Abstract
Thirty-one new theophylline derivatives have been synthesized and evaluated for their hypoglycemic activity. Compounds 24 (56% reduction) and 31 (57% reduction) showed better hypoglycemic activity than the standard drug glibenclamide which showed 52% reduction in serum glucose level. Compound 27 remarkably reduced serum glucose level by 53%. Ten compounds showed varying degrees of hypoglycemic activity ranging from 20 to 37% reduction in serum glucose level compared to the standard drug. The aromatic amide functionality is the common feature of these theophylline hypoglycemic derivatives. However, anthranilamide and or aliphatic amides proved to be the least active compounds in the present series.
Introduction
Non-insulin dependent diabetes mellitus (NIDDM) is a complex disorder of heterogeneous etiology and characterized by abnormal insulin secretion, decrease in the response of peripheral tissue to insulin (insulin resistance) and increased hepatic glucose production. These metabolic abnormalities cause hyperglycemia in NIDDM patients, and this hyperglycemia is regarded as the most important cause of diabetic complications.
Therefore, a major therapeutic goal in NIDDM patients is to optimize blood glucose control to prevent the risk of complications resulting from vascular diseaseCitation1.
It was reported that substitution at the eighth position of theophylline, mainly with phenyl or cycloalkyl groups increases the activity at adenosine receptorsCitation2. Significant effort has been spent in defining more precisely a physiological role for adenosine receptor related processes in the cardiovascular system and in the central nervous system. The purine bases also have effects on the pituitary–adrenocortical axis, increasing the release of a number of hormones, including dopamine, as part of a stress-related responseCitation3,Citation4.
Adenosine is involved in glucose homeostasisCitation5. It has been reported that A1 adenosine receptor antagonism improves glucose tolerance by increasing glucose uptake in skeletal muscle in Zucker rats. Studies with specific agonists and antagonists suggested that adenosine hepatic effects are mediated by the A2 receptors. It has also been reported that abnormal hepatic glucose production rather than decreased muscle glucose uptake is the major factor responsible for both fasting and postprandial hyperglycemia in NIDDMCitation6–8.
9-Methyladenine derivatives have been reported to act as antagonists of adenosine, but not agonists. 2-Substituted 9-alkyladenine and 2-alkyl-8-aryl-9-methyladenine derivatives have been reported as adenosine antagonistsCitation9,Citation10.
8-Phenyltheophylline inhibited a nonselective, N-ethylcarboxamidoadenosine (NECA) induced glucose production in primary cultured rat hepatocytes, both in a dose-dependent mannerCitation11. This Accumulation of facts promoted us to synthesize some new theophylline derivatives modified at position 7 with acetohydrazide derivatives and investigate the possibility of developing new antidiabetic agent.
Results and discussion
Chemistry
The fact that substituted theophylline derivatives exerted interesting biological activitiesCitation12–16 promoted us to prepare the 7-acetohydrazide derivative 3 by hydrazinolysis of the ethyl acetate ester derivative 2.
The synthetic strategy to prepare the target compounds 4–21 is depicted in and . The theophylline acetohydrazide derivative 3 was reacted with the appropriate reagents, e.g. benzene-1,2-diamine, some aldehydes, ketones, acid chlorides and anhydrides to afford the corresponding final derivatives in considerable yields. The structures of the intermediates and final compounds were confirmed by elemental analysis, 1H, 13C NMR and Mass spectra (MS) and were found in accordance with the proposed structures ().
Figure 1. Reagents and conditions: a = BrCH2CO2Et/K2CO3/reflux. b = benzene-1,2-diamine/fusion. c = N2H4ċH2O/reflux. d = aldehyde and/or /ketone /C2H5OH/ reflux. e = pyridine/reflux. f = benzoxazone/pyridine/reflux.
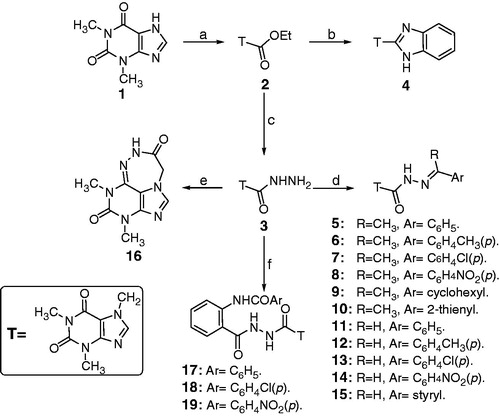
Figure 2. Reagents and conditions: a = phenylisothiocyanate/dioxane/TEA/reflux. b = acid anhydride/CH3CO2H/ CH3CO2Na/ reflux. c = acid chloride/DMF/see experimental. d = aromatic acid anhydride/CH3CO2H/ CH3CO2Na/ reflux. e = sulfonyl chloride derivative/pyridine/reflux.
Table 1. Melting points, crystallization solvents, yield percentages, molecular formulae and molecular weights of compounds (4–31).
