Abstract
Sulfonamide containing molecules are of sound biomedical interest. This work comprises the synthesis and in vitro antitumor testing of new library of 20 such molecules. These compounds were screened for cytotoxic activity against three tumor cell lines MCF-7, HeLa, and HepG2 using MTT assay. The yield was low but all the target compounds exhibited antiproliferative activity better than the standard drug Doxorubicin (CAS-23214-92-8). Seven compounds were more potent and four compounds were as active as the standard drug. There were no great difference between compounds obtained from dimedone and those obtained from cyclohexandione. Also no significant difference found in activity between compounds bearing o-amino ethyl ester side chain and compounds bearing o-amino amide derivatives. However, compounds bearing o-amino-cyano group, although retained considerable activity they were far less active than the preceding two. It was clear that monohydroxy aldehyde derivatives were less active compared with the di and trihydroxy ones.
Introduction
Tumor growth and metastasis depend on multiple factors, including several physiological processes. For example, elevated expression of different growth factors is associated with tumor angiogenesis, metastases, survival, and resistance to apoptosisCitation1–3. Therefore, these growth factors have represented potential molecular targets for inhibition of tumor growth and progression. Mono-targeted therapies have in many cases shown clinical utility with many drawbacks. This is because tumors harbor manyirregular growth, survival, invasion, and metastasis pathways, which may develop during the course of treatmentCitation4. In addition, data accumulate that substantial levels of cross talk exist within and between signaling networks in most tumors, necessitating the combined inhibition of multiple targets in order to establish efficient tumor growth inhibitionCitation5. Consequently, cell signaling network models indicate that partial inhibition of a number of targets is more effective than the complete inhibition of a single targetCitation6. Therefore, various tactics were followed to inhibit multiple steps by the development of multi-targeted drugs or the combination of single targeted agents. The use of a combination of different compounds can introduce adverse effects related to pharmacokinetics, toxicity, and patient compliance. Alternatively, the use of multi-targeted agents can avoid most of the problems due to combination therapyCitation7.
Our interest was to develop a series of some small molecules as potential multi-targeted agents that can block biologically relevant molecular targets. In this application, we prepared certain quinoline containing sulfonamide pharmacophores and/or their isosteres. The quinoline core has been shown to be a suitable carrier that allows for an effective DNA intercalation. In that context, several quinoline derivatives have been shown to exhibit high affinity for receptor tyrosine kinases (RTKs), as well as other oncogenic targets, and to possess remarkable antitumor activity both in vitro and in vivoCitation1–7.
Many new sulfonamide derivatives, such as HMN-214, E7010 (ABT-751), and E7070 (Indisulam), have shown substantial antitumor activity via different mechanisms as multidrug resistance down-regulation, tubulin polymerization, inhibition of receptor tyrosine kinases (RTKs) among othersCitation8–21. Recently, the mechanism of sulfonamide antitumor activity has been reported to involve inhibition of carbonic anhydrase (CA) isoforms IX and XIICitation19–21. Therefore, in this work, we will prepare some acrylamide containing sulfonamide derivatives and will study their activity against some selected cell lines.
We proposed that the quinoline backbone will act as a carrier for the sulfonamide moiety, and being selectively trapped within cancerous cells, it will target other oncogenic targets, as RTKs (Scheme 1).
Experimental protocols
Chemistry
All reactions were performed in an efficient fume hood. Solvents and reagents were purchased from commercial sources and were used without further purification. Melting points (°C, uncorrected) were determined in open capillaries on a Gallenkamp melting point apparatus (Sanyo Gallenkamp, Southborough, UK) and were uncorrected. Precoated silica gel plates (silica gel 0.25 mm, 60 G F254; Merck, Darmstadt, Germany) were used for thin layer chromatography, dichloromethane/methanol (9.5:0.5) mixture was used as a developing solvent system and the spots were visualized by ultraviolet light and/or iodine. Infra red spectra were recorded in KBr discs using IR-470 Shimadzu spectrometer (Shimadzu, Tokyo, Japan). 1H NMR spectra (DMSO-d6) were acquired at ambient temperatures on Bruker AC-300 Ultra Shield NMR spectrometer (Bruker, Flawil, Switzerland, δ ppm) at 300 MHz for 1H and 75 MHz for 13C, using TMS as internal standardand peak multiplicities are designed as follows: s-singlet; d-doublet; t-triplet and m-multiplet. Electron impact Mass Spectra were recorded on a Shimadzu GC-MS-QP 5000 instrument (Shimadzu, Tokyo, Japan). Elemental analyses were performed on Carlo Erba 1108 Elemental Analyzer (Heraeus, Hanau, Germany) at the Microanalytical Unit, Faculty of Science, Cairo University, Cairo, Egypt, and the results were within ±0.4% of the theoretical values.
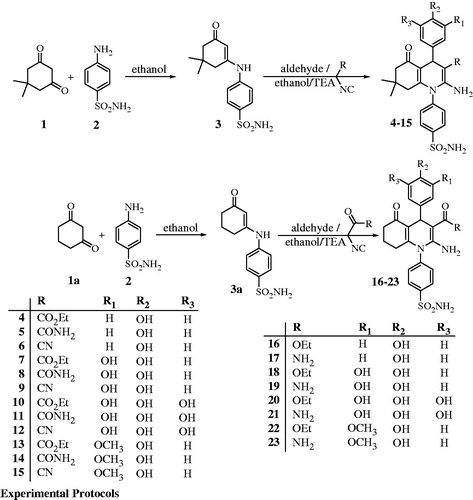
General procedures for the preparation of compounds 4-(3-oxo-5,5-dimethylcyclohex-1-enylamino)benzenesulfonamide (3) and 4-(3-oxo-cyclohex-1-enylamino)benzene sulfonamide (3a)
A mixture of 5,5-dimethylcyclohexane-1,3-dione 1 and/or cyclohexane-1,3-dione 1a (0.01 mol) and sulfanilamide 2 (1.72 g, 0.01 mol) in ethanol (30 mL) was refluxed for 3 h. The reaction mixture was cooled and then poured onto cold water and the obtained solid was crystallized from ethanol to give 3 and/or 3aCitation22.
