Abstract
A novel set of 16 hybrids of bromopyrrole alkaloids with aroyl hydrazone were designed, synthesized and evaluated for antibacterial and antibiofilm activities against methicillin-resistant Staphylococcus aureus (MRSA; ATCC 43866), methicillin-susceptible Staphylococcus aureus (MSSA; ATCC 35556) and Staphylococcus epidermidis (SE, S. epidermidis ATCC 35984). Of the 16 tested hybrids, 14 exhibited equal or superior antibiofilm activity against MSSA and MRSA relative to standard vancomycin. Compound 4m showed highest potency with antibiofilm activity of 0.39 µg/mL and 0.78 µg/mL against MSSA and MRSA, respectively. Thus, this compound could act as a potential lead for further development of new antistaphylococcal drugs.
Introduction
Staphylococcus aureus is a major cause of metal-biomaterial infections, whereas Staphylococcus epidermidis (SE) is found mostly in polymer-biomaterial-associated infectionsCitation1. Biofilm growth of methicillin-resistant S. aureus (MRSA) is common during hemodialysisCitation2 and among patients bearing indwelling medical devicesCitation3. In the USA, 12–25% of MRSA catheter-related blood stream infections result in mortality, with annual patient care costs projected to be as high as $460 millionCitation4. When enclosed within a biofilm, MRSA exhibits increased resistance to multiple classes of antibioticsCitation5. Current strategies for targeting staphylococcal pathogens includes interference with cell–cell signaling by modulating quorum sensingCitation6, targeting slime formation by inhibiting bacterial cell wall synthesisCitation7, impairing adhesion using biosurfactantsCitation8 and immunotherapyCitation9. The challenge of staphylococcal strains resistant to most or all conventional antibiotics could be faced by developing new antibacterial agents with different chemical characteristics and mechanism of action with respect to current antibiotics.
Bromopyrrole alkaloids represent an important family of marine alkaloids produced by marine sponges with diverse biological activitiesCitation10–13. Many of these molecules are identified by the presence of 4,5-dibromopyrrole ring with oroidin as their prototype alkaloid known for its antibiofilm activityCitation10. Recently, a few reports on synthesis of marine bromopyrrole alkaloid derivatives containing 1,3,4-oxadiazole and thiazolidinone features for their antimicrobial and antibiofilm properties have been publishedCitation14,Citation15. Synthesized 1,3,4-oxadiazole derivatives (E1–E8) showed antimicrobial activity against representative Gram-negative and Gram-positive bacteria. Herein, eight of twenty tested derivatives showed equal antibacterial activity compared to standard ciprofloxacin against S. aureus (MIC – 1.56 μg/mL). We further derivatized 1-methyl-4,5-dibromopyrrole core with 4-thiazolidinone feature and tested for its antibiofilm potential against few Gram-positive bacteria. Antibiofilm activity of 4-thiazolidinones, F1 and F2 (MIC = 0.78 μg/mL) was 3-fold superior than standard vancomycin (MIC = 3.125 μg/mL), while activity of compounds F3, F4, F5 and F6 was 2-fold (MIC = 1.56 μg/mL) against S. aureus biofilm. Compounds F1–F7 showed equal antibiofilm activity against S. epidermidis compared to standard Vancomycin (MIC = 3.125 μg/mL) ().
Figure 1. (a) Reported bromopyrrole alkaloids and aroyl hydrazones with antibacterial and antibiofilm activities. (b) Design of novel N′-arylidene-4,5-dibromo-1-methyl-1H-pyrrole-2-carbohydrazides using molecular hybridization approach.
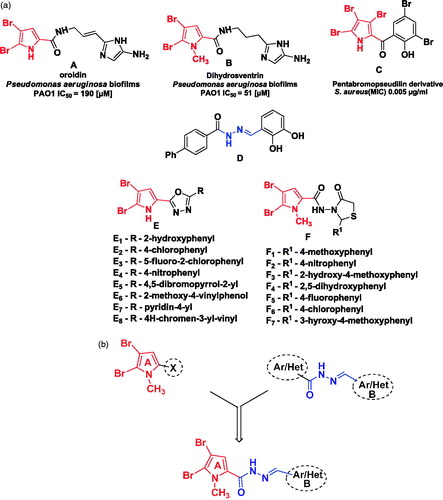
Aroyl hydrazones constitute an important class of privileged scaffold for new drug development. Aroyl hydrazone derivatives have been known to possess diverse array of biological effects like anticonvulsant, analgesic, anti-inflammatory, antimalarial and antitumor activity. Also, aroyl hydrazone moiety is common among many of antimicrobial and antimycobacterial agentsCitation16. High-throughput screening performed on a library of small molecules indicated aroyl hydrazone as biofilm development inhibitorCitation17 ().
