Abstract
Carbonic anhydrases (CAs, EC 4.2.1.1) catalyze a simple reaction in all life domains: the carbon dioxide hydration to bicarbonate and protons: CO2 + H2O → + H+. Six different, genetically distinct CA families are known to date, the α-, β-, γ-, δ-, ζ- and η-CAs. Bacteria encode for CAs belong to the α-, β- and γ-classes. Recently, our groups investigated the presence of CAs in two bacteria belonging to the genus Sulfurihydrogenibium living in hot springs all over the world, at temperatures of up to 110 °C. The α-CAs from Sulfurihydrogenibium yellowstonense and Sulfurihydrogenibium azorense, denominated SspCA and SazCA, respectively, are highly thermostable, maintaining a good catalytic activity even after being heated for a prolonged period. Moreover, SazCA was to be the fastest CA known to date with a kcat value of 4.40 × 106 s−1 and a kcat/KM value of 3.5 × 108 M−1 s−1. SspCA also showed a good catalytic activity for the same reaction, with a kcat value of 9.35 × 105 s−1 and a kcat/KM value of 1.1 × 108 M−1 s−1, proving that the “extremo-α-CAs” are between the most effective CAs known to date. Here, we describe a failed tentative to obtain a super-CA, SupCA, by combining the amino acid sequence of SazCA and SspCA. To achieve this goal we introduced six His residues in N-terminal sequence of SspCA. However the obtained SupCA showed lower catalytic activity and thermostability compared to both extremophilic enzymes from which it has been designed. We rationalized the biochemical reasons of this failure, which may be useful to design enzymes with a better catalytic activity.
Introduction
High-pressure, high levels of radiation, high concentration of toxic compounds, high (55–121 °C) and low (−2 to 20 °C) temperatures, high salinity (2–5 M NaCl) and either high alkalinity (pH > 8) or high acidity (pH < 4)Citation1–3 are examples of extreme habitats. Harsh habitats might be compared to the industrial process conditions, which require biocatalysts with an elevated stability under extreme conditions. Extremophiles are microorganisms that can tolerate the aforementioned habitats and are thus an important source of extremo-biocatalysts, known as “extremozymes”Citation4–7.
Recently, our groups investigated the presence of carbonic anhydrases (CAs, EC 4.2.1.1) in two bacteria belonging to the genus Sulfurihydrogenibium living in hot springs all over the world, at temperatures of up to 110 °CCitation8–21. Genome inspection of Sulfurihydrogenibium yellowstonense and Sulfurihydrogenibium azorense revealed the presence of α- and γ-CAs. Generally, Bacteria encode for CAs belong to the α-, β- and γ-classesCitation8–21. Six different, genetically distinct CA families are known to date, the α-, β-, γ-, δ-, ζ- and η-CAsCitation22–25. Whereas α-, β-, δ- and η-CAs use Zn(II) ions at the active site, the γ-CAs are probably Fe(II) enzymes (but they are also active with bound Zn(II) or Co(II) ions), whereas the ζ-class uses Cd(II) or Zn(II) to perform the physiologic reaction catalysisCitation8–10,Citation25–40. CAs catalyze a simple reaction in all life domains: the carbon dioxide hydration to bicarbonate and protons: CO2 + H2O → + H+Citation41–44. These enzymes are thus involved in many physiologic processes, such as photosynthesis, respiration, CO2 transport, as well as metabolism of xenobiotics (e.g. cyanate in Escherichia coli)Citation36,Citation41–46. Many classes of CA inhibitors (CAIs) are known to date: the metal complexing anions and the unsubstituted sulfonamides which bind to the Zn(II) ion of the enzyme either by substituting the non-protein zinc ligand or add to the metal coordination sphere, generating trigonal–bipyramidal species are the classical, are the classical and the most frequently investigated onesCitation12–16,Citation29,Citation47–58. In addition to them, coumarins, phenols, dithiocarbamates and xanthates as well as polyamines, are new classes of CAIs reported in the last years by these groupsCitation23,Citation59–69. Many representatives of all CA classes have been crystallized and characterized in detail, except δ- and η-CAs. The three-dimensional structure of CAs presents a substantial variability between the different classes: the overall shape of the molecules, the protein folding patterns as well as the oligomeric organization of the six genetic CA families are very much differentCitation8–10,Citation25–40. For example, α-CAs are normally monomers and rarely dimers; β-CAs are dimers, tetramers or octamers; γ-CAs are trimers, whereas the δ- and ζ-CAs are less well understood at the moment. The only ζ-CA crystallized so far has three slightly different active sites on the same polypeptide chain whereas no X-ray crystal structures of δ- and η-CAs are available so farCitation26–28,Citation70–76. Some of the catalytically active α-CAs can also catalyze the hydrolysis of esters, for example 4-nitrophenyl acetate (4-NpA) (and other hydrolytic reactions as well). However, no esterase activity was detected so far for enzymes belonging to other classes than the α-CAsCitation28,Citation77–79. Moreover, bacterial α-CAs are characterized by a signal peptide indicating a periplasmic or extracellular location of these enzymesCitation33,Citation35,Citation37,Citation40,Citation41,Citation43,Citation61,Citation62.