4-(3-Oxo-5,5-dimethylcyclohex-1-enylamino)benzenesulfonamide (3)
Yield: 82%, M.P.: 164–66 °C. IR, υ (cm−1): 3411, 3326 (SO2NH2), 3063 (Ar-CH), 2947 (Aliph. CH), 1758 (C=O). 1H NMR (DMSOd6): δ 1.12 (s, 6H, 2CH3), 1.42 (s, 2H, CH2), 2.39 (s, 2H, CH2), 2.51 (s, 2H, CH2), 6.14 (s, 2H, SO2NH2), 7.20 (d, J = 7.5 Hz, 2H, H-3 and H-5, C6H4SO2), 7.58 (d, J = 7.5 Hz, 2H, H-2 and H-6, C6H4SO2). 13C NMR: δ 27.8 (2CH3), 32.2 (C(CH3)2), 42.0, 50.0, (2CH2), 98.6 (vinyl = CH), 112.4, 127.4, 129.9, 151.8, 158.8 (Ar-C), 196.2 (C=O). MS m/z (Rel. Int.): 294 (M+, 67). Anal. (C14H18N2O3S), Calcd/Found C: 57.12 (56.88); H: 6.16 (5.92); N: 9.52 (9.75); S: 10.89 (11.08).
4-[2-Amino-3-substituted-4-aryl-5-oxo-5,6,7,8-tetrahydroquinolin-1(4H)-yl]benzene sulfonamide (4–23)
A mixture of compound 3 (2.66 g, 0.01 mol) the appropriate aldehyde (0.01 mol) and the active methylene component (0.01 mol) in absolute ethanol (30 mL) containing three drops of triethylamine, was refluxed for 5 h. The solid obtained after concentration and cooling, was filtered, and crystallized from 1-butanol to give 4–23, respectively.
4-[2-Amino-3-(ethoxycarbonyl)-4-(4-hydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro-7,7-dimethyl quinolin-1(4H)-yl]benzenesulfonamide (4)
Yield: 20%, M.P.: 157–59 °C. IR, υ (cm−1): 3452, 3381, 3264 (OH, NH2, SO2NH2), 3061 (Ar-CH), 2965 (Aliph. CH), 1754, 1736 (2C=O). 1H NMR (DMSOd6): δ 1.01 (s, 6H, 2CH3), 1.23 (t, J = 2.8 Hz,3H, CH3), 1.90 (s, 2H, CH2),2.31 (s, 2H, CH2), 4.27 (q, 2H, CH2), 4.55 (s, 1H, CH), 5.39 (s, 1H, OH), 6.13 (s, 2H, SO2NH2),6.77–7.60 (m, 8H, Ar-H), 8.31 (s, 2H, NH2). 13C NMR: δ15.1,23.5, 35.2, 38.5, 45.7,55.0, 62.6 (CH and CH2,CH3), 86.2, 114.5, 126.3, 128.0, 128.4, 133.7,136.5, 142.0, 144.9, 147.2 (Ar-C), 166.7,181.5 (2C=O). MS m/z (Rel. Int.): 511 (M+, 34). Anal. (C26H29N3O6S), Calcd/Found C: 61.04 (61.27); H: 5.71 (5.54); N: 8.21 (8.47); S: 6.27 (6.09).
4-[2-Amino-3-(aminocarbonyl)-4-(4-hydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro-7,7-dimethylquinolin-1(4H)-yl]benzenesulfonamide (5)
Yield: 20%, M.P.: 273–75 °C. IR, υ (cm−1): 3409, 3372, 3258 (OH, NH2, SO2NH2), 3055 (Ar-CH), 2965 (Aliph. CH), 1751, 1738 (2C=O). 1H NMR (DMSOd6): δ 1.04 (s, 6H, 2CH3), 1.89 (s, 2H, CH2), 2.33 (s, 2H, CH2), 4.54 (s, 1H, CH), 5.42 (s, 1H, OH), 6.11 (s, 2H, SO2NH2), 6.78–7.60 (m, 10H, Ar-H, NH2), 8.32 (s, 2H, NH2). 13C NMR: δ 26.5, 33.2, 38.5, 44.3, 52.1 (CH, CH2, CH3), 81.8, 110.5, 115.2, 117.0, 130.0, 130.4, 130.9, 137.3, 153.1, 155.7, 159.3 (Ar-C), 176.4, 194.1 (2C=O). MS m/z (Rel. Int.): 482 (M+, 61). Anal. (C24H26N4O5S), Calcd/Found: C, 59.74 (59.49); H, 5.43 (5.15); N, 11.61 (11.39); S, 6.64 (6.85).