As extension of our previous work and with the aim to obtain more effective compounds, we thought to be of interest to design and synthesize a series of molecular hybrids of 1-methyl-4,5-dibrompyrrole core and aroyl hydrazone as anti-staphylococcal agents (). The phenyl ring of these hybrids is substituted with different groups or atoms in order to obtain a good discrimination of antistaphylococcal activity among the compounds. All the new compounds were tested for antibacterial and antibiofilm activity against bacterial strains of S. aureus, including MRSA and SE.
Materials and methods
Chemistry
Instrumentation
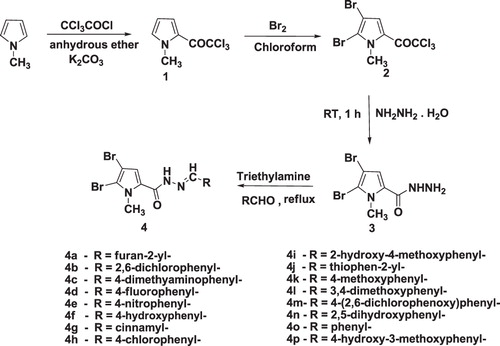
All reactions were carried out under an inert nitrogen atmosphere under anhydrous conditions and by using molecular sieves (4 Å 1/16 inches pellets), ethanol and acetonitrile were freshly distilled from CaCl2. All chromatographic solvents were distilled before use. Silica gel of 60–120 and 200–400 mesh was used for column and flash chromatography. Some of the starting materials were obtained from S. D. Fine Pvt. Ltd. (Mumbai, India), SRL (Mumbai, India), Spectrochem (Mumbai, India)/Aldrich (India) and some of them were prepared in the laboratory and were used without further purification. All melting points were recorded on Thermomik Campbell electronics (India), having oil-heating system and were uncorrected. Analytical thinlayer chromatography (TLC) was carried out on precoated SiO2 plates (silica gel 60, F 254, Merck, India). Fourier transform infrared spectroscopy spectra were recorded on Perkin Elmer RX I spectrometer using KBr pellets. All the nuclear magnetic resonance (NMR) spectra were recorded on JEOL AL-300 FT-NMR spectrometer (Tokyo, Japan) with dimethyl sulfoxide (DMSO)-d6 as solvent using tetramethyl silane as internal reference. Mass spectra were obtained on Thermo Finnigan LCQ advantage max (LCMS) (Washington). Compounds 1, 2 and 3 were prepared according to reported methodsCitation9,Citation10. Evaporations of final product solutions were done under vacuum with a rotatory evaporator.
Synthesis of 4,5-dibromo-N′-arylidene-1-methyl-1H-pyrrole-2-carbohydrazide analogs 4
Appropriate aldehydes (27.5 mmol) and compound 3 (6, 27.5 mmol), were stirred in 30 mL absolute ethanol in a round-bottomed flask. A quantity of 1–2 mL of triethylamine was added to the reaction mixture and was refluxed for 4–7 h. Most of the 4,5-dibromo-1-methyl-1H-pyrrole-2-carbohydrazide was consumed at this time (monitored by TLC), the reaction mixture was concentrated under reduced pressure and the obtained solid residue was partitioned between 50 mL water and 50 mL chloroform to compound 4 in organic layer. Column purification using flash chromatography (SiO2, 10% MeOH/CHCl3) of the crude product yielded pure 4,5-dibromo-N′-arylidene-1-methyl-1Hpyrrole-2-carbohydrazide. The obtained solid was recrystallized from MeOH.
4,5-dibromo-N′-(furan-2-ylmethylene)-1-methyl-1H-pyrrole-2-carbohydrazide (4a)
Yield: 78%; m.p.: 188–190 °C; IR (KBr): Vmax/cm 1620 (C=C), 1643 (CH=N), 1715 (C=O), 3206 (NH); 1HNMR (300 MHz, DMSO-d6): δ 3.59 (s, 3H pyrrole N-CH3), 6.43 (s, 1H pyrrole 3H), 6.7–7.4 (m, 3H ArH), 8.40 (s, 1H azometine H), 11.52 (s, 1H CONH); 13C NMR (75 MHz, DMSO-d6): δ 157.4, 149.3, 144.1, 134.3, 127.8, 126.3, 118.6, 114.5, 112.6, 94.6, 37.8; MS m/z: 373.9152 [M]+, 375.9059 [M + 2]+, 377.9063 [M + 4]+.