Hypoglycemic activity
All the target compounds (4–21) were screened for their hypoglycemic activities. Fundamental signs of diabetes mellitus such as hyperglycemia, loss of body weight, polyphagia and polydipsia were induced in rats by injection of streptozotocinCitation17–19. Subsequently, animals were treated with the compounds under test and glibenclamide as a positive control. Ten compounds 12–14, 16, 17, 22, 23, 26, 29 and 30, in the present study, were able to ameliorate the signs of diabetes mellitus as compared to the diabetic untreated rats. Glibenclamide produced a significant blood glucose lowering effect compared to untreated rats which is mainly due to its insulin-like actionCitation20. These 10 compounds showed 26, 25, 20, 35, 20, 24, 37, 30, 31 and 33% reduction in serum glucose level, respectively; however, compounds 24, 27 and 31 were the most effective hypoglycemic agent in this work by exerting 56%, 53% and 57% reduction in serum glucose. Glibenclamide showed 52% reduction in glucose blood level (). The hypoglycemic effect could result from either β-cells stimulation, and/or the insulin-like action on peripheral tissues or through both mechanisms. However, work is going on to find out the exact mechanism of action.
Table 2. Effect of 14 days oral treatment of test compounds 4–31 (10 mg/kg) or glibenclamide (10 mg/kg) on serum glucose (mean − SD).
Structure-activity correlation
Three compounds showed interesting hypoglycemic activities. Compounds 24 and 27 incorporated the phthalimide and benzamide moieties; however compound 31 contained 4-nitro-benzene sulfonamide joined to the theophylline backbone. These compounds proved to be the most active members in our study. Compounds 12–14, 16, 17, 22, 23, 26, 29 and 30 showed 20–37% reduction in serum glucose level. Structure–activity correlation among this series showed that the type of phthalimide and/or benzamide functionality is crucial for activity as presented by compound 24 (theophylline-diimide 56%) and 27 (theophylline-benzamide 53%); however, compound 31 (sulfonamide containing theophylline, 57%) was almost as effective as its amide and diimide congeners. Except for compound 26, replacement of the amide, sulfamide or diimide with anthranilamide or aliphatic ones has little to do with blood glucose lowering effect according to the results obtained in the current study. From these observations, it became clear that our future efforts have to be devoted towards developing aromatic amides and/or sulfonamides bearing theophylline derivatives in order to elaborate more on the structure–activity relationship of such interesting class of hypoglycemic compounds.
Conclusion
In the present study, certain new amide, diimide and their related sulfonamide isosters were synthesized and evaluated for their hypoglycemic activity. Most of the synthesized compounds showed promising hypoglycemic effect. Compound 2-(1,2,3,4,5,6-hexahydro-1,3-dimethyl-2,6-dioxopurin-7-yl)-N-(5-nitro-1,3-dioxoiso-indolin-2-yl)acetamide (24), N′-benzoyl-2-(1,2,3,4,5,6-hexahydro-1,3-dimethyl-2,6-dioxopurin-7-yl)acetoohydrazide (27) and N′-4-nitrobenzenesulfonyl-2-(1,2,3,4,5,6-hexahydro-1,3-dimethyl-2,6-dioxopurin-7-yl)acetoohydrazide (31) showed 56, 53, and 57% reduction in serum glucose level, respectively. Glibenclamide showed only 52% reduction. The obtained results showed that compounds 24, 27 and 31 could be useful as a template for future development, modification and exploration to produce more active analogs.
Experimental
Chemistry
All melting points (°C, uncorrected) were determined on a Stuart melting point apparatus (Stuart Scientific, Redhill, UK). Elemental analyses (C, H, N) were performed on Perkin-Elmer 2400 analyzer (Perkin-Elmer, Norwalk, CT) and were in full agreement with the proposed structures within 0.4% of the theoretical values. NMR spectra (DMSOd6) were obtained on a Bruker AC 300 Ultra Shield NMR spectrometer (Bruker, Munich, Germany) at 300 MHz for 1H and 75 MHz for 13C, the chemical shifts are expressed in δ (ppm) downfield from tetramethylsilane (TMS). Splitting patterns were designated as follows: s: singlet; d: doublet; t: triplet; m: multiplet. Electron impact mass spectra were recorded on a Varian Mat 311-A70eV instrument (Varian, Fort Collins, CO). Chemicals used are supplied from Sigma-Aldrich (Sigma-Aldrich Chemie GmbH, Steinheim, Germany).
Adult Wistar albino male rats, weighing (200–250 g body weight; 10–12 weeks old), were provided by Experimental Animal Care Center, College of Pharmacy, King Saud University, Riyadh, Kingdom of Saudi Arabia. The animals were housed in cages under standard controlled environmental conditions of humidity, temperature (25 ± 2 °C) and light (12 h light/12 h dark). They were allowed free access to pulverized standard rat pellet diet and tap water ad lib. All animal experiments were according to the accepted standards of human animal care in accordance with the NIH guidelines and the legal requirements in Kingdom of Saudi Arabia. Streptozotocin (4% w/v) was dissolved in fresh citrate-dextrose buffer (pH 4.5–5.0). Glibenclamide was dissolved in distilled water and administered immediately after preparation. The test compounds were formulated with 1.0–1.5% sodium carboxymethyl cellulose (CMC) in distilled water mixed on a magnetic stirrer at 50 °C for 30 min prior to gavages administration. The glucose kit was purchased from Randox Laboratories LTD (Crumlin, UK)Citation21.