4-[2-Amino-3-cyano-4-(4-hydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro-7,7-dimethyl quinolin-1(4H)-yl]benzenesulfonamide (6)
Yield: 23%, M.P.: 281–83 °C. IR, υ (cm−1): 3452, 3381, 3264 (OH, NH2, SO2NH2), 3061 (Ar-CH), 2969 (Aliph. CH), 2234 (C≡N), 1749 (C=O). 1H NMR (DMSOd6): δ 1.06 (s, 6H, 2CH3), 1.86 (s, 2H, CH2), 2.64 (s, 2H, CH2), 4.47 (s, 1H, CH), 5.61 (s, 1H, OH), 6.14 (s, 2H, SO2NH2), 6.79–7.63 (m, 8H, Ar-H), 8.41 (s, 2H, NH2). 13C NMR: δ 26.9, 33.5, 38.2, 40.7, 51.4(CH, CH2, CH3), 58.1 (C-CN), 111.4, 115.2, 116.5, 117.1, 128.3, 129.5, 130.5, 134.6, 145.1, 153.6, 160.9 (Ar-C), 195.1 (C=O). MS m/z (Rel. Int.): 464 (M+, 47). Anal. (C24H24N4O4S), Calcd/Found: C, 62.05 (61.79); H, 5.21 (4.98); N, 12.06 (11.85); S, 6.90 (7.14).
4-[2-Amino-3-(ethoxycarbonyl)-4-(4-hydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro quinolin-1(4H)-yl]benzenesulfonamide (7)
Yield: 25%, M.P.: 183–85 °C. IR, υ (cm−1): 3455, 3380, 3267 (OH, NH2, SO2NH2), 3069 (Ar-CH), 2968 (Aliph. CH), 1759, 1737 (2C=O). 1H NMR (DMSOd6): δ 1.01 (s, 6H, 2CH3), 1.22 (t, J = 2.7 Hz, 3H, CH3), 1.91 (s, 2H, CH2), 2.30 (s, 2H, CH2), 4.27 (q, J = 3.2, Hz, 2H, CH2), 4.57 (s, 1H, CH), 5.41 (s, 2H, 2OH), 6.10 (s, 2H, SO2NH2), 6.79–7.56 (m, 7H, Ar-H), 8.34 (s, 2H, NH2). 13C NMR: δ 14.7, 25.3, 34.5, 38.7, 43.9, 52.1, 62.5(CH, CH2, CH3), 82.7, 112.0, 114.3, 116.1, 116.6, 124.4, 130.1, 130.5, 136.6, 143.5, 144.2, 145.9, 152.1, 159.5 (Ar-C), 166.2, 192.3 (2C=O). MS m/z (Rel. Int.): 527 (M+, 34). Anal. (C26H29N3O7S), Calcd/Found: C, 59.19 (58.93); H, 5.54 (5.80); N,7.96 (8.14); S, 6.08 (5.86).
4-[2-Amino-3-(aminocarbonyl)-4-(4-hydroxyphenyl)-5-oxo-5,6,7,8-tetrahydroquinolin-1(4H)-yl]benzenesulfonamide (8)
Yield: 24%, M.P.: 252–54 °C. IR, υ (cm−1): 3451, 3380, 3266 (OH, NH2, SO2NH2), 3063 (Ar-CH), 2966 (Aliph. CH), 1752, 1740 (2C=O). 1H NMR (DMSOd6): δ0.99 (s, 6H, 2CH3), 1.87 (s, 2H, CH2), 2.34 (s, 2H, CH2), 4.51 (s, 1H, CH), 5.43 (s, 2H, 2OH), 6.11 (s, 2H, SO2NH2), 6.79–7.58 (m, 9H, Ar-H, NH2), 8.32 (s, 2H, NH2). 13C NMR: δ 26.4, 33.3, 38.2, 44.5, 52.6(CH, CH2, CH3), 82.5, 111.3, 115.5, 116.3, 117.0, 123.0, 130.3, 130.7, 137.1, 144.3, 144.8, 146.0 153.3, 159.1 (Ar-C), 173.8, 192.8 (2C=O). MS m/z (Rel. Int.): 498 (M+, 55). Anal. (C24H26N4O6S), Calcd/Found: C, 57.82 (57.61); H, 5.26 (5.58); N, 11.24 (11.54); S, 6.43 (6.65).
4-[2-Amino-3-(ethoxycarbonyl)-4-(3,4-dihydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro-7,7-dimethylquinolin-1(4H)-yl]benzenesulfonamide (9)
Yield: 23%, M.P.: 294–96 °C. IR, υ (cm−1): 3450, 3383, 3265 (OH, NH2, SO2NH2), 3063 (Ar-CH), 2961 (Aliph. CH), 2228 (C≡N), 1748 (C=O). 1H NMR (DMSOd6): δ 1.03 (s, 6H, 2CH3), 1.87 (s, 2H, CH2), 2.61 (s, 2H, CH2), 4.46 (s, 1H, CH), 5.60 (s, 2H, 2OH), 6.16 (s, 2H, SO2NH2), 6.76–7.62 (m, 7H, Ar-H), 8.47 (s, 2H, NH2). 13C NMR: δ 26.8, 33.8, 38.5, 40.9, 51.4 (CH, CH2, CH3), 58.7 (C-CN), 112.3, 116.3, 116.8, 117.4, 124.0, 128.1, 129.4, 135.9, 144.9, 145.2, 147.1, 153.8, 162.3 (Ar-C), 196.0 (C=O). MS m/z (Rel. Int.): 480 (M+, 47). Anal. (C24H24N4O5S), Calcd/Found: C, 59.99 (60.27); H, 5.03 (4.77); N, 11.66 (11.92); S, 6.67 (6.95).