4,5-dibromo-N′-(2,6-dichlorobenzylidene)-1-methyl-1H-pyrrole-2-carbohydrazide (4b)
Yield: 78%; m.p.: 256–258 °C; IR (KBr): Vmax/cm 1628 (C=C), 1644 (CH=N), 1719 (C=O), 3209 (NH); 1HNMR (300 MHz, DMSO-d6): δ 3.56 (s, 3H pyrrole N-CH3), 6.46 (s, 1H pyrrole 3H), 6.9–7.67 (m, 3H ArH), 8.42 (s, 1H azometine H), 11.57 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 157.4, 138.1, 133.5, 130.5, 129.4, 128.3, 127.6, 126.2, 114.5, 94.5, 37.7; MS m/z: 451.8491 [M]+, 453.8492 [M + 2]+, 455.8494 [M + 4]+.
4,5-dibromo-N′-(4-(dimethylamino)benzylidene)-1-methyl-1H-pyrrole-2-carbohydrazide (4c)
Yield: 80%; m.p.: 216–218 °C; IR (KBr): Vmax/cm 1623 (C=C), 1646 (CH=N), 1718 (C=O), 3212 (NH); 1HNMR (300 MHz, DMSO-d6): δ 3.12 (s, 6H CH3), 3.58 (s, 3H pyrrole N-CH3), 6.42 (s, 1H pyrrole 3H), 6.7–7.4 (m, 4H ArH), 8.43 (s, 1H azometine H), 11.67 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 157.8, 153.5, 146.9, 128.1, 127.5, 126.1, 123.4, 114.6, 112.1, 94.7, 42, 37.6; MS m/z: 426.9690 [M]+, 428.9693 [M + 2]+, 430.9697 [M + 4]+.
4,5-dibromo-N′-(4-fluorobenzylidene)-1-methyl-1H-pyrrole-2-carbohydrazide (4d)
Yield: 74%; m.p.: 204–206 °C; IR (KBr): Vmax/cm 1618 (C=C), 1641 (CH=N), 1715 (C=O), 3206 (NH); 1HNMR (300 MHz, DMSO-d6): δ 3.59 (s, 3H pyrrole N-CH3), 6.43 (s, 1H pyrrole 3H), 6.9–7.8 (m, 4H ArH), 8.40 (s, 1H azometine H), 11.64 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 165.3,157.5,146.5, 130.3, 129.4, 127.4, 126.3, 115, 114.2, 94.6, 37.7; MS m/z: 401.9178 [M]+, 403.9180 [M + 2]+, 405.9182 [M + 4]+.
4,5-dibromo-1-methyl-N′-(4-nitrobenzylidene)-1H-pyrrole-2-carbohydrazide (4e)
Yield: 80%; m.p.: 242–246 °C; IR (KBr): Vmax/cm 1620 (C=C), 1644 (CH=N), 1714 (C=O), 3204 (NH); 1HNMR (300 MHz, DMSO-d6): δ 3.53 (s, 3H pyrrole N-CH3), 6.41 (s, 1H pyrrole 3H), 6.8–7.8 (m, 4H ArH), 8.41 (s, 1H azometine H), 11.58 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 157.6, 150.4, 146.5, 139.7, 127.4, 126.4, 124, 123.8, 114.5, 94.3, 37.8; MS m/z: 428.9124 [M]+, 430.9127 [M + 2]+, 432.9129 [M + 4]+.
4,5-dibromo-N′-(4-hydroxybenzylidene)-1-methyl-1H-pyrrole-2-carbohydrazide (4f)
Yield: 88%; m.p.: 234–236 °C; IR (KBr): Vmax/cm 1624 (C=C), 1643 (CH=N), 1721 (C=O), 3204 (NH), 3410 (OH); 1HNMR (300 MHz, DMSO-d6): δ 3.58 (s, 3H pyrrole N-CH3), 6.49 (s, 1H pyrrole 3H), 5.34 (s,1H OH), 6.64–7.63 (m, 4H ArH), 8.48 (s, 1H azometine H), 11.66 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 160.3, 157.8, 146.5, 130.4, 127.5, 126.1, 125.9, 116.5, 114, 94.3, 37.8; MS m/z: 399.9221 [M]+, 401.9223 [M + 2]+, 403.9225 [M + 4]+.
4,5-dibromo-1-methyl-N′-(3-phenylallylidene)-1H-pyrrole-2-carbohydrazide (4g)
Yield: 78%; m.p.: 218–220 °C; IR (KBr): Vmax/cm 1610 (C=C), 1649 (CH=N), 1723 (C=O), 3213 (NH); 1HNMR (300 MHz, DMSO-d6): δ 3.55 (s, 3H pyrrole N-CH3), 6.44 (s, 1H pyrrole 3H), 6.2-7.12 (dd, 2H CH=CH), 7.4–7.8 (m, 5H ArH), 8.43 (s, 1H azometine H), 11.62 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 157.3, 137.2, 135.6, 134.1, 128.6, 128.4, 127.9, 127.4, 126.4, 126, 114.5, 94.6, 37.9; MS m/z: 409.9427 [M]+, 411.9430 [M + 2]+, 413.9432 [M + 4]+.