Synthesis
7-[(1H-benzo[d]imidazol-2-yl)-methyl]-1,3-dimethyl-1H-purine 2,6(3H,7H)-dione 4
An equimolecular amount of compound 2 and benzene-1,2-diamine (0.005 mol) was mixed thoroughly, ground and fused at 200 °C for 30 min in a sand bath. The reaction mixture was extracted with boiling acetic acid (30 ml), cooled and poured into ice water (50 ml). The solid obtained was filtered, washed with water, dried and crystallized from acetic acid to afford 4.
4: 1H-NMR δ: 2.79 (s, 6H, 2NCH3CO), 4.88 (s, 2H, NCH2), 7.26–7.73 (m, 4H, Ar-H), 8.01 (s, 1H, N = CH), 9.37 (brs, 1H, CONH).). 13C NMR: δ 29.0 (CH3), 31.6 (CH3), 43.7 (NCH2), 107.2, 116.5, 124.1, 140.7, 143.4, 146.1, 150.0 (Ar-C), 152.4, 154.7 (2C = O). MS (EI): m/z 310 [M+]. Anal. (C15H14N6O2) Calcd/Found: C, 58.06 (57.88); H, 4.55 (4.72); N, 27.08 (26.95).
2-(1,3-Dimethyl-2,6-dioxo-1,2,3,6-tetrahydropurin-7-yl)-N′-(1-substituted phenylethylidene)acetohydrazide 5–15
A mixture of acetohydrazide 3 (0.005 mol) and the appropriate aldehyde/or ketone derivative (0.0075 mol) was heated under reflux in ethanol (50 ml) for 24 h. The reaction mixture was concentrated in vacuum and the solid obtained was filtered, dried and crystallized from acetic acid to give compounds 5–15.
5: 1H-NMR δ: 1.31 (s, 3H, CH3), 2.75 (s, 6H, 2NCH3CO), 5.51 (s, 2H, NCH2CO), 7.20–7.76 (m, 5H, Ar-H), 8.11 (s, 1H, N = CH), 9.38 (brs, 1H, CONH). 13C NMR: δ 13.6 (CH3), 28.8(CH3), 30.1(CH3), 32.6(NCH2), 107.8, 128.5, 129.6, 131.5, 135.2, 145.3, 150.7, 151.4 (Ar-C), 151.9, 154.2, 163.9 (3C = O). MS (EI): m/z 354 [M+]. Anal. (C17H18N6O3) Calcd/Found: C, 57.62 (57.81); H, 5.12 (5.37); N, 23.72 (23.92).
6: 1H-NMR δ: 1.28 (s, 3H, CH3), 2.29 (s, 4H, Ar-CH3), 2.80 (s, 6H, 2NCH3CO), 5.52 (s, 2H, NCH2CO), 7.20–7.81 (m, 4H, Ar-H), 8.11 (s, 1H, N = CH), 9.20 (brs, 1H, CONH). 13C NMR: δ 13.9 (CH3), 29.3 (CH3), 30.3 (CH3), 31.6 (CH2N), 107.9, 126.0, 129.1, 129.7, 131.6, 139.1, 145.9, 150.3, 151.8 (Ar-C), 154.6, 163.2, 165.8 (3C = O). MS (EI): m/z 368 [M+]. Anal. (C18H20N6O3) Calcd/Found: C, 58.69 (58.46); H, 5.47 (5.51); N, 22.81 (23.01).
7: 1H-NMR δ: 1.27 (s, 3H, CH3), 2.77 (s, 6H, 2NCH3CO), 5.52 (s, 2H, NCH2CO), 7.21–7.79 (m, 4H, Ar-H), 8.11 (s, 1H, N = CH), 9.21 (brs, 1H, CONH). 13C NMR: δ 13.6 (CH3), 29.5 (CH3), 30.2 (CH3), 32.5 (CH2N), 107.8, 127.6, 129.5, 130.8, 132.7, 135.1, 138.3, 146.1, 150.2, 151.5, 154.7 (Ar-C), 159.6, 162.9, 167.7 (3C = O). MS (EI): m/z 388 [M+]. Anal. (C17H17ClN6O3) Calcd/Found: C, 52.51 (52.77); H, 4.41 (4.64); N, 21.61 (21.57).
8: 1H-NMR δ: 1.14 (s, 3H, CH3), 2.81 (s, 6H, 2NCH3CO), 5.52 (s, 2H, NCH2CO), 7.20–7.81 (m, 4H, Ar-H), 8.11 (s, 1H, N = CH), 8.80 (brs, 1H, CONH). 13C NMR: δ 13.5 (CH3), 29.3(CH3), 30.2(CH3), 32.7(CH2N), 108.7, 115.1, 117.6, 122.2, 129.7, 132.8, 145.6, 149.9, 151.2, 154.3, 158.2 (Ar-C), 154.5, 163.8, 167.2 (3C = O). MS (EI): m/z 399 [M+]. Anal. (C17H17N7O5) Calcd/Found: C, 51.13 (50.92); H, 4.29 (4.53); N, 24.55 (24.80).
9: 1H-NMR δ: 1.05 (s, 3H, CH3), 1.39–1.61 (m, 11H, cyclohexyl-H), 2.75 (s, 6H, 2NCH3CO), 4.74 (s, 2H, NCH2CO), 8.18 (s, 1H, N = CH), 9.41 (br s, 1H, CONH). 13C NMR: δ 19.0 (CH3), 25.2 (CH3), 26.0 (CH2), 28.0 (CH2), 36.5 (CH2), 47.3 (CH), 107.8, 147.8, 149.0, 150.8 (Ar-C), 152.4, 154.6, 167.2 (3C = O). MS (EI): m/z 360 [M+]. Anal. (C17H24N6O3) Calcd/Found: C, 56.65 (56.87); H, 6.71 (6.55); N, 23.32 (23.17).
10: 1H-NMR δ: 1.05 (s, 3H, CH3), 2.79 (s, 6H, 2NCH3CO), 3.55 (S, 3H, N3-CH3), 5.50 (s, 2H, NCH2CO), 7.20–7.76 (m, 3H, Ar-H), 8.12 (s, 1H, N = CH), 9.18 (brs, 1H, CONH). 13C NMR: δ 13.5 (CH3), 28.6(CH3), 30.1(CH3), 32.8(CH2N), 108.3, 125.3, 127.0, 128.5, 129.4, 145.8, 151.0, 151.8 (Ar-C), 153.9, 155.2, 163.5 (3C = O). MS (EI): m/z 360 [M+]. Anal. (C15H16N6O3S) Calcd/Found: C, 49.99 (50.24); H, 4.47 (4.69); N, 23.32 (23.59); S, 8.90 (9.14).
N′-Benzylidene-2-(1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropurin-7-yl) acetohydrazide 11–15
A mixture of 3 (0.005 mol) and the appropriate aldehyde (0.0075 mol) in glacial acetic acid (20 ml) was heated under reflux for 24 h. The reaction mixture was cooled and the solid separated was filtered, dried and crystallized from acetic acid to obtain 11–15.
11: 1H-NMR δ: 2.73 (s, 6H, 2NCH3CO), 4.52 (s, 2H, NCH2CO), 7.56–8.52 (m, 6H, Ar-H), 8.73 (s, 1H, N = CH), 9.16 (brs, 1H, CONH). 13C NMR: δ 29.2 (CH3), 30.2 (CH3), 32.5 (CH2N), 107.9, 128.3, 129.7, 131.6, 134.0, 143.5, 147.1, 150.6 (Ar-C), 152.8, 155.1, 163.5 (3C = O). MS (EI): m/z 340 [M+]. Anal. (C16H16N6O3) Calcd/Found: C, 56.47 (56.67); H, 4.74 (4.51); N, 24.69 (24.85).
12: 1H-NMR δ: 2.50 (s, 3H, Ar-CH3), 2.74 (s, 6H, 2NCH3CO), 4.55 (s, 2H, NCH2CO), 7.14–8.15 (m, 5H, Ar-H), 8.53 (s, 1H, N = CH), 9.12 (brs, 1H, CONH). 13C NMR: δ 25.0 (CH3), 29.1 (CH3), 30.3 (CH3), 32.4 (CH2N), 107.7, 129.3, 129.7, 130.6, 140.9, 143.6, 146.3, 150.7 (Ar-C), 152.5, 154.3, 163.7 (3C = O). MS (EI): m/z 354 [M+]. Anal. (C17H18N6O3) Calcd/Found: C, 57.62 (57.33); H, 5.12 (5.65); N, 23.72 (23.90).
13: 1H-NMR δ: 2.71 (s, 6H, 2NCH3CO), 4.54 (s, 2H, NCH2CO), 7.32–8.11 (m, 5H, Ar-H), 8.45 (s, 1H, N = CH), 9.11 (brs, 1H, CONH). 13C NMR: δ 29.1 (CH3), 30.2 (CH3), 32.3 (CH2N), 108.0, 129.3, 129.7, 131.5, 137.2, 143.5, 146.2, 150.1 (Ar-C), 152.4, 154.6, 163.2 (3C = O). MS (EI): m/z 374 [M+]. Anal. (C16H15ClN6O3) C, H, N.
14: 1H-NMR δ: 2.76 (s, 6H, 2NCH3CO), 4.51 (s, 2H, NCH2CO), 7.80–8.22 (m, 5H, Ar-H), 8.37 (s, 1H, N = CH), 9.13 (brs, 1H, CONH). 13C NMR: δ 29.4 (CH3), 30.7 (CH3), 32.3 (CH2N), 107.8, 125.8, 127.0, 132.5, 133.8, 143.7, 146.4, 150.0 (Ar-C), 152.3, 154.9, 163.5 (3C = O). MS (EI): m/z 385 [M+]. Anal. (C16H15N7O5) Calcd/Found: C, 49.87 (50.01); H, 3.92 (3.78); N, 25.44 (25.17).