4-[2-Amino-3-(aminocarbonyl)-4-(3,4-dihydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro-7,7-dimethylquinolin-1(4H)-yl]benzenesulfonamide (10)
Yield: 28%, M.P.: 273–75 °C. IR, υ (cm−1): 3447, 3378, 3267 (OH, NH2, SO2NH2), 3067 (Ar-CH), 2958 (Aliph. CH), 1769, 1747 (2C=O). 1H NMR (DMSOd6): δ1.13 (s, 6H, 2CH3), 1.25 (t, J = 2.7 Hz, 3H, CH3), 1.88 (s, 2H, CH2), 2.68 (s, 2H, CH2), 4.28 (q, J = 4.2 Hz, 2H, CH2), 4.55 (s, 1H, CH), 5.53 (s, 3H, 3OH), 5.96 (s, 2H, Ar-H), 6.18 (s, 2H, SO2NH2), 6.91–7.53 (m, 4H, Ar-H), 8.39 (s, 2H, NH2). 13C NMR: δ 14.6, 26.4, 32.6, 39.9, 40.5, 52.1, 62.4 (CH and CH2, CH3), 81.5, 109.4, 112.3, 116.8, 127.6, 130.0, 134.2, 137.5, 144.3, 149.0, 152.8, 159.3 (Ar-C), 164.9, 196.5 (2C=O). MS m/z (Rel. Int.): 543 (M+, 38). Anal. (C26H29N3O8S), Calcd/Found: C, 57.45 (57.14); H, 5.38 (5.09); N, 7.73 (7.52); S, 5.90 (5.66).
4-[2-Amino-3-cyano-4-(3,4-dihydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro-7,7-dimethyl quinolin-1(4H)-yl]benzenesulfonamide (11)
Yield: 25%, M.P.: >300 °C. IR, υ (cm−1): 3405, 3362, 3267 (OH, NH2, SO2NH2), 3067 (Ar-CH), 2968 (Aliph. CH), 1751, 1744 (2C=O). 1H NMR (DMSOd6): δ 1.10 (s, 6H, 2CH3), 1.86 (s, 2H, CH2), 2.76 (s, 2H, CH2), 4.45 (s, 1H, CH), 5.47 (s, 3H, 3OH), 5.97 (s, 2H, Ar-H), 6.16 (s, 2H, SO2NH2), 6.81–7.59 (m, 6H, Ar-H, NH2), 8.36 (s, 2H, NH2). 13C NMR: δ 26.7, 32.9, 40.2, 40.6, 52.3 (CH, CH2, CH3), 82.6, 109.1, 111.6,116.5, 128.3, 129.9,134.0, 137.3, 144.7, 144.5, 153.5, 159.3 (Ar-C), 169.8, 196.0 (2C=O). MS m/z (Rel. Int.): 514 (M+, 59). Anal. (C24H26N4O7S), Calcd/Found: C, 56.02 (55.74); H, 5.09 (5.32); N, 10.89 (11.05); S, 6.23 (6.47).
4-[2-Amino-3-(ethoxycarbonyl)–(3,4-dihydroxyphenyl)-5-oxo-5,6,7,8-tetrahydroquinolin-1(4H)-yl]benzenesulfonamide (12)
Yield: 27%, M.P.: >300 °C. IR, υ (cm−1): 3454, 3383, 3267 (OH, NH2, SO2NH2), 3063 (Ar-CH), 2967 (Aliph. CH), 2224 (C≡N), 1752 (C=O). 1H NMR (DMSOd6): δ 1.38 (m, 2H, CH2), 1.89 (t, J = 2.3 Hz, 2H, CH2), 2.94 (t, 2H, J = 2.6 Hz, CH2), 4.46 (s, 1H, CH), 5.44 (s, 3H, 3OH), 5.96 (s, 2H, Ar-H), 6.15 (s, 2H, SO2NH2), 6.79–7.63 (m, 4H, Ar-H), 8.39 (s, 2H, NH2). 13C NMR: δ21.0, 26.8, 36.5, 38.9 (CH, CH2), 58.1 (C-CN), 109.1, 111.3, 116.5, 117.1, 128.2, 129.4, 133.5, 137.2, 144.5, 148.7, 153.5, 163.7 (Ar-C), 196.1 (C=O). MS m/z (Rel. Int.): 468 (M+, 47). Anal. (C22H20N4O6S), Calcd/Found: C, 56.40 (56.69); H, 4.30 (4.52); N, 11.96 (12.19); S, 6.84 (6.59).
4-[2-Amino-3-(aminocarbonyl)-(3,4-dihydroxyphenyl)-5-oxo-5,6,7,8-tetrahydroquinolin-1(4H)-yl]benzenesulfonamide (13)
Yield: 28%, M.P.: 212–214 °C. IR, υ (cm−1): 3453, 3381, 3265 (OH, NH2, SO2NH2), 3062 (Ar-CH), 2966 (Aliph. CH), 1760, 1741 (2C=O). 1H NMR (DMSOd6): δ 1.01 (s, 6H, 2CH3), 1.22 (t, J = 2.6 Hz, 3H, CH3), 1.92 (s, 2H, CH2), 2.31 (s, 2H, CH2), 3.78 (s, 3H, OCH3) 4.28 (q, J = 2.9 Hz, 2H, CH2), 4.54 (s, 1H, CH), 5.41 (s, 1H, OH), 6.11 (s, 2H, SO2NH2), 6.79–7.57 (m, 7H, Ar-H), 8.35 (s, 2H, NH2). 13C NMR: δ 14.6, 27.3, 33.4, 39.8, 41.0, 51.6, 57.1, 61.6 (CH, CH2, and CH3), 81.6, 112.0, 114.7, 116.3, 116.5, 123.4, 128.1, 129.4, 135.6, 142.5, 144.9, 150.9, 153.0, 159.8 (Ar-C), 166.5, 196.3 (2C=O). MS m/z (Rel. Int.): 541 (M+, 34). Anal. (C27H31N3O7S), Calcd/Found: C, 59.87 (60.08); H, 5.77 (6.02); N, 7.76 (7.50); S, 5.92 (6.17).
4-[2-Amino-3-(ethoxycarbonyl)-4-(3,4,5-trihydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro-7,7-dimethylquinolin-1(4H)-yl]benzenesulfonamide (14)
Yield: 25%, M.P.: 262–64 °C. IR, υ (cm−1): 3415, 3381, 3268 (OH, NH2, SO2NH2), 3061 (Ar-CH), 2969 (Aliph. CH), 1753, 1741 (2C=O). 1H NMR (DMSOd6): δ 1.02 (s, 6H, 2CH3), 1.86 (s, 2H, CH2), 2.34 (s, 2H, CH2), 3.80 (s, 3H, OCH3), 4.52 (s, 1H, CH), 5.42 (s, 1H, OH), 6.14 (s, 2H, SO2NH2), 6.79–7.56 (m, 9H, Ar-H, NH2), 8.33 (s, 2H, NH2). 13C NMR: δ 26.7, 32.6, 39.7, 40.3, 51.6, 57.0 (CH, CH2, CH3), 82.6, 111.5, 114.6, 116.3, 116.8, 122.1, 128.0, 129.6 135.3, 142.4, 144.5, 150.9,153.2, 159.0 (Ar-C), 169.9, 196.7 (2C=O). MS m/z (Rel. Int.): 512 (M+, 62). Anal. (C25H28N4O6S), Calcd/Found: C, 58.58 (58.32); H, 5.51 (5.28); N, 10.93 (11.15); S, 6.26 (6.51).
4-[2-Amino-3-(aminocarbonyl)-4-(3,4,5-trihydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro-7,7-dimethylquinolin-1(4H)-yl]benzenesulfonamide (15)
Yield: 27%, M.P.: >300 °C. IR, υ (cm−1): 3421, 3385, 3263 (OH, NH2, SO2NH2), 3066 (Ar-CH), 2971 (Aliph. CH), 2225 (C≡N), 1752 (C=O). 1H NMR (DMSOd6): δ 1.07 (s, 6H, 2CH3), 1.85 (s, 2H, CH2), 2.65 (s, 2H, CH2), 3.78 (s, 3H, OCH3), 4.44 (s, 1H, CH), 5.62 (s, 1H, OH), 6.17 (s, 2H, SO2NH2), 6.79–7.63 (m, 7H, Ar-H), 8.47 (s, 2H, NH2). 13C NMR: δ 27.2, 33.0, 39.2, 40.5, 51.4, 55.9 (CH, CH2,CH3), 58.0 (C-CN), 111.4, 115.1, 116.3, 116.5, 117.1, 122.5, 128.1, 129.4, 134.5, 142.7, 144.6, 152.0, 153.6, 162.3 (Ar-C), 196.2 (C=O). MS m/z (Rel. Int.): 494 (M+, 54). Anal. (C25H26N4O5S), Calcd/Found: C, 60.71 (60.94); H, 5.30 (5.54); N, 11.33 (11.05); S, 6.48 (6.75).
4-[2-Amino-3-cyano-4-(3,4,5-trihydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro-7,7-dimethyl quinolin-1(4H)-yl]benzenesulfonamide (16)
Yield: 22%, M.P.: 165–67 °C. IR, υ (cm−1): 3450, 3382, 3266 (OH, NH2, SO2NH2), 3068 (Ar-CH), 2973 (Aliph. CH), 1755, 1742 (2C=O). 1H NMR (DMSOd6): δ 1.21 (t, J = 3.0 Hz, 3H, CH3), 1.43 (m, 2H, CH2), 1.97 (t, J = 2.5 Hz, 2H, CH2), 2.93 (t, J = 2.8, Hz, 2H, CH2), 4.29 (q, J = 3.5 Hz, 2H, CH2), 4.36 (s, 1H, CH), 5.40 (s, 1H, OH), 6.17 (s, 1H, SO2NH2), 6.74–7.68 (m, 8H, Ar-H), 8.33 (s, 2H, NH2). 13C NMR: δ 14.5, 20.8, 27.1, 36.4, 39.7, 62.6 (CH, CH2, and CH3), 81.1, 112.3, 115.6, 116.0, 128.6, 129.1, 130.7, 134.2, 144.6, 154.0, 155.8, 159.7 (Ar-C), 166.1, 194.2 (2C=O). MS m/z (Rel. Int.): 483 (M+, 34). Anal. (C24H25N3O6S), Calcd/Found: C, 59.61 (59.35); H, 5.21 (4.99); N, 8.69 (8.40); S, 6.63 (6.37).
4-[2-Amino-3-(ethoxycarbonyl)-4-(3,4,5-trihydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro quinolin-1(4H)-yl]benzenesulfonamide (17)
Yield: 26%, M.P.: 261–63 °C. IR, υ (cm−1): 3416, 3389, 3261 (OH, NH2, SO2NH2), 3062 (Ar-CH), 2964 (Aliph. CH), 1754, 1739 (2C=O). 1H NMR (DMSOd6): δ 1.41 (m, 2H, CH2), 1.98 (t, J = 2.9, Hz, 2H, CH2), 2.95 (t, J = 3.0, Hz, 2H, CH2), 4.47 (s, 1H, CH), 5.45 (s, 1H, OH), 6.13 (s, 2H, SO2NH2), 6.75–7.62 (m, 10H, Ar-H, NH2), 8.50 (s, 2H, NH2). 13C NMR: δ 20.7, 26.4, 36.6, 39.2, (CH, CH2), 82.1, 111.6, 115.6, 116.9, 128.1, 129.5, 130.6, 135.2, 145.6, 153.3, 155.1, 159.5 (Ar-C), 174.7, 195.5 (2C=O). MS m/z (Rel. Int.): 454 (M+, 61). Anal. (C22H22N4O5S), Calcd/Found: C, 58.14 (57.83); H, 4.88 (4.63); N, 12.33 (12.06); S, 7.06 (6.75).
4-[2-Amino-3-(aminocarbonyl)-4-(3,4,5-trihydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro quinolin-1(4H)-yl]benzenesulfonamide (18)
Yield: 25%, M.P.: 169–71 °C. IR, υ (cm−1): 3453, 3381, 3266 (OH, NH2, SO2NH2), 3069 (Ar-CH), 2972 (Aliph. CH), 1756, 1740 (2C=O). 1H NMR (DMSOd6): δ 1.20 (t, J = 3.1 Hz, 3H, CH3), 1.45 (m, 2H, CH2), 1.98 (t, J = 2.2 Hz, 2H, CH2), 2.95 (t, J = 2.8, Hz, 2H, CH2), 4.26 (q, J = 3.1 Hz, 2H, CH2), 4.37 (s, 1H, CH), 5.46 (s, 2H, 2OH), 6.19 (s, 1H, SO2NH2), 6.76–7.69 (m, 7H, Ar-H), 8.34 (s, 2H, NH2). 13C NMR: δ 15.2, 21.0, 27.5, 36.3, 39.5, 62.4 (CH, CH2, and CH3), 81.0, 112.3, 115.7, 116.1, 128.5, 128.9, 130.6, 134.1, 144.7, 153.6, 156.2, 159.8 (Ar-C), 169.0, 195.6 (2C=O). MS m/z (Rel. Int.): 499 (M+, 41). Anal. (C24H25N3O7S), Calcd/Found: C, 57.70 (57.96); H, 5.04 (4.77); N, 8.41 (8.69); S, 6.42 (6.17).
4-[2-Amino-3-(ethoxycarbonyl)-4-(3-methoxy-4-hydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro-7,7-dmethyliquinolin-1(4H)-yl]benzenesulfonamide (19)
Yield: 25%, M.P.: 266–68 °C. IR, υ (cm−1): 3427, 3390, 3274 (OH, NH2, SO2NH2), 3065 (Ar-CH), 2961 (Aliph. CH), 1756, 1742 (2C=O). 1H NMR (DMSOd6): δ 1.41 (m, 2H, CH2), 1.98 (t, J = 2.9, Hz, 2H, CH2), 2.95 (t, J = 3.0, Hz, 2H, CH2), 4.47 (s, 1H, CH), 5.45 (s, 2H, 2OH), 6.13 (s, 2H, SO2NH2), 6.74–7.63 (m, 9H, Ar-H, NH2), 8.51 (s, 2H, NH2). 13C NMR: δ 20.8, 26.9, 37.0, 39.8, (CH, CH2), 82.6, 112.2, 116.1, 116.8, 117.1, 124.1, 128.2, 129.4, 136.0, 145.2, 145.6, 147.2, 154.1, 159.4 (Ar-C), 171.7, 194.8 (2C=O). MS m/z (Rel. Int.): 470 (M+, 67). Anal. (C22H22N4O6S), Calcd/Found: C, 56.16 (55.92); H, 4.71 (5.04); N, 11.91 (12.16); S, 6.82 (7.08).
4-[2-Amino-3-(aminocarbonyl)-4-(3methoxy-4-hydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro-7,7-dimethylquinolin-1(4H)-yl]benzenesulfonamide (20)
Yield: 24%, M.P.: 221–23 °C. IR, υ (cm−1): 3450, 3381, 3265 (OH, NH2, SO2NH2), 3067 (Ar-CH), 2972 (Aliph. CH), 1756, 1743 (2C=O). 1H NMR (DMSOd6): δ 1.19 (t, J = 3.0 Hz, 3H, CH3), 1.42 (m, 2H, CH2), 1.96 (t, J = 2.7 Hz, 2H, CH2), 2.91 (t, J = 2.6, Hz, 2H, CH2), 4.27 (q, J = 3.6 Hz, 2H, CH2), 4.38 (s, 1H, CH), 5.43 (s, 3H, 3OH), 5.97 (s, 2H, Ar-H), 6.18 (s, 1H, SO2NH2), 6.79–7.65 (m, 4H, Ar-H), 8.36 (s, 2H, NH2). 13C NMR: δ 14.56, 20.5, 27.3, 37.1, 39.9, 62.1 (CH, CH2, and CH3), 81.2, 109.0, 111.4, 116.1, 128.5, 129.3, 134.1, 137.5, 144.7, 148.3, 154.5, 159.6 (Ar-C), 167.2, 194.8 (2C=O). MS m/z (Rel. Int.): 515 (M+, 34). Anal. (C24H25N3O8S), Calcd/Found: C, 55.91 (56.17); H, 4.89 (5.08); N, 8.15 (7.96); S, 6.22 (5.96).