4,5-dibromo-N′-(4-chlorobenzylidene)-1-methyl-1H-pyrrole-2-carbohydrazide (4h)
Yield: 87%; m.p.: 216–218 °C; IR (KBr): Vmax/cm 1612 (C=C), 1644 (CH=N), 1712 (C=O), 3209 (NH); 1HNMR (300 MHz, DMSO-d6): δ 3.58 (s, 3H pyrrole N-CH3), 6.46 (s, 1H pyrrole 3H), 6.87–7.4 (m, 4H ArH), 8.42 (s, 1H azometine H), 11.59 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 157.2, 146.9, 136.3, 131.2, 130.2, 128.4, 127.4, 126.4, 114.5, 94.5, 37.8; MS m/z: 417.8879 [M]+, 419.8881 [M + 2]+, 421.8883 [M + 4]+.
4,5-dibromo-N′-(2-hydroxy-4-methoxybenzylidene)-1-methyl-1H-pyrrole-2-carbohydrazide (4i)
Yield: 76%; m.p.: 194–196 °C; IR (KBr): Vmax/cm 1620 (C=C), 1641 (CH=N), 1719 (C=O), 3212 (NH), 3440 (OH); 1HNMR (300 MHz, DMSO-d6): δ 3.28 (s, 3H OCH3), 3.57 (s, 3H pyrrole N-CH3), 6.47 (s, 1H pyrrole 3H), 5.38 (s, 1H OH), 6.9–7.42 (m, 3H ArH), 8.38 (s, 1H azometine H), 11.54 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 164.4, 162.2, 157.3, 146.2, 133.2, 127.9, 126.2, 114.2, 110.8,107.2,103.2, 94.2, 55.8, 37.7; MS m/z: 429.9325 [M]+, 431.9328 [M + 2]+, 433.9331 [M + 4]+.
4,5-dibromo-1-methyl-N′-(thiophen-2-ylmethylene)-1H-pyrrole-2-carbohydrazide (4j)
Yield: 78%; m.p.: 230–232 °C; IR (KBr): Vmax/cm 1614 (C=C), 1637 (CH=N), 1716 (C=O), 3208 (NH); 1HNMR (300 MHz, DMSO-d6): δ 3.58 (s, 3H pyrrole N-CH3), 6.47 (s, 1H pyrrole 3H), 6.67–7.2 (m, 3H ArH), 8.41 (s, 1H azometine H), 11.60 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 157.4, 144.8, 130.6, 128, 127.4, 126.9, 125, 124.9, 114.5, 94.6, 37.8; MS m/z: 389.8836 [M]+, 391.8839 [M + 2]+, 393.8841 [M + 4]+.
4,5-dibromo-N′-(4-methoxybenzylidene)-1-methyl-1H-pyrrole-2-carbohydrazide (4k)
Yield: 80%; m.p.: 115–117 °C; IR (KBr): Vmax/cm 1619 (C=C), 1646 (CH=N), 1710 (C=O), 3204 (NH); 1HNMR (300 MHz, DMSO-d6): δ 3.12 (s, 3H OCH3), 3.54 (s, 3H pyrrole N-CH3), 6.43 (s, 1H pyrrole 3H), 6.74–7.1 (m, 4H ArH), 8.49 (s, 1H azometine H), 11.58 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 162.8, 157.7, 146.7, 130.1, 127.3, 126.0, 114.5, 114.3, 94.3, 55.7, 37.8; MS m/z: 413.9376 [M]+, 415.9379 [M + 2]+, 417.9382 [M + 4]+.
4,5-dibromo-N′-(3,4-dimethoxybenzylidene)-1-methyl-1H-pyrrole-2-carbohydrazide (4l)
Yield: 85%; m.p.: 220–222 °C; IR (KBr): Vmax/cm 1621 (C=C), 1644 (CH=N), 1713 (C=O), 3214 (NH); 1HNMR (300 MHz, DMSO-d6): δ 3.14 (s, 3H OCH3), 3.23 (s, 3H OCH3), 3.59 (s, 3H pyrrole N-CH3), 6.46 (s, 1H pyrrole 3H), 6.67–7.14 (m, 3H ArH), 8.48 (s, 1H azometine e H), 11.62 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 157.8, 152.2, 149.8, 146.7, 130.7, 127.5, 126.2,122.6, 114.6, 111.8, 109.3, 94.6, 56.2, 37.8; MS m/z: 443.9482 [M]+, 445.9484 [M + 2]+, 447.9487 [M + 4]+.