15: 1H-NMR δ: 2.70 (s, 6H, 2NCH3CO), 4.53 (s, 2H, NCH2CO), 5.50–5.78 (m, 2H, Olefinic-H), 7.52–8.01 (m, 6H, Ar-H), 8.15 (s, 1H, N = CH), 8.75 (brs, 1H, NHCO). 13C NMR: δ 29.2 (CH3), 30.2 (CH3), 32.5 (CH2), 108.1, 126.4, 128.4, 128.9, 135.2, 137.7, 139.4, 146.8, 150.8 (Ar-C), 151.9, 155.4, 164.6 (3C = O). MS (EI): m/z 366 [M+]. Anal. (C18H18N6O3) Calcd/Found: C, 59.01 (58.86); H, 4.95 (5.16); N, 22.94 (23.12).
8,10-Dimethyl-[1,2,5]triazepino[4,3,5-hi]purine-3,9(2H,4H,8H,10H)-dione 16
The acid hydrazide 3 (0.001 mol) in glacial acetic acid (20 ml) containing fused sodium acetate (1.0 g) was heated under reflux for 24 h. The reaction mixture was cooled and the solid separated was filtered, dried and crystallized from acetic acid to obtain 16.
16: 1H-NMR δ: 2.74 (s, 6H, 2NCH3CO), 4.63 (s, 2H, NCH2CO), 7.31 (s, 1H, Ar-H), 7.55 (brs, 1H, NHCO). 13C NMR: δ 29.1 (CH3), 31.3 (CH3), 48.5 (CH2N), 111.3, 137.1, 137.8, 150.5 (Ar-C), 153.7, 164.8 (2C = O). MS (EI): m/z 234 [M +]. Anal. (C9H10N6O2) Calcd/Found: C, 46.15 (45.92); H, 4.30 (4.61); N, 35.88 (36.07).
N-[2-(2 -(2-(1,3-Dimethyl-2,6-dioxo-1,2,3,6-tetrahydropurin-7-yl)acetyl)-hydrazinecarbonyl)substitutedphenyl]benzamide 17–19
A mixture (0.00s mol) of equimolecular amount of 3 and the appropriate benzoxazone derivative was heated under reflux for 24 h in pyridine (30 ml). The reaction mixture was concentrated in vacuum and the solid obtained was crystallized from acetic acid to give 17–19.
17: 1H-NMR δ: 2.73 (s, 6H, 2NCH3CO), 4.65 (s, 2H, NCH2CO), 7.51–8.0 (m, 10H, Ar-H), 8.50 (brs, 3H, NH). 13C NMR: δ 28.5 (CH3), 29.7 (CH3), 32.3 (CH2N), 108.0, 122.4, 124.7, 126.1, 127.3, 129.6, 133.0, 134.6, 138.2, 146.6, 150.1 (Ar-C), 153.8, 155.2, 164.2, 164.5, 164.6 (5C = O). MS (EI): m/z 475 [M+]. Anal. (C23H21N7O5) Calcd/Found: C, 58.10 (57.93); H, 4.45 (4.73); N, 20.62 (20.34).
18: 1H-NMR δ: 2.71 (s, 6H, 2NCH3CO), 4.63 (s, 2H, NCH2CO), 7.44–8.0 (m, 9H, Ar-H), 8.54 (brs, 3H, NH). 13C NMR: δ 28.4 (CH3), 29.7 (CH3), 32.3 (CH2N), 107.7, 121.9, 124.5, 127.3, 129.1, 132.5, 133.5, 138.0, 146.5, 150.3 (Ar-C), 153.7, 155.1, 164.2, 164.4, 164.5 (5C=O). MS (EI): m/z 509 [M+]. Anal. (C23H20ClN7O5) Calcd/Found: C, 54.18 (54.37); H, 3.95 (4.08); N, 19.23 (18.95).
19: 1H-NMR δ: 1H-NMR δ: 2.71 (s, 6H, 2NCH3CO), 4.62 (s, 2H, NCH2CO), 7.45–8.31(m, 9H, Ar-H), 8.51 (brs, 3H, NH). 13C NMR: δ 28.7 (CH3), 29.5 (CH3), 32.2 (CH2N), 107.8, 121.0, 121.8, 123.7, 124.3, 128.0, 129.1, 132.6, 137.5, 141.2, 147.0, 150.2, 151.5 (Ar-C), 152.9, 154.0, 164.2, 164.3, 164.4 (5C = O). MS (EI): m/z 520 [M+]. Anal. (C23H20N8O7) Calcd/Found: C, 53.08 (53.31); H, 3.87 (3.69); N, 21.53 (21.77).
2-(2-(1,3-Dimethyl-2,6-dioxo-1,2,3,6-tetrahydropurin-7-yl)acetyl)-N-phenyl hydrazine carbothioamide 20
A mixture of the hydrazide 3 (0.005 mol) and phenylisothiocyanate (0.006 mol) in dioxane was heated under reflux for 24 h. The reaction mixture was concentrated in vacuum and the solid obtained was crystallized from acetic acid ().
20: 2.75 (s, 6H, 2NCH3CO), 4.63 (s, 2H, NCH2CO), 7.25–8.11(m, 6H, Ar-H), 8.51 (m, 3H, NH). 13C NMR: δ 28.8 (CH3), 29.1 (CH3), 32.1 (CH2N), 107.8, 123.6, 124.9, 129.7, 137.2, 145.2, 150.3, 151.5 (Ar-C), 152.5, 154.2, 164.5 (3 C = O), 172.2 (C = S). MS (EI): m/z 387 [M+]. Anal. (C16H17N7O3S) Calcd/Found: C, 49.60 (49.32); H, 4.42 (4.19); N, 25.31 (25.09); S, 8.28 (8.05).
2-(1,3-Dimethyl-2,6-dioxo-1,2,3,6-tetrahydropurin-7-yl)-N-(substituted dioxo-heterocyclic-1/or 2-yl) acetamide 21–24
A mixture of compound 3 (0.01 mol), the appropriate anhydride (0.01 mol) and glacial acetic acid (30 ml), was heated under reflux for 24 h. The precipitated solid was filtered while hot, dried and crystallized from acetic acid to afford 21–24.
21: 1H-NMR δ: 2.69 (s, 6H, 2NCH3CO), 2.76 (m, 4H, 2CH2CO), 4.69 (s, 2H, NCH2CO), 8.09 (s, 1H, N = CH), 8.91 (s, 1H, NHCO). 13C NMR: δ 29.0 (CH3), 30.2 (CH3), 30.7 (CH2), 32.0 (CH2), 107.9, 146.8, 150.7 (Ar-C), 151.3, 153.6, 164.6, 171.2 (5C = O). MS (EI): m/z 334 [M+]. Anal. (C13H14N6O5) Calcd/Found: C, 46.71 (46.54); H, 4.22 (4.51); N, 25.14 (24.98).
22: 1H-NMR δ: 2.71 (s, 6H, 2NCH3CO), 4.60 (s, 2H, NCH2CO), 7.64–8.10 (m, 5H, Ar-H), 8.25 (brs, 1H, NHCO). 13C NMR: δ 29.1 (CH3), 29.9 (CH3), 32.4 (CH2N), 107.9, 128.0, 132.1, 132.9, 146.2, 150.2 (Ar-C), 152.5, 154.3, 164.2, 164.3 (5C = O). MS (EI): m/z 382 [M+]. Anal. (C17H14N6O5) Calcd/Found: C, 53.40 (53.61); H, 3.69 (3.85); N, 21.98 (22.07).
23: 1H-NMR δ: 2.71 (s, 6H, 2NCH3CO), 4.61 (s, 2H, NCH2CO), 7.76–8.01 (m, 4H, Ar-H), 8.27 (brs, 1H, NH). 13C NMR: δ 29.3 (CH3), 30.0 (CH3), 32.5 (CH2N), 108.1, 126.0, 132.5, 133.2, 133.6, 146.3, 150.1 (Ar-C), 152.4, 154.5, 164.0, 164.3 (5C = O). MS (EI): m/z 416 [M+]. Anal. (C17H13ClN6O5) Calcd/Found: C, 48.99 (49.26); H, 3.14; N (3.37), 20.16 (19.88).
24: 1H-NMR δ: 2.74 (s, 6H, 2NCH3CO), 4.63 (s, 2H, NCH2CO), 7.96–8.56 (m, 4H, Ar-H), 8.80 (brs, 1H, NH). 13C NMR: δ 29.3 (CH3), 30.1 (CH3), 32.7 (CH2N), 108.5, 125.3, 127.8, 133.0, 133.2, 133.5, 145.8, 148.0, 150.3 (Ar-C), 153.4, 154.9, 164.1, 164.3 (5C = O). MS (EI): m/z 427 [M+]. Anal. (C17H13N7O7) Calcd/Found: C, 47.78 (47.94); H, 3.07 (2.75); N, 22.94 (23.19).
N′-Acetyl-2-(1,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxopurin-7-yl)aceto-hydrazide 25–28
A mixture of the hydrazide 3 (0.005 mol) and the appropriate acid chloride (0.0065 mol) in pyridine (15 ml) was heated under reflux for 1 h. The solid obtained on cooling was crystallized from ethanol to afford compounds 25–28 ().
25: 1H-NMR δ: 2.25 (s, 3H, CH3CO), 2.77 (s, 6H, 2NCH3CO), 4.63 (s, 2H, NCH2CO), 8.09 (s, 1H, N = CH). 13C NMR: δ 24.6 (CH3), 28.2 (CH3), 29.5 (CH3), 32.4 (CH2), 107.8, 144.8, 150.8 (Ar-C), 152.0, 155.1, 164.3, 168.0 (4C = O). MS (EI): m/z 294 [M+]. Anal. (C11H14N6O4) Calcd/Found: C, 44.90 (45.18); H, 4.80 (5.11); N, 28.56 (28.86).