The α-CAs from S. yellowstonense and S. azorense, denominated as SspCA and SazCA, respectively, are highly thermostable, maintaining a good catalytic activity even after being heated for a prolonged period (more than 3 h) to 100 °C. They are also highly effective catalysts for the CO2 hydration reactionCitation33,Citation35,Citation37,Citation40,Citation41,Citation43,Citation61,Citation62. SazCA is the fastest CA known to date, and the second most efficient enzyme (after superoxide dismutase), with a kcat value of 4.40 × 106 s−1 and a kcat/KM value of 3.5 × 108 M−1 s−1. SspCA also showed a good catalytic activity for the same reaction, with a kcat value of 9.35 × 105 s−1 and a kcat/KM value of 1.1 × 108 M−1 s−1, proving that the “extremo-α-CAs” are indeed among the most effective CAs known to dateCitation33,Citation35,Citation37,Citation40,Citation41,Citation43,Citation61,Citation62.
Here, we describe the tentative to produce a chimeric CA with the biochemical properties of the most heat stable CA (SspCA) and the fastest CA (SazCA). To achieve this goal we aligned the primary structures of SspCA, SazCA and hCA II and changed specific amino acid residues of SspCA into those present in the SazCA or hCA II of the N-terminal sequence. The resulting chimeric CA, named SupCA, was analyzed for its thermostability and thermoactivity, but proved to be both less thermostable and less catalytically active compared to the two enzymes used to design it. We thus analyze the biochemical reasons of this failed tentative to obtain a superCA.
Materials and methods
Mammalian bCA
Bovine α-CA (bCA) was supplied by Sigma-Aldrich (St. Louis, MO) as crude extract from bovine erythrocytes (lyophilized powder). SspCA, SazCA and SupCA were recombinant proteins prepared as described earlierCitation13,Citation36.
Sequence analysis
Multialignment of the amino acid sequences was performed using the programs MUSCLE version 3.7. The phylogenetic tree was constructed with the program PhylML version 3.0.
Design of the chimeric-α-CA gene
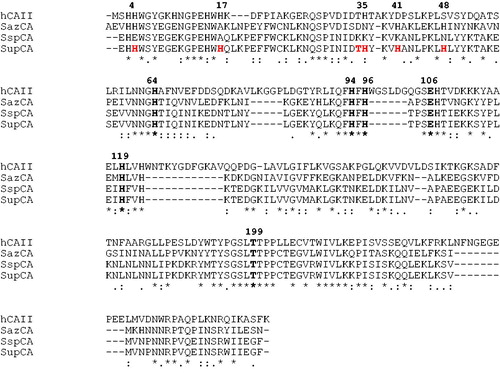
Gene was designed starting from the nucleotide sequence of the most thermostable α-CA, SspCA. Nucleotide triplets of the SspCA gene, localized in the 5′ region, have been changed to obtain a nucleotide sequence encoding for the chimeric α-CA, named from our group SupCA, and characterized by six different amino acid residues in the N-terminal region respect to SspCA (). The GeneArtcompany, specialized in gene synthesis, synthesized SupCAgene encoding for the chimeric-α-CA.
Figure 1. Multialignment of the amino acid sequences of α-CAs from different sources was performed with the program MUSCLE. Legend: hCA II, Homo sapiens, isoform II (accession no. AAH11949.1); SspCA, Sulfurihydrogenibium sp. YO3AOP1 (accession no. ACD66216.1); SazCA, Sulfurihydrogenibium azorense (accession no. ACN99362.1); SupCA, super-CA (chimeric α-CA). The zinc ligands (His94, His96 and His119), the gatekeeper residues (Glu106 and Thr199) and the proton shuttle residue (His64) are conserved in all these enzymes and are indicated in bold. hCAI numbering system was used. Histidines in position 4, 17, 35, 41 and 48 were inserted to create the super-CA. The asterisk (*) indicates identity at all aligned positions. The symbol (:) relates to conserved substitutions, while (.) means that semi conserved substitutions are observed.
Construct preparation for SupCA expression
SupCA gene contained a NdeI and XhoI sites at the 5′ and 3′ end of the SupCA gene, respectively. The resulting plasmid was amplified into E. coli DH5 α cells. The SupCADNA fragments were separated on 1% agarose gel. The recovered SupCAgene and the linearized expression vector (pET15-b) were ligated by T4 DNA ligase to form the expression vector pET15-b/SupCA. In order to confirm the integrity of the SupCAgene and that no errors occurred at the ligation sites, the pET15-b/SupCA vector was sequenced.
Expression and purification of SupCA
Competent E. coli BL21 (DE3) cells were transformed with pET15-b/SupCA, grown at 37 °C, induced with 1 mM IPTG and grown for 5 h. After additional growth for 5 h, cells were harvested and disrupted by sonication at 4 °C. Following centrifugation, the cell extract was resuspended in 20 mM buffer phosphate, pH 8.0 and loaded onto a His-select HF Nickel affinity gel. The protein was eluted with 250 mM imidazole. At this stage of purification the enzyme was at least 95% pure and 5 mg of the recombinant SupCA was obtained.
CA assay
An Applied Photophysics stopped-flow instrument was used for assaying the CA catalyzed CO2 hydration activityCitation50. Phenol red (at 0.2 mM) was used as indicator, working at the absorbance maximum of 557 nm with 10 mM Hepes (pH 7.5) as buffer and 0.1 M NaClO4 (for maintaining constant ionic strength), at 20 °C, following the CA-catalyzed CO2 hydration reaction for a period of 10–100 s (the uncatalyzed reaction needs around 60–100 s in the assay conditions, whereas the catalyzed ones are of around 6–10 s). The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Enzyme concentrations in the assay system were about10 nM for SspCA, SazCA, SupCA and hCA II.
Thermoactivity
The temperature dependence activity of SupCA was measured using p-nitrophenylacetate (p-NpA) as substrate. Protein concentration used in the assay was 300 ng. The activity was measured in the temperature range from 25 to 100 °C. All the reactions were performed in triplicate and in parallel with bCA II, SspCA and SazCA.
Thermostability
To compare the stability of SupCA, bCA II, SazCA and SspCA at different temperatures, enzymes at the concentration of 3 μg/mL in 10 mM Tris/HCl, pH 8.3 were incubated at 40, 50, 60, 70, 80, 90 and 100 °C for different time (30, 60, 120 and 180 min). Enzyme aliquots (30 ng) were withdrawn at appropriate times and the residual activity was measured at 0 °C using CO2 as substrate. Thermostability of SupCA, SspCA, SazCA and bCA II were performed in triplicate and in parallel.
Results and discussion
Design and expression of the chimeric-α-CA
In the present paper, we describe the tentative to obtain a chimeric α-CA having the biochemical characteristics of the most heat stable CA (SspCA) and the fastest CA (SazCA)Citation10,Citation14, which we thought will possess the features of a super CA. To achieve this goal, we aligned the primary structures of SspCA, SazCA and hCA II and changed specific amino acid residues of SspCA into those present in SazCA or hCA II, which are catalytically more efficient than SspCA (). We substituted six amino acid residues in the N-terminal sequence of SspCA with five histidines and one residue with a threonine (). All these residues are localized before the proton shuttle residue His64 which is involved in the rate-limiting step of the catalytic process, i.e. the shuttling of protons from the water coordinated to the zinc ion out of the active site with formation of the nucleophilic, catalytically active zinc hydroxide species of the enzymeCitation24. Our hypothesis was to prove if these substitutions could increase the catalytic constant (kcat) of the SspCA, transforming the enzyme into the most stable and the fastest α-CA. We designed a synthetic gene encoding for SspCA and containing the substitution indicated in . The gene was inserted in an expression vector (pET-15 b) and the protein expressed in E. coli as described in the section “Materials and methods”. The chimeric-α-CA, SupCA, was isolated and purified to homogeneity at room temperature from E. coli (DE3) cell extract. The carbonic anhydrase activity was recovered in the soluble fraction of cell extract after sonication and centrifugation as described in “Materials and methods” section. Using the affinity column (His-select HF Nickel affinity gel), SupCA was purified to apparent homogeneity, as indicated by a single protein band after SDS-PAGE (data not shown).
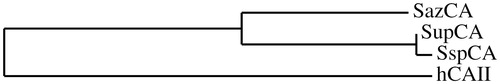
We constructed a phylogenetic tree for showing that SupCA with the inserted residues continued to cluster in the group of the thermophilic bacterial CAs (SspCA and SazCA), a cluster different from that formed by the human enzyme, hCA II ().
Figure 2. Phylogenetic tree of SupCA, SazCA, SspCA and hCA II. Tree was constructed using the program PhyML3.0. Sequence names and accession numbers are reported in .
A stopped-flowCO2 hydrase assay has been used to measure the catalyticactivity of SupCA, which was compared with those of SspCA, SazCA and hCA II. shows the catalytic activity of these enzymes for the CO2 hydration reaction. It may be observed that SupCA has kinetic parameters, which prove that this enzyme is much less efficient compared to the two bacterial enzymes from which it has been designed, i.e. SspCA and SazCA. Indeed, both the kinetic constant (kcat) as well as KM of SupCA are quite different compared to SspCA and SazCA (as well as hCA II). Overall, SupCA is more than 10 times less effective as a catalyst for the hydration of carbon dioxide compared to SazCA, considering the kcat/KM ratio (). The main factor explaining this reduced catalytic efficacy is the more than 10 times lower kinetic constant of the chimeric enzyme compared to SazCA. In addition, the affinity of the chimeric enzyme for the sulfonamide inhibitor acetazolamide (AAZ) was also diminished compared to SazCA, SspCA or hCA II. Indeed, as seen from , AAZ is a very effective, subnanomolar SazCA inhibitor, being a low nanomolar SspCA and hCA II inhibitor too. However, its affinity for SupCA was of only 113 nM orders of magnitude lower compared to the previous enzymes. Probably the introduction of the amino acids within its active site completely perturbed the conformation/orientation of amino acid residues involved in the catalytic cycle and/or the binding of inhibitors.
Table 1. Comparison of the CO2-hydrase activities of SazCA, SspCA (from thermophilic bacteria), SupCA (chimeric enzyme) and hCA II. The kinetic parameters were determined by using a stopped-flow assay. Inhibition data with the sulfonamide acetazolamide (5-acetamido-1,3,4-thiadiazole-2-sulfonamide) is also provided.
Stability studies
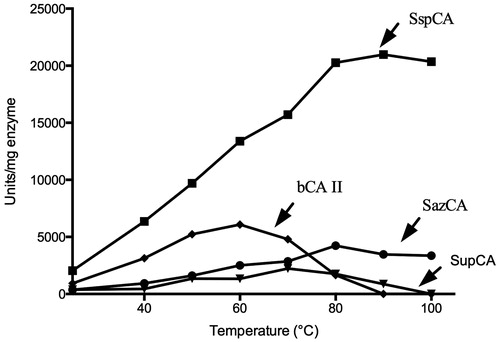
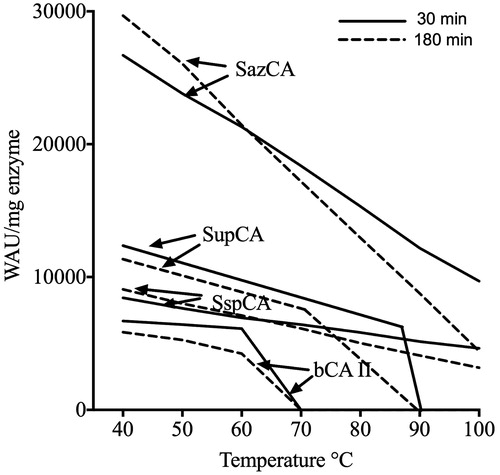
The temperature dependent activity of SupCA was assayed using the p-NpA as substrate, and measurements were performed in parallel with bCA II, SspCA and SazCA using 300 ng of each enzyme (). The optimum temperature range for the CAs aforementioned was determined by incubating enzymes at 25, 40, 50, 60, 70, 80, 90 and 100 °C. Reaction was monitored following the absorbance at 348 nm for 5 min. As shown in , the optimum temperature for SupCA was found to be 70 °C, 10 °C higher than the bovine enzyme (bCA II) with an optimum temperature of 60 °C; while SspCA and SazCA, the two extremophile enzymes, showed an optimum temperature of 90 and 80 °C, respectively. Intriguingly, SspCA showed a specific activity for the esterase reaction higher than the other α-CAs considered in the experiment. The chimeric-α-CA (SupCA) had an esterase specific activity very similar to that of the fastest α-CA reported in literature, SazCA (). We analyzed the temperature effect on the stability of SupCA and compared it with that obtained for bCA II, SspCA and SazCA. The stabilities of SupCA, bCA II, SspCA and SazCA were measured and compared at the temperatures indicated on the X-axis (). After 30 and 180 min of incubation at the temperature ranging from 40 to 100 °C, the activities of aforementioned α-CAs were determined using CO2 as substrate. After 30 min of incubation, SupCA was inactivated at temperature higher than 90 °C, while inactivation temperature became less than 75 °C after 180 min of incubation (). The bovine enzyme was inactivated at temperature higher than 60 °C () for all incubation times. Noticeably, the bacterial enzymes are still active at temperature higher than 90 °C (), being the SspCA more stable than SazCA as already described by our groups. These studies indicate that the chimeric-α-CA (SupCA) was more stable than the bovine enzyme but the enzyme did not retain the activity for a longer time (e.g. 180 min), unlike SspCA and SazCA which were stable at temperature higher than 90 °C for a long time ().
Conclusions
The main goal of this work was to combine in a chimeric enzyme the very high catalytic activity of SazCA with the excellent thermostability of SspCA, creating a chimeric, super-CA, denominated tentatively SupCA. For doing this, we used the SspCA polypeptide chain in which five histidines and one threonine residues were inserted, thus substituting the residues at positions 4, 17, 35, 41, 48 (His) and 34 (Thr). We thought that the introduction of more His residues which eventually can participate in proton transfer reactions between the active site and the enzymeenvironment, may enhance the rate-determining step in the catalytic cycle, making the mutant more effective compared to the other two enzymes from which it has been derived. Indeed, in hCA II and SazCA, two of the most effective CAs known to date, a large number of His residues are present in the N-terminal part of the protein. However, this scenario did not work, since the chimeric enzyme had a kcat at least one order of magnitude lower than SazCA, proving thus that the number of His residues surrounding the active site (or at its entrance) is not a significant parameter for the catalytic activity. Furthermore, probably the hydrogen bonds network of the active site environment was also strongly perturbed by the substitutions we did, since the chimeric enzyme also showed a diminished thermostability compared to the parent CAs from which it has been designed. Overall, despite the negative result, the experiments that we report here are important for understanding how proteins work, and how perfect the evolution of these catalysts is. Indeed, a small number of mutations of amino acids far away from the catalytic center, completely changed the kinetic properties, inhibitor affinity and thermal stability of the protein. Sometimes failures are also important for successful endeavors, and this negative lesson can probably be used to design catalysts with an enhanced activity and stability.
Declaration of interest
The authors declare no conflict of interest.
References
- Demirjian DC, Moris-Varas F, Cassidy CS. Enzymes from extremophiles. Curr Opin Chem Biol 2001;5:144–51
- Gabani P, Singh OV. Radiation-resistant extremophiles and their potential in biotechnology and therapeutics. Appl Microbiol Biotechnol 2013;97:993–1004
- Madigan MT, Marrs BL. Extremophiles. Sci Am 1997;276:82–7
- Egorova K, Antranikian G. Industrial relevance of thermophilic Archaea. Curr Opin Microbiol 2005;8:649–55
- Eichler J. Biotechnological uses of archaealextremozymes. Biotechnol Adv 2001;19:261–78
- Marhuenda-Egea FC, Bonete MJ. Extreme halophilic enzymes in organic solvents. Curr Opin Biotechnol 2002;13:385–9
- Schumacher K, Heine E, Hocker H. Extremozymes for improving wool properties. J Biotechnol 2001;89:281–8
- Vullo D, Del Prete S, Osman SM, et al. Sulfonamide inhibition studies of the gamma-carbonic anhydrase from the oral pathogen Porphyromonas gingivalis. Bioorg Med Chem Lett 2014;24:240–4
- Nishimori I, Vullo D, Minakuchi T, et al. Anion inhibition studies of two new beta-carbonic anhydrases from the bacterial pathogen Legionella pneumophila. Bioorg Med Chem Lett 2014;24:1127–32
- Del Prete S, De Luca V, Scozzafava A, et al. Biochemical properties of a new alpha-carbonic anhydrase from the human pathogenic bacterium, Vibrio cholerae. J Enzyme Inhib Med Chem 2014;29:23–7
- Alafeefy AM, Abdel-Aziz HA, Vullo D, et al. Inhibition of carbonic anhydrases from the extremophilic bacteria Sulfurihydrogenibium yellostonense (SspCA) and S. azorense (SazCA) with a new series of sulfonamides incorporating aroylhydrazone-, [1,2,4]triazolo[3,4-b][1,3,4]thiadiazinyl- or 2-(cyanophenylmethylene)-1,3,4-thiadiazol-3(2H)-yl moieties. Bioorg Med Chem 2014;22:141–7
- Vullo D, Sai Kumar RS, Scozzafava A, et al. Anion inhibition studies of a beta-carbonic anhydrase from Clostridium perfringens. Bioorg Med Chem Lett 2013;23:6706–10
- Vullo D, De Luca V, Scozzafava A, et al. The extremo-alpha-carbonic anhydrase from the thermophilic bacterium Sulfurihydrogenibium azorense is highly inhibited by sulfonamides. Bioorg Med Chem 2013;21:4521–5
- Luca VD, Vullo D, Scozzafava A, et al. An alpha-carbonic anhydrase from the thermophilic bacterium Sulphurihydrogenibium azorense is the fastest enzyme known for the CO2 hydration reaction. Bioorg Med Chem 2013;21:1465–9
- Di Fiore A, Capasso C, De Luca V, et al. X-ray structure of the first ‘extremo-alpha-carbonic anhydrase’, a dimeric enzyme from the thermophilic bacterium Sulfurihydrogenibium yellowstonense YO3AOP1. Acta Crystallogr D Biol Crystallogr 2013;69:1150–9
- Del Prete S, Vullo D, De Luca V, et al. A highly catalytically active gamma-carbonic anhydrase from the pathogenic anaerobe Porphyromonas gingivalis and its inhibition profile with anions and small molecules. Bioorg Med Chem Lett 2013;23:4067–71
- Del Prete S, De Luca V, Vullo D, et al. Biochemical characterization of the gamma-carbonic anhydrase from the oral pathogen Porphyromonas gingivalis, PgiCA. J Enzyme Inhib Med Chem 2014;29:532–7
- Vullo D, De Luca V, Scozzafava A, et al. The first activation study of a bacterial carbonic anhydrase (CA). The thermostable alpha-CA from Sulfurihydrogenibium yellowstonense YO3AOP1 is highly activated by amino acids and amines. Bioorg Med Chem Lett 2012;22:6324–7
- Vullo D, De Luca V, Scozzafava A, et al. Anion inhibition studies of the fastest carbonic anhydrase (CA) known, the extremo-CA from the bacterium Sulfurihydrogenibium azorense. Bioorg Med Chem Lett 2012;22:7142–5
- Del Prete S, Isik S, Vullo D, et al. DNA cloning, characterization, and inhibition studies of an alpha-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. J Med Chem 2012;55:10742–8
- De Luca V, Vullo D, Scozzafava A, et al. Anion inhibition studies of an alpha-carbonic anhydrase from the thermophilic bacterium Sulfurihydrogenibium yellowstonense YO3AOP1. Bioorg Med Chem Lett 2012;22:5630–4
- Supuran CT. Carbonic anhydrase inhibitors: an editorial. Expert Opin Ther Pat 2013;23:677–9
- De Simone G, Alterio V, Supuran CT. Exploiting the hydrophobic and hydrophilic binding sites for designing carbonic anhydrase inhibitors. Expert Opin Drug Discov 2013;8:793–810
- Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72
- Del Prete S, Vullo D, Fisher GM, et al. Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum – the eta-carbonic anhydrases. Bioorg Med Chem Lett 2014;24:4389–96
- Vullo D, Del Prete S, Osman SM, et al. Sulfonamide inhibition studies of the delta-carbonic anhydrase from the diatom Thalassiosira weissflogii. Bioorg Med Chem Lett 2014;24:275–9
- Del Prete S, Vullo D, Scozzafava A, et al. Cloning, characterization and anion inhibition study of the delta-class carbonic anhydrase (TweCA) from the marine diatom Thalassiosira weissflogii. Bioorg Med Chem 2014;22:531–7
- Del Prete S, Vullo D, De Luca V, et al. Biochemical characterization of the delta-carbonic anhydrase from the marine diatom Thalassiosira weissflogii, TweCA. J Enzyme Inhib Med Chem 2014;29:906–11
- Capasso C, Supuran CT. Anti-infective carbonic anhydrase inhibitors: a patent and literature review. Expert Opin Ther Pat 2013;23:693–704
- Vullo D, Kupriyanova EV, Scozzafava A, et al. Anion inhibition study of the beta-carbonic anhydrase (CahB1) from the cyanobacterium Coleofasciculus chthonoplastes (ex-Microcoleus chthonoplastes). Bioorg Med Chem 2014;22:1667–71
- Vullo D, Flemetakis E, Scozzafava A, et al. Anion inhibition studies of two alpha-carbonic anhydrases from Lotus japonicus, LjCAA1 and LjCAA2. J Inorg Biochem 2014;136:67–72
- Vullo D, Del Prete S, Osman SM, et al. Anion inhibition study of the beta-class carbonic anhydrase (PgiCAb) from the oral pathogen Porphyromonas gingivalis. Bioorg Med Chem Lett 2014;24:4402–6
- Rodrigues GC, Feijo DF, Bozza MT, et al. Design, synthesis, and evaluation of hydroxamic acid derivatives as promising agents for the management of Chagas disease. J Med Chem 2014;57:298–308
- Prete SD, Vullo D, Osman SM, et al. Sulfonamide inhibition study of the carbonic anhydrases from the bacterial pathogen Porphyromonas gingivalis: the beta-class (PgiCAb) versus the gamma-class (PgiCA) enzymes. Bioorg Med Chem 2014;22:4537–43
- Nishimori I, Vullo D, Minakuchi T, et al. Sulfonamide inhibition studies of two beta-carbonic anhydrases from the bacterial pathogen Legionella pneumophila. Bioorg Med Chem 2014;22:2939–46
- Migliardini F, De Luca V, Carginale V, et al. Biomimetic CO2 capture using a highly thermostable bacterial alpha-carbonic anhydrase immobilized on a polyurethane foam. J Enzyme Inhib Med Chem 2014;29:146–50