4-[2-Amino-3-cyano-4-(3-methoxy-4-hydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro-7,7-dimethyl quinolin-1(4H)-yl]benzenesulfonamide (21)
Yield: 27%, M.P.: >300 °C. IR, υ (cm−1): 3451, 3386, 3263 (OH, NH2, SO2NH2), 3061 (Ar-CH), 2967 (Aliph. CH), 1757, 1738 (2C=O). 1H NMR (DMSOd6): δ 1.40 (m, 2H, CH2), 1.97 (t, J = 2.8, Hz, 2H, CH2), 2.94 (t, J = 3.2, Hz, 2H, CH2), 4.46 (s, 1H, CH), 5.47 (s, 3H, 3OH), 5.97 (s, 2H, Ar-H), 6.14 (s, 2H, SO2NH2), 6.84–7.62 (m, 6H, Ar-H, NH2), 8.52 (s, 2H, NH2). 13C NMR: δ 20.5, 26.7, 36.5, 39.9, (CH, CH2, CH3), 82.2, 112.0, 116.7, 128.3, 129.2, 134.4, 138.1, 145.0, 147.6, 153.7, 159.8 (Ar-C), 170.6, 196.3 (2C=O). MS m/z (Rel. Int.): 486 (M+, 61). Anal. (C22H22N4O7S), Calcd/Found: C, 54.31 (54.55); H, 4.56 (4.74); N, 11.52 (11.78); S, 6.59 (6.31).
4-[2-Amino-3-(ethoxycarbonyl)-4-(3-methoxy-4-hydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro quinolin-1(4H)-yl]benzenesulfonamide (22)
Yield: 25%, M.P.: 206–208 °C. IR, υ (cm−1): 3438, 3374, 3265 (OH, NH2, SO2NH2), 3067 (Ar-CH), 2973 (Aliph. CH), 1758, 1746 (2C=O). 1H NMR (DMSOd6): δ 1.16 (t, J = 3.0 Hz, 3H, CH3), 1.38 (m, 2H, CH2), 1.93 (t, J = 2.9 Hz, 2H, CH2), 2.88 (t, J = 2.6, Hz, 2H, CH2), 3.77 (s, 3H, OCH3), 4.29 (q, J = 3.7 Hz, 2H, CH2), 4.39 (s, 1H, CH), 5.47 (s, 1H, OH), 6.15 (s, 1H, SO2NH2), 6.81–7.65 (m, 7H, Ar-H), 8.37 (s, 2H, NH2). 13C NMR: δ 14.6, 20.3, 27.1, 36.7, 39.4, 57.0, 61.8 (CH, CH2, and CH3), 80.9, 111.5, 114.3, 116.1, 116.5, 122.4, 128.6, 129.1, 136.0, 142.7, 145.1, 151.0, 153.6, 160.1 (Ar-C), 167.0, 195.3 (2C=O). MS m/z (Rel. Int.): 513 (M+, 34). Anal. (C25H27N3O7S), Calcd/Found: C, 58.47 (58.19); H, 5.30 (5.11); N, 8.18 (7.92); S, 6.24 (5.95).
4-[2-Amino-3-(aminocarbonyl)-4(3-methoxy-4-hydroxyphenyl)-5-oxo-5,6,7,8-tetrahydro quinolin-1(4H)-yl]benzenesulfonamide (23)
Yield: 27%, M.P.: >300 °C. IR, υ (cm−1): 3425, 3374, 3267 (OH, NH2, SO2NH2), 3064 (Ar-CH), 2968 (Aliph. CH), 1760, 1739 (2C=O). 1H NMR (DMSOd6): δ 1.40 (m, 2H, CH2), 1.95 (t, J = 2.7, Hz, 2H, CH2), 2.92 (t, J = 3.0, Hz, 2H, CH2), 3.76 (s, 3H, OCH3), 4.47 (s, 1H, CH), 5.45 (s, 1H, OH), 6.15 (s, 2H, SO2NH2), 6.83–7.63 (m, 9H, Ar-H, NH2), 8.47 (s, 2H, NH2). 13C NMR: δ 20.3, 26.7, 36.4, 39.9, 57.0 (CH, CH2, CH3), 82.1, 112.0, 115.1, 116.5, 116.9, 122.5, 128.3, 129.2, 134.9, 142.7, 144.8, 152.0, 153.6, 160.2 (Ar-C), 172.5, 196.8 (2C=O). MS m/z (Rel. Int.): 484 (M+, 69). Anal. (C23H24N4O6S), Calcd/Found: C, 57.01 (56.78); H, 4.99 (5.22); N, 11.56 (11.72); S, 6.62 (6.85).
In vitro anticancer screening23,24
The stock solutions of the tested compounds were prepared in DMSO and were used for serial dilutions in the culture medium (the final concentration of DMSO was adjusted as not to exceed 0.3%). The three chosen cell lines were grown in RPMI-1640 medium supplemented with 10% calf serum. For growth assays, exponentially growing cells were suspended in the mentioned medium at a density of 4 × 104 cells per mL and were seeded onto 96-well plates well (200 µL/well), and incubated at 37 °C in a humidified 5% CO2 atmosphere for 24 h. The cell medium in test wells was then changed to another culture medium containing different concentrations of the compounds under test. The control wells were changed to new medium with an equivalent amount of solvent. After incubation at 37 °C in a humidified 5% CO2 atmosphere for 3 d, 100 µL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, 0.5 μg/mL) in the serum-free medium was added to each well and incubated at 37 °C for an additional 4 h. Subsequently, 200 µL of DMSO was added to each well and thoroughly mixed. The cell viability was then evaluated by measuring the optical density at 544 nm using a Microelisa Reader. The percentage of inhibition of cell growth was calculated as follows:
The response parameter calculated was IC50 value (), which corresponds to the compound concentration causing 50% mortality in net cells.
