1030.
- Chen L, Xue Z, Jiang H. Effect of propofol on pathologic time-course and apoptosis after cerebral ischemia-reperfusion injury. Acta Anaesthesiol Scand 2008;52:413–419.
- Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of Oxidative Stress, Apoptosis, PGC-1a and Mitochondrial Biogenesis in Cerebral Ischemia. Int J Mol Sci 2011;12:7199–7215.
- Thornberry NA. Caspases: key mediators of apoptosis. Chem Biol 1998;5:R97–103.
- Knoblach SM, Alroy DA, Nikolaeva M, Cernak I, Stoica BA, Faden AI. Caspase inhibitor z-DEVD-fmk attenuates calpain and necrotic cell death in vitro and after traumatic brain injury. J Cereb Blood Flow Metab 2004;24:1119–1132.
- Li H, Colbourne F, Sun P, Zhao Z, Buchan AM, Iadecola C. Caspase inhibitors reduce neuronal injury after focal but not global cerebral ischemia in rats. Stroke 2000;31:176–182.
- Ray SK. Currently evaluated calpain and caspase inhibitors for neuroprotection in experimental brain ischemia. Curr Med Chem 2006;13:3425–3440.
- Wiessner C, Sauer D, Alaimo D, Allegrini PR. Protective effect of a caspase inhibitor in models for cerebral ischemia in vitro and in vivo. Cell Mol Biol (Noisy-le-grand) 2000;46:53–62.
- Hara H, Friedlander RM, Gagliardini V, Ayata C, Fink K, Huang Z et al. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci USA 1997;94:2007–2012.
- Schielke GP, Yang GY, Shivers BD, Betz AL. Reduced ischemic brain injury in interleukin-1 beta converting enzyme-deficient mice. J Cereb Blood Flow Metab 1998;18:180–185.
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20:84–91.
- Mackay KB, Bailey SJ, King PD, Patel S, Hamilton TC, Campbell CA. Neuroprotective effect of recombinant neutrophil inhibitory factor in transient focal cerebral ischaemia in the rat. Neurodegeneration 1996;5:319–323.
- Mouton PR. (2002) Principles and Practices of Unbiased Stereology. An introduction for bioscientistss. Baltimore, MD, USA: The Johns Hopkins University Press.
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience 2005;130:813–831.
- Schmitz C, Hof PR. (2007). Design-based stereology in brain aging research. In: Riddle DR. Brain Aging: Models, Methods, and Mechanisms. Boca Raton, FL, USA: Frontiers in Neuroscience.
- Abulrob A, Brunette E, Slinn J, Baumann E, Stanimirovic D. Dynamic analysis of the blood-brain barrier disruption in experimental stroke using time domain in vivo fluorescence imaging. Mol Imaging 2008;7:248–262.
- Mattila OS, Strbian D, Saksi J, Pikkarainen TO, Rantanen V, Tatlisumak T et al. Cerebral mast cells mediate blood-brain barrier disruption in acute experimental ischemic stroke through perivascular gelatinase activation. Stroke 2011;42:3600–3605.
- Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol 2004;56:468–477.
- Haqqani AS, Nesic M, Preston E, Baumann E, Kelly J, Stanimirovic D. Characterization of vascular protein expression patterns in cerebral ischemia/reperfusion using laser capture microdissection and ICAT-nanoLC-MS/MS. FASEB J 2005;19:1809–1821.
- Inoue S, Drummond JC, Davis DP, Cole DJ, Patel PM. Combination of isoflurane and caspase inhibition reduces cerebral injury in rats subjected to focal cerebral ischemia. Anesthesiology 2004;101:75–81.
- Li H, Colbourne F, Sun P, Zhao Z, Buchan AM, Iadecola C. Caspase inhibitors reduce neuronal injury after focal but not global cerebral ischemia in rats. Stroke 2000; 31:176–182.