4,5-dibromo-N′-(4-(2,6-dichlorophenoxy)benzylidene)-1-methyl-1H-pyrrole-2-carbohydrazide (4m)
Yield: 88%; m.p.: 212–214 °C; IR (KBr): Vmax/cm 1620 (C=C), 1642 (CH=N), 1717 (C=O), 3207 (NH); 1HNMR (300 MHz, DMSO-d6): δ 3.55 (s, 3H pyrrole N-CH3), 6.47 (s, 1H pyrrole 3H), 7.2–7.9 (m, 7H ArH), 8.47 (s, 1H azometine H), 11.64 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 159.4, 157.7, 146.8, 146.6, 129.8, 129.1, 128.9, 127.5, 127.2, 126.8, 126.0, 117.7, 114.5, 94.6, 37.9; MS m/z: 543.8755 [M]+, 545.8757 [M + 2]+, 547.8760 [M + 4]+.
4,5-dibromo-N′-(2,5-dihydroxybenzylidene)-1-methyl-1H-pyrrole-2-carbohydrazide (4n)
Yeild: 80%; m.p.: 230–232 °C; IR (KBr): Vmax/cm 1622 (C=C), 1645 (CH=N), 1709 (C=O), 3209 (NH), 3440 (OH); 1HNMR (300 MHz, DMSO-d6): δ 3.54 (s, 3H pyrrole N-CH3), 5.28 (s, 1H OH), 5.36 (s, 1H OH), 6.43 (s, 1H pyrrole 3H), 6.97–7.84 (m, 3H ArH), 8.41 (s, 1H azometine H), 11.66 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 157.8, 153.8, 151.3, 146.0, 127.5, 126.0, 120.5, 119.9, 119.5, 116.4, 114.6, 94.6, 37.8; MS m/z: 415.9168 [M]+, 417.9169 [M + 2]+, 419.9171 [M + 4]+.
4,5-dibromo-N′-benzylidene-1-methyl-1H-pyrrole-2-carbohydrazide (4o)
Yield: 86%; m.p.: 212–214 °C; IR (KBr): Vmax/cm 1625 (C=C), 1641 (CH=N), 1711 (C=O), 3214 (NH); 1HNMR (300 MHz, DMSO-d6): δ 3.56 (s, 3H pyrrole N-CH3), 6.42 (s, 1H pyrrole 3H), 6.67–7.36 (m, 5H ArH), 8.42 (s, 1H azometine H), 11.62 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 157.5, 146.7, 133.6, 131.1, 129.1, 128.7, 127.5, 126.1, 114.6, 94.6, 37.9; MS m/z: 383.9270 [M]+, 385.9273 [M + 2]+, 387.9276 [M + 4]+.
4,5-dibromo-N′-(4-hydroxy-3-methoxybenzylidene)-1-methyl-1H-pyrrole-2-carbohydrazide (4p)
Yield: 80%; m.p.: 248–250 °C; IR (KBr): Vmax/cm 1627 (C=C), 1649 (CH=N), 1715 (C=O), 3211 (NH), 3436 (OH); 1HNMR (300 MHz, DMSO-d6): δ 3.16 (s, 3H OCH3), 3.58 (s, 3H pyrrole N-CH3), 5.34 (s, 1H OH) 6.47 (s, 1H pyrrole 3H), 6.7–7.4 (m, 3H ArH), 8.49 (s, 1H azometine H), 11.60 (s, 1H CONH); Citation13C NMR (75 MHz, DMSO-d6): δ 157.7, 151.2, 149.4, 146.9, 131.0, 127.5, 126.1, 122.9, 117.1, 114.6, 112.2, 94.6, 56.2, 37.6; MS m/z: 429.9326 [M]+, 431.9328 [M + 2]+, 433.9330 [M + 4]+.
Antibacterial study
The newly prepared compounds were screened for their minimal inhibitory concentration (MIC), minimal bactericidal concentration (MBC) and antibiofilm concentration assay against MRSA (ATCC 43866), methicillin-susceptible S. aureus (MSSA, ATCC 35556) and SE (ATCC 35984) bacterium using a standard tube-dilution assay (). MIC assays for the antibacterial activities of the compounds were performed according to the broth microdilution (in tubes) method of the Clinical and Laboratory Standards Institute (CLSI) of AmericaCitation18. MBC of the compounds was obtained by subculturing 100 µL from each negative (no visible bacterial growth) tube from the MIC assay, onto substance-free Mueller–Hinton agar plates. The plates were incubated at 37 °C for 24 h, and the MBC was defined as the lowest concentration of compounds that produced subcultures growing no more than five colonies on each plate.
Table 1. Antibacterial and antibiofilm test results*.
Antibiofilm study
Detection of biofilm formation by tissue culture plate method
Tissue culture plate (TCP) assay described by Christensen et al.Citation19 is most widely used and was considered as standard test for detection of biofilm formation. In present study, we screened MRSA (ATCC 43866), MSSA (ATCC 35556) and SE (ATCC 35984) bacterium for their ability to form biofilm by TCP method as described by Christensen et al. with a modification in duration of incubation, which was extended to 24 h. Isolates from fresh agar plates were inoculated in respective media and incubated for 18 h at 37 °C in stationary condition and diluted 1 in 100 with tryptic soy broth (TSB). Individual wells of sterile, polystyrene, 96-well flat-bottom tissue culture plates were filled with 0.2 mL aliquots of the diluted cultures and only broth served as control to check sterility and nonspecific binding of media.
The tissue culture plates were incubated for 18 h and 24 h at 37 °C. After incubation, content of each well was gently removed by tapping the plates. The wells were washed four times with 0.2 mL of phosphate buffer saline (PBS, pH 7.2) to remove free-floating “planktonic” bacteria. Biofilms formed by adherent “sessile” organisms in the plate were fixed with sodium acetate (2%) and stained with crystal violet (0.1% w/v). Excess stain was rinsed off by thorough washing with de-ionized water and plates were kept for drying. Adherent staphylococcal cells usually formed biofilm on all side wells and were uniformly stained with crystal violet. Optical densities (ODs) of stained adherent bacteria were determined with a micro ELISA auto reader (California) at wavelength of 570 nm (OD 570 nm). These OD values were considered as an index of bacteria adhering to surface and forming biofilms. Experiment was performed in triplicate and repeated three times, the data were then averaged and standard deviation was calculated. To compensate for background absorbance, OD readings from sterile medium, fixative and dye were averaged and subtracted from all test values. The mean OD value obtained from media control well was deducted from all the test OD values ().
Table 2. Screening of various staphylococcal strains for biofilm formation by TCP method in tryticase soy broth with 1% glucose at 18 and 24 h of incubation.
Antibiofilm assay
In the antibiofilm concentration experiment, overnight culture of bacterium (MRSA (ATCC 43866), MSSA (ATCC 35556) and SE (ATCC 35984) was diluted 1:10 in TSB (OD 600 = 0.6–0.8), then was diluted 1:200 in Mueller--Hinton. The bacterial suspension was inoculated into the wells of sterile 96-well polystyrene microtiter plates (Falcon) incubated at 37 °C for 6 h. The plates with young biofilm were washed gently four times with sterile PBS before adding fresh TSB containing the various concentrations of compounds and incubated at 37 °C for 16 h. The initiate dilution was 200 µM. MIC values were defined as the lowest concentration at which no visible growth is observed. For all assays, reference standard used was vancomycinCitation20.
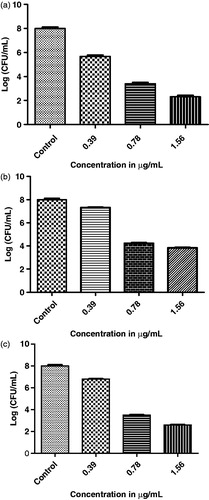
In order to study effectiveness of most active compound 4m on older biofilm (24 h) of MSSA, MRSA and SE, log reduction in the viable count was determined according to Filoche et al.Citation21 with some modifications. In brief, 100 µL of nutrient medium and 10 µL of microorganism cultures were placed in 96-well plates (Cellstar®) and incubated for 24 h at 37 °C in order to establish biofilm formation. Following growth (24 h), the supernatant was removed and each well was rinsed with sterile saline three times. Subsequently, the first experimental well was filled with double-strength nutrient medium, while the other wells were filled with single-strength nutrient medium (150 µL). The ethanol extract or volatile oil (150 µL) was added to the first well. Positive and negative controls were used. Double-fold serial dilution was then carried out across the plate and the plates were incubated for 24 h at 37 °C. After incubation, cultures were removed from the wells showing no turbidity and wells were rinsed three times with sterile saline to remove unattached bacteria. Attached cells were removed and quantified by viable cell counts. In order to do this, attached cells were removed from wells’ surface using a sterile needle. This step was validated by staining the recovered wells with crystal violet (1%) and visual examination after rinsing three times with saline. Viable counts were performed using 10-fold serial dilution. Attached microbial populations were expressed as colony forming unit per well (CFU/well). The method for each strain was carried out in triplicate and the standard mean of error was calculated. The graphical result for log reduction of CFU/mL versus concentration is presented in .
Figure 2. (a) Evaluation of antibiofilm activity of compound 4m by Log reduction in viable counts of MSSA (ATCC 35556). (b) Evaluation of antibiofilm activity of compound 4m by Log reduction in viable counts of MRSA (ATCC 43866). (c) Evaluation of antibiofilm activity of compound 4m by Log reduction in viable counts of SE (ATCC 35984).
Results and discussion
Chemistry
In this study, 1-methyl-4,5-dibromo-1H-pyrrole-2-carbohydrazide derivatives were synthesized utilizing the reaction sequence, as shown in . Trichloroacetylation of 1-methylpyrrole gave an excellent yield of compound 1Citation22. Compound 1 was brominated using bromine in chloroform to give compound 2Citation23. Compound 2 was further converted into corresponding hydrazide compound 3 by stirring with hydrazine hydrate at room temperature. Compound 3 on condensation with respective aldehydes gave 4,5-dibromo-1-methyl-1H-pyrrole-2-carbohydrazides, i.e. compound 4. The spectral data (infra red (IR), 1H NMR and mass spectra (MS)) of all synthesized compounds were in agreement with the proposed structures. The MS of all the compounds exhibited the [M+] as molecular ion peak, confirming the molecular weight. Presence of dibromo was confirmed with the presence of [M + 2]+, [M + 4]+ peaks in mass spectra. The 1H-NMR spectra of all compounds 4a–4p showed single at δ 3.5 corresponding to N-CH3 attached to pyrrole core. Proton at third position of pyrrole core resonates at δ6.4. N-H proton of CONHN=CH group resonated in between δ11.7 and 11.5. The azometine proton to which aromatic ring B is attached showed a singlet at around δ 8.5–8.3. The aryl protons of ring B resonated in the range of δ 7.9–6.6.
Biological results
The synthesized series of compounds showed some promising antibiofilm activity especially against MSSA and MRSA. As indicated in , most of synthesized compounds showed potent to moderate antibacterial and antibiofilm activity against all tested Gram-positive bacterium. These derivatives are more active against biofilm produced by MRSA and MSSA compared toSE. All tested compounds exhibited equal or more antibiofilm potency against MSSA and MRSA compared to standard vancomycin, except compounds 4g and 4o. In general, substitution of benzene ring at 4-position is favorable for antibacterial and antibiofilm activity. Substituents likes fluoro, chloro, nitro, hydroxyl, methoxy and 4-(2,6-dichlorophenoxy)phenyl placed at 4-postion of benzene ring, resulted in favorable effect on antibiofilm activity relative to the unsubstituted compound 4o. Herein, 4-(2,6-dichlorophenoxy)phenyl-substituted compound 4m was more active compared to other compounds (MIC: 0.39 µg/mL MSSA, 0.79 µg/mL MRSA). Activity of compound 4m may be attributed to the presence of para-substituted phenyl ring with oxygen linker that adds drug-like features to it. Next, the molecules with substitution at ortho- or meta-position of aromatic rings did not exhibit increased potency relative to para-substituted molecules. Further, heteroaromatic ring system was incorporated as in compounds 4a and 4j instead of phenyl ring. Both these derivatives showed better activity indicating that a suitable substituted heteroaromatic ring system can increase the potency of the compounds. Increasing the length of aroyl hydrazone linker using cinnamyl substituent decreases the antibiofilm activity relative to compound 4o.
Antibiofilm activity of compounds 4a, 4c, 4d, 4e, 4f and 4h was 3-fold superior (MIC = 0.78 µg/mL) to that of standard vancomycin (MIC = 3.125 µg/mL) against MSSA, while that of compounds 4b, 4i, 4j, 4k, 4l and 4n (MIC = 1.56 µg/mL) was 2-fold superior than MSSA. For antibiofilm activity against MRSA; compounds 4a, 4b, 4c, 4f and 4j exhibited 3-fold potency (MIC = 1.56 µg/mL) compared to standard vancomycin (MIC = 6.25 µg/mL), whereas activity of compounds 4d, 4e, 4h, 4i, 4k, 4l, 4n and 4p was found to be 2-fold superior to that of standard. Hybrid compound (4m) displayed four times the potency relative to standard vancomycin against biofilms of MSSA and MRSA with MIC of 0.39 µg/mL and 0.78 µg/mL, respectively. Further, the antistaphylococcal potential of most active hybrid compound (4m) was validated by determining antibacterial and antibiofilm MICs of its intermediates. The antibacterial and antibiofilm MICs obtained for 1-methyl-1H-pyrrole-2-carbohydrazide compound 3 and 4-(2,6-dichlorphenoxy)-benzaldehyde against all tested bacterium were ≥100 µg/mL. All the tested compounds showed less antibiofilm activity against SE (MIC = 25 µg/mL) compared to standard vancomycin. All the compounds exhibited similar antibacterial profile on planktonic and biofilm stages, which can be beneficial as it increases their spectrum of activities.
Conclusion
In conclusion, 16 novel hybrids of aroyl hydrazone and marine bromopyrrole alkaloids were designed, synthesized and evaluated for their antistaphylococcal activity. The synthesized hybrids showed promising activity against MSSA and MRSA. Biological data revealed that substitution of phenyl ring at 4-postion is favorable for antibiofilm activity against MSSA and MRSA. The antibiofilm activity at concentrations as low as 0.39 µg/mL and 0.78 µg/mL shown by compound 4m against MSSA and MRSA, respectively, indicates that this compound can act as novel lead for development of more potent antistaphylococcal agent. Further studies on these compounds and optimization of their structures leading to novel analogs with superior antimicrobial properties are ongoing in our laboratories.
Declaration of interest
There is no conflict of interest.
Acknowledgements
R.A.R. thanks Professor R. S. Gaud for providing facility to carry out these experiments.
References
- Leclercq R. Epidemiological and resistance issues in multidrug-resistant staphylococci and enterococci. Clin Microbiol Infect 2009;15:224–31
- Reed SD, Friedman JY, Engemann JJ, et al. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. J Infect Control Hosp Epidemiol 2005;26:175–83
- Mohan SS, McDermott BP, Cunha BA. Methicillin-resistant Staphylococcus aureus prosthetic aortic valve endocarditis with paravalvular abscess treated with daptomycin. Heart Lung 2005;34:69–71
- Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med 2000;132:391–402
- Raad I, Hanna H, Jiang Y, et al. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant staphylococcus bacteremic isolates embedded in biofilm. Antimicrob Agents Chemother 2007;51:1656–60
- Cirioni O, Giacometti A, Ghiselli R, et al. Prophylactic efficacy of topical temporin A and RNAIIIinhibiting peptide in a subcutaneous rat Pouch model of graft infection attributable to staphylococci with intermediate resistance to glycopeptides. Circulation 2003;108:767–71
- Burton E, Gawande PV, Yakandawala N, et al. Antibiofilm activity of GlmU enzyme inhibitors against catheterassociated uropathogens. Antimicrob Agents Chemother 2006;50:1835–40
- Rodrigues LR, Banat IM, van der Mei HC, et al. Interference in adhesion of bacteria and yeasts isolated from explanted voice prostheses to silicone rubber by rhamnolipid biosurfactants. J Appl Microbiol 2006;100:470–80
- Bryers JD, Ratner BD. Bioinspired implant materials befuddle bacteria. ASM News 2004;70:232–7
- Aiello A, Fattorusso E, Menna M, Taglialatela-Scafati O. Modern alkaloids structure, isolation, synthesis and biology. Weinheim: Wiley-VCH; 2008:271
- Tasdemir D, Topaloglu B, Perozzo R, et al. Marine natural products from the Turkish sponge Agelas oroides that inhibit the enoyl reductases from Plasmodium falciparum, Mycobacterium tuberculosis and Escherichia coli. Bioorg Med Chem 2007;15:6834–45
- Xiong S, Pang HD, Fan J, et al. In vitro and in vivo antineoplastic activity of a novel bromopyrrole and its potential mechanism of action. Br J Pharmacol 2010;159:909–18
- Schillaci D, Petruso S, Cascioferro S, Raimondi MV. In vitro anti-Gram-positive and antistaphylococcal biofilm activity of newly halogenated pyrroles related to pyrrolomycins. Int J Antimicrob Agents 2008;31:380–99
- Rane RA, Gutte SD, Sahu NU. Synthesis and evaluation of novel 1,3,4-oxadiazole derivatives of marine bromopyrrole alkaloids as antimicrobial agent. Bioorg Med Chem Lett 2012;22:6429–32
- Rane RA, Sahu NU, Shah CP. Synthesis and antibiofilm activity of marine natural product-based 4-thiazolidinones derivatives. Bioorg Med Chem Lett 2012;22:7131–4
- Rollas S, Küçükgüzel ŞG. Biological activities of hydrazone derivatives. Molecules 2007;12:1910–39
- Junker LM, Clardy J. High-throughput screens for small-molecule inhibitors of Pseudomonas aeruginosa biofilm development. J Antimicrob Agents and Chemother 2007;51:3582–90
- Matthew A, Cockerill FR, Craig WA, et al. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, M7-A7. 7th ed. Vol. 26. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2006: 1–49
- Christensen GD, Simpson WA, Younger JA, et al. Adherence of cogulase negative Staphylococi to plastic tissue cultures: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 1985;22:996–1006
- Pan B, Huang R, Han S, et al. Design, synthesis, and antibiofilm activity of 2-arylimino-3-aryl-thiazolidine-4-ones. Bioorg Med Chem Lett 2010;20:2461–4
- Filoche SK, Soma K, Sissons CH. Antimicrobial effects of essential oils in combination with chlorhexidine digluconate. Oral Microbiol Immunol 2005;20:221–5
- Bailey DM, Johnson RE, Salvador JU. Pyrrole antibacterial agents. 1. Compounds related to pyoluteorin. J Med Chem 1973;16:1298–300
- Linington RG, Williams DE, Tahir A, et al. Latonduines A and B, new alkaloids isolated from the marine sponge Stylissa carteri: structure elucidation, synthesis, and biogenetic implications. Org Lett 2003;5:2735–8