26: 1H-NMR δ: 2.73 (s, 6H, 2NCH3CO), 3.36 (s, 2H, CH2CO), 4.70 (s, 2H, NCH2CO), 7.23–7.95 (m, 6H, Ar-H), 9.31 (br s, 2H, CONHNHCO). 13C NMR: δ 29.0 (CH3), 29.4 (CH3), 32.3 (NCH2), 43.3 (CH2), 107.9, 127.5, 129.1, 129.9, 135.9, 146.3, 150.8 (Ar-C), 152.2, 155.0, 164.3, 168.1 (4C = O). MS (EI): m/z 370 [M+]. Anal. (C17H18N6O4) Calcd/Found: C, 55.13 (54.85); H, 4.90 (5.17); N, 22.69 (22.90).
27: 1H-NMR δ: 2.71 (s, 6H, 2NCH3CO), 4.70 (s, 2H, NCH2CO), 7.59–8.01 (m, 6H, Ar-H), 9.30 (brs, 2H, 2CONH). 13C NMR: δ 29.0 (CH3), 29.8 (CH3), 32.8 (CH2), 108.1, 129.2, 133.8, 135.4, 146.5, 150.3 (Ar-C), 152.1, 154.6, 162.5, 164.7 (4C = O). MS (EI): m/z 356 [M+]. Anal. (C16H16N6O4) Calcd/Found: C, 53.93 (54.21); H, 4.53 (4.27); N, 23.58 (23.74).
28: 1H-NMR δ: 2.74 (s, 6H, 2NCH3CO), 4.72 (s, 2H, NCH2CO), 7.67–8.08 (m, 4H, Ar-H), 9.31 (brs, 2H, 2CONH). 13C NMR: δ 29.1 (CH3), 29.8 (CH3), 32.9 (CH2), 107.5, 129.1, 135.7, 137.2, 137.9, 146.9, 150.1 (Ar-C), 152.0, 154.7, 160.7, 164.6 (4C = O). MS (EI): m/z 362 [M+]. Anal. (C14H14N6O4S) Calcd/Found: C, 46.40 (46.68); H, 3.89 (4.11); N, 23.19 (22.95); S, 8.85 (9.08).
N′-(2-(1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropurin-7-yl)acetyl)-4-substituted benzenesulfonohydrazide 29–31
A mixture of the hydrazide 3 (0.005 mol) and the appropriate benzenesulfonyl chloride (0.0065 mol) in pyridine (20 ml) was heated under reflux for 3 h. The solvent was evaporated under reduced pressure and the solid obtained was crystallized from ethanol to afford compounds 29–31.
29: 1H-NMR δ: 2.82 (s, 6H, 2NCH3CO), 4.95 (br s, 1H, SO2NH), 4.66 (s, 2H, NCH2CO), 7.53–8.10 (m, 6H, Ar-H), 9.60 (br s, 1H, CONH). 13C NMR: δ 29.0 (CH3), 29.7 (CH3), 32.0 (CH2), 107.6, 127.1, 129.4, 132.4, 139.5, 145.8, 150.2 (Ar-C), 151.3, 154.6, 165.3 (3C = O). MS (EI): m/z 392 [M+]. Anal. (C15H16N6O5S) Calcd/Found: C, 45.91 (46.08); H, 4.11 (4.35); N, 21.42 (21.77); S, 8.17 (8.41).
30: 1H-NMR δ: 2.72 (s, 6H, 2NCH3CO), 2.85 (s, 3H, CH3), 4.94 (br s, 1H, SO2NH), 4.64 (s, 2H, NCH2CO), 7.52–8.10 (m, 5H, Ar-H), 9.61 (br s, 1H, CONH). 13C NMR: δ 25.3 (CH3), 29.1 (CH3), 29.8 (CH3), 32.3 (CH2), 107.5, 127.4, 129.6, 137.1, 141.2, 145.7, 150.3 (Ar-C), 151.1, 154.5, 164.7 (3C = O). MS (EI): m/z 406 [M+]. Anal. (C16H18N6O5S) Calcd/Found: C, 47.28 (46.99); H, 4.46 (4.68); N, 20.68 (20.39); S, 7.89 (8.08).
31: 1H-NMR δ: 2.70 (s, 6H, 2NCH3CO), 4.90 (br s, 1H, SO2NH), 4.65 (s, 2H, NCH2CO), 7.55–8.01 (m, 5H, Ar-H), 9.60 (br s, 1H, CONH). 13C NMR: δ 29.0 (CH3), 29.9 (CH3), 32.5 (CH2), 107.6, 122.0, 128.1, 145.9, 150.2, 152.0 (Ar-C), 152.1, 154.6, 164.5 (3C = O). MS (EI): m/z 437 [M+]. Anal. (C15H15N7O7S) Calcd/Found: C, 41.19 (40.88); H, 3.46 (3.72); N, 22.42 (22.15); S, 7.33 (7.06).
Evaluation of serum glucose level
Diabetes model was induced by a single intraperitonial injection of streptozotocin at a dosage of 70 mg/kgCitation22. Streptozotocin-injected animals were given 20% glucose solution for 24 h to prevent initial drug-induced hypoglycemic mortality. Three days after streptozotocin administration, the blood glucose level of each rat was determined. Rats with blood glucose levels >350 mg/dL were considered diabetic and included in the present study. Diabetic animals were randomly divided into three main groups (N = 6 animals/group): untreated (received 1% CMC), treated with test compounds (10 mg/kg/day) or treated with a positive control (glibenclamide, 10 mg/kg/day). The experiment included also a healthy group received equal amounts of 1% CMC as a vehicle and served as a control group. All treatments were by gavages over a period of 14 days.
During this period, the animals were kept on food and water given ad libitum. Parameters such as changes in body weight, food intake, water intake and mortality were recorded. At the end of treatment the rats were fasted for 12 h and blood samples were collected from the retro-orbital plexus then serum was separated. Serum glucose level was determined using Randox Laboratories LTD kits on Shimadzu UV-1202 Spectrophotometer according to the manufacturers’ instructions.
Declaration of interest
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RGP-VPP-060.
References
- De Fronzo RA, Bonasonna RC, Ferrannini E. Pathogenesis of INDDM: a balanced overview. Diabetes Care 1992;15:318–68
- Jacobson KA, van Galen PJ, Williams M. Adenosine receptors: pharmacology, structure-activity relationships, and therapeutic potential. J Med Chem 1992;35:407–22
- Scaccianoce S, Navarra D, Di Sciullo A, et al. Adenosine and pituitary-adrenocortical axis activity in the rat. Neuroendocrinology 1989;50:464–8
- Schettini G, Landolfi E, Meucci O, et al. Adenosine and its analogue (−)-N6-phenylisopropyladenosine modulate anterior pituitary adenylate cyclase activity and prolactin secretion in the rat. J Mol Endocrinol 1990;5:69–76
- Buxton DB, Fisher RA, Robertson SM, Olson MS. Stimulation of glycognolysis and vasoconstriction by adenosine and adenosine analogues in the perfused liver. Biochem J 1987;248:35–41
- Mclane MP, Black PR, Law WR, Raymond RM. Adenosine reversal of in vivo hepatic responsiveness to insulin. Diabetes 1990;39:62–9
- Chaliss RAJ, Budohiski L, MacManus B, Newsholme EA. Effects of an adenosine receptor antagonist in insulin resistance in Soleus Muscle from Obese Zucker rats. Biochem J 1984;221:915–17
- Crist GH, LaNoue KF, Lang CH. Tissue-specific effects of in vivo adenosine receptor blockage on glucose uptake in Zucker rats. FASEB J 1998;12:1301–8
- Camaioni E, Costanzi S, Vittori S, et al. New substituted 9-alkylpurinies as adenosine receptor ligands. Bioorg Med Chem. 1998;6:523–33
- Harada H, Asano O, Hoshino Y, et al. 2-Alkynyl-8-aryl-9-methyladenine as novel adenosine receptor antagonists: their synthesis and structure-activity relationships toward hepatic glucose production induced via agonism of the A2B receptors. J Med Chem 2001;44:170–9
- Jeong LS, Choe SA, Kim AY, et al. Synthesis of N6-substituted 3′-ureidoadenosine derivatives as highly potent agonists at the mutant A3 adenosine receptor. Nucleosides Nucleotides Nucleic Acids 2007;26:717–19
- Stefanachi A, Nicolotti O, Leonetti F, et al. 1,3-Dialkyl-8-(hetero)aryl-9-OH-9-deazaxanthines as potent A2B adenosine receptor antagonists: design, synthesis, structure-affinity and structure-selectivity relationships. Bioorg Med Chem 2008;16:9780–9
- Katyal J, Gupta YK. Dopamine release is involved in antinociceptive effect of theophylline. Int J Neurosci 2012;122:17–21
- Hierrezuelo J, Manuel López-Romero J, Rico R, et al. Synthesis of theophylline derivatives and study of their activity as antagonists at adenosine receptors. Bioorg Med Chem 2010;18:2081–8
- Watanabe S, Yamakami J, Tsuchiya M, et al. Anti-inflammatory effect of theophylline in rats and its involvement of the glucocorticoid-glucocorticoid receptor system. J Pharmacol Sci 2008;106:566–70
- Hasegawa M, Hada J, Abe T, et al. Theophylline attenuates hippocampal blood flow responses induced by tooth pulp stimulation in rats. Neurosci Res 2009;65:156–9
- Cunha RA, Ferré S, Vaugeois JM, Chen JF. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des 2008;14:1512–24
- Umrani DN, Goyal RK. Beneficial effects of fenoldopam treatment on renal function in streptozotocin-induced diabetic rats. Clin Exp Hypertens 2002;24:207–19
- Akhani SP, Vishwakarma SL, Goyal RK. Anti-diabetic activity of Zingiber officinale in streptozotocin-induced type I diabetic rats. J Pharm Pharmacol 2004;56:101–5
- Majithiya JB, Balaraman R. Metformin reduces blood pressure and restores endothelial function in aorta of streptozotocin-induced diabetic rats. Life Sci 2006;78:2615–24
- Tayek JA. Low-dose oral glyburide reduces fasting blood glucose by decreasing hepatic glucose production in healthy volunteers without increasing carbohydrate oxidation. Am J Med Sci 1995;309:134–9
- El Tahir KE, Williams KI, Betteridge DJ. The effect of experimental diabetes on prostacyclin production by tissues from pregnant rats. Prostaglandins Leukot Med 1982;8:429–35