- Del Prete S, Vullo D, De Luca V, et al. Biochemical characterization of recombinant beta-carbonic anhydrase (PgiCAb) identified in the genome of the oral pathogenic bacterium Porphyromonas gingivalis. J Enzyme Inhib Med Chem 2014. [Epub ahead of print]. DOI:10.3109/14756366.2014.931383
- Del Prete S, De Luca V, Vullo D, et al. Biochemical characterization of the gamma-carbonic anhydrase from the oral pathogen Porphyromonas gingivalis, PgiCA. J Enzyme Inhib Med Chem 2014;29:532–7
- De Luca V, Del Prete S, Supuran CT, Capasso C. Protonography, a new technique for the analysis of carbonic anhydrase activity. J Enzyme Inhib Med Chem 2014. [Epub ahead of print].doi:10.3109/14756366.2014.917085
- Ceruso M, Del Prete S, AlOthman Z, et al. Synthesis of sulfonamides with effective inhibitory action against Porphyromonas gingivalis gamma-carbonic anhydrase. Bioorg Med Chem Lett 2014;24:4006–10
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov, 2008;7:168–81
- Supuran CT. Bacterial carbonic anhydrases as drug targets: toward novel antibiotics? Front Pharmacol 2011;2:34
- Capasso C, Supuran CT. An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2014. [Epub ahead of print]. doi:10.3109/14756366.2014.910202
- Capasso C, Supuran CT. An overview of the selectivity and efficiency of the bacterial carbonic anhydrase inhibitors. Curr Med Chem 2014. [Epub ahead of print]
- Ceruso M, Del Prete S, Alothman Z, et al. Sulfonamides with potent inhibitory action and selectivity against the alpha-carbonic anhydrase from Vibrio cholerae. ACS Med Chem Lett 2014;5:826–30
- Capasso C, Supuran CT. Sulfa and trimethoprim-like drugs – antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J Enzyme Inhib Med Chem 2014;29:379–87
- Vullo D, Luca VD, Scozzafava A, et al. The alpha-carbonic anhydrase from the thermophilic bacterium Sulfurihydrogenibium yellowstonense YO3AOP1 is highly susceptible to inhibition by sulfonamides. Bioorg Med Chem 2013;21:1534–8
- Vullo D, Leewattanapasuk W, Muhlschlegel FA, et al. Carbonic anhydrase inhibitors: inhibition of the beta-class enzyme from the pathogenic yeast Candida glabrata with sulfonamides, sulfamates and sulfamides. Bioorg Med Chem Lett 2013;23:2647–52
- Vullo D, Isik S, Del Prete S, et al. Anion inhibition studies of the alpha-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Bioorg Med Chem Lett 2013;23:1636–8
- Syrjanen L, Vermelho AB, Rodrigues Ide A, et al. Cloning, characterization, and inhibition studies of a beta-carbonic anhydrase from Leishmania donovani chagasi, the protozoan parasite responsible for leishmaniasis. J Med Chem 2013;56:7372–81
- Pan P, Vermelho AB, Scozzafava A, et al. Anion inhibition studies of the alpha-carbonic anhydrase from the protozoan pathogen Trypanosoma cruzi, the causative agent of Chagas disease. Bioorg Med Chem 2013;21:4472–6
- Pan P, Vermelho AB, Capaci Rodrigues G, et al. Cloning, characterization, and sulfonamide and thiol inhibition studies of an alpha-carbonic anhydrase from Trypanosoma cruzi, the causative agent of Chagas disease. J Med Chem 2013;56:1761–71
- Nishimori I, Vullo D, Minakuchi T, et al. Restoring catalytic activity to the human carbonic anhydrase (CA) related proteins VIII, X and XI affords isoforms with high catalytic efficiency and susceptibility to anion inhibition. Bioorg Med Chem Lett 2013;23:256–60
- Monti SM, De Simone G, Dathan NA, et al. Kinetic and anion inhibition studies of a beta-carbonic anhydrase (FbiCA 1) from the C4 plant Flaveria bidentis. Bioorg Med Chem Lett 2013;23:1626–30
- Guzel-Akdemir O, Akdemir A, Pan P, et al. A class of sulfonamides with strong inhibitory action against the alpha-carbonic anhydrase from Trypanosoma cruzi. J Med Chem 2013;56:5773–81
- Alafeefy AM, Isik S, Al-Jaber NA, et al. Carbonic anhydrase inhibitors. Benzenesulfonamides incorporating cyanoacrylamide moieties strongly inhibit Saccharomyces cerevisiae beta-carbonic anhydrase. Bioorg Med Chem Lett 2013;23:3570–5
- Akdemir A, Vullo D, De Luca V, et al. The extremo-alpha-carbonic anhydrase (CA) from Sulfurihydrogenibium azorense, the fastest CA known, is highly activated by amino acids and amines. Bioorg Med Chem Lett 2013;23:1087–90
- Akdemir A, Guzel-Akdemir O, Scozzafava A, et al. Inhibition of tumor-associated human carbonic anhydrase isozymes IX and XII by a new class of substituted-phenylacetamido aromatic sulfonamides. Bioorg Med Chem 2013;21:5228–32
- McKenna R, Supuran CT. Carbonic anhydrase inhibitors drug design. SubcellBiochem 2014;75:291–323
- Carta F, Aggarwal M, Maresca A, et al. Dithiocarbamates: a new class of carbonic anhydrase inhibitors. Crystallographic and kinetic investigations. Chem Commun (Camb) 2012;48:1868–70
- Winum JY, Supuran CT. Recent advances in the discovery of zinc-binding motifs for the development of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2014. [Epub ahead of print]. doi:10.3109/14756366.2014.913587
- Carta F, Akdemir A, Scozzafava A, et al. Xanthates and trithiocarbonates strongly inhibit carbonic anhydrases and show antiglaucoma effects in vivo. J Med Chem 2013;56:4691–700
- Davis RA, Vullo D, Supuran CT, Poulsen SA. Natural product polyamines that inhibit human carbonic anhydrases. Biomed Res Int 2014;2014:374079. doi:10.1155/2014/374079
- Carradori S, De Monte C, D'Ascenzio M, et al. Salen and tetrahydrosalen derivatives act as effective inhibitors of the tumor-associated carbonic anhydrase XII – a new scaffold for designing isoform-selective inhibitors. Bioorg Med Chem Lett 2013;23:6759–63
- Supuran CT. Amide derivatives of benzene-sulfonanilide, pharmaceutical composition thereof and method for cancer treatment using the same (US20120095092). Expert Opin Ther Pat 2012;22:1251–5
- Supuran CT. Carbonic anhydrase inhibitors and activators for novel therapeutic applications. Future Med Chem 2011;3:1165–80
- Durdagi S, Senturk M, Ekinci D, et al. Kinetic and docking studies of phenol-based inhibitors of carbonic anhydrase isoforms I, II, IX and XII evidence a new binding mode within the enzyme active site. Bioorg Med Chem 2011;19:1381–9
- Supuran CT. Carbonic anhydrase inhibition with natural products: novel chemotypes and inhibition mechanisms. Mol Divers 2011;15:305–16
- Carta F, Temperini C, Innocenti A, et al. Polyamines inhibit carbonic anhydrases by anchoring to the zinc-coordinated water molecule. J Med Chem 2010;53:5511–22
- Lehneck R, Neumann P, Vullo D, et al. Crystal structures of two tetrameric beta-carbonic anhydrases from the filamentous ascomycete Sordaria macrospora. FEBS J 2014;281:1759–72
- Di Fiore A, Truppo E, Supuran CT, et al. Crystal structure of the C183S/C217S mutant of human CA VII in complex with acetazolamide. Bioorg Med Chem Lett 2010;20:5023–6
- Avvaru BS, Wagner JM, Maresca A, et al. Carbonic anhydrase inhibitors. The X-ray crystal structure of human isoform II in adduct with an adamantyl analogue of acetazolamide resides in a less utilized binding pocket than most hydrophobic inhibitors. Bioorg Med Chem Lett 2010;20:4376–81
- Temperini C, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. X-ray crystal studies of the carbonic anhydrase II-trithiocarbonate adduct – an inhibitor mimicking the sulfonamide and urea binding to the enzyme. Bioorg Med Chem Lett 2010;20:474–8
- Alterio V, Hilvo M, Di Fiore A, et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc Natl Acad Sci USA 2009;106:16233–8
- Di Fiore A, Monti SM, Hilvo M, et al. Crystal structure of human carbonic anhydrase XIII and its complex with the inhibitor acetazolamide. Proteins 2009;74:164–75
- Temperini C, Scozzafava A, Puccetti L, Supuran CT. Carbonic anhydrase activators: X-ray crystal structure of the adduct of human isozyme II with L-histidine as a platform for the design of stronger activators. Bioorg Med Chem Lett 2005;15:5136–41
- Guzel O, Innocenti A, Vullo D, et al. 3-Phenyl-1H-indole-5-sulfonamides: structure-based drug design of a promising class of carbonic anhydrase inhibitors. Curr Pharm Des 2010;16:3317–26
- Temperini C, Innocenti A, Guerri A, et al. Phosph(on)ate as a zinc-binding group in metalloenzyme inhibitors: X-ray crystal structure of the antiviral drug foscarnet complexed to human carbonic anhydrase I. Bioorg Med Chem Lett 2007;17:2210–15
- Winum JY, Temperini C, El Cheikh K, et al. Carbonic anhydrase inhibitors: clash with Ala65 as a means for designing inhibitors with low affinity for the ubiquitous isozyme II, exemplified by the crystal structure of the topiramate sulfamide analogue. J Med Chem 2006;49:7024–31