Table 1. In vitro antitumor activity of the designed tetrahydroquinoline derivatives 4–23.
Results and discussion
Chemistry
The synthetic strategies adopted for the synthesis of the target compounds are depicted in Scheme 1, the key starting material the enaminone 3 was obtained by condensation of the appropriate di-carbonyl compound with sulfanilamide in absolute ethanol. The structure of the enaminone derivative was confirmed by elemental analysis and spectral data which were in accordance with the proposed structures. The target compounds 4–23 were obtained by one-pot synthesis through base-catalyzed-condensation reaction of the appropriate enaminone, active methylene, and aldehyde in absolute ethanol. IR spectra for all the compounds showed the presence of aliphatic carbons at around 2950 cm−1, one carbonyl was common and appeared at around 1700 cm−1 in addition to the SO2NH2 amino group at around 3250 cm−1. Compounds 4, 7, 9, 12, 14, 17, and 22 showed the presence of an additional OH group absorption band at around 3400 cm−1. The NMR spectra of these compounds showed the presence of the required hydrogens and their carrying carbons in the expected regions. The amide derivatives 5, 8, 10, 13, 15, 18, 20, and 23 were characterized by an additional NH2 absorption band in the IR spectra and the corresponding protons in the 1H NMR spectra in addition, the absence of the characteristic triplet-quartet pattern of the ethyl ester functionality. The 2-amino-cyano derivatives 6, 11, 16, and 21 were characterized by the presence of the absorption band at around 2225 cm−1 as an indicative for the cyano group and its 13C at around 116 ppm. The MS and elemental analyses were fully in accordance with suggested molecular structures.
In vitro cytotoxicity screening
The target compounds 4–23 were initially screened at concentration of 0.06 mM using the colorimetric MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to test their activity against human breast cancer cell line (MCF-7), human cervix cancer (HeLa), and human hepatocellular liver carcinoma (HepG2). Doxorubicin was employed as a reference standard drug. The cytotoxic activity of these compounds was evaluated in terms of percent growth inhibition compared with the control cells. The candidate compound which produces ≥50% inhibition in the primary dose was retested by serial dilution from 0.06 to 0.002 mM. The results were expressed as IC50, and the data are shown in .
Close scrutiny of the cytotoxic data revealed that all the compounds exhibited significant activity against the three tested cell lines. Seven compounds were more potent than the standard drug. The activity of which could be arranged in the following order: 9 = 12 > 13 = 17 > 18 = 22 = 23. Four compounds were more or less as active as the standard drug. These compounds may be also arranged in the following order: 14 > 10 = 20 > 15. It is also worth to mention that there were no great difference in activity between those compounds obtained from dimedone and those obtained from cyclohexandione. Also, compounds (4, 7, 9, 12, 14, 17, 19, and 22) bearing 2-amino ethyl ester side chain and compounds (5, 8, 10, 13, 15, 18, 20, and 23) bearing 2-amino amide derivatives, both groups showed almost equal antiproliferative activity against the three tested cell lines without significant difference. However, compounds (6, 11, 16, and 21) bearing the 2-amino-cyano group, although retained considerable activity they were far less active than the preceding two. With regard to the effect of aldehyde substituents, it was clear that monohydroxy derivatives were less active compared to the di and trihydroxy derivatives.
The observation that most of the compounds in this study showed considerable activity could be attributed to the great structural similarity and the inherent activity of the current nuclei where the sulfonamide and catechol are molded in the tetrahydroquinoline backbone.
Conclusion
The present work adopted simple chemical procedures to develop new small molecules comprising sulfonamide functionality, catechol in a tetrahydroquinoline scafold. The structure of the obtained compounds was established by different instrumental analytical techniques. All the obtained very similar derivatives revealed outstanding close activities against three tested tumor cell lines at low micromolar concentration.
Declaration of interest
This work was supported by a grant from the Graduate Studies and Scientific Research Agency (Grant no. 4.H.33), Salman Bin Abdulaziz University, Alkharj, Saudi Arabia. Thanks are extended to Prof. Dr. Sherif Y. S. Department of Cancer Research, National Cancer Institute, Research Center, Cairo, Egypt, for carrying out the anticancer activities of the tested compounds.
References
- Clark DD, Peterson BR. Analysis of protein tyrosine kinase inhibitors in recombinant yeast lacking the ERG6 gene. Chem Bio Chem 2003;4:101–7
- Lombardo LJ, Camuso A, Clark J, et al. Design, synthesis, and structure-activity relationships of tetrahydroquinoline-based farnesyltransferase inhibitors. Bioorg Med Chem Lett 2005;15:1895–9
- Bendale P, Olepu S, Suryadevara PK, et al. Second generation tetrahydroquinoline-based protein farnesyltransferase inhibitors as antimalarials. J Med Chem 2007;50:4585–605
- Muñoz A, Sojo F. Cytotoxic effects of new trans-2,4-diaryl-r-3-methyl-1,2,3,4-tetrahydroquinolines and their interaction with antitumoral drugs gemcitabine and paclitaxel on cellular lines of human breast cancer. Chem Biol Interact 2011;189:215–21
- Jiang C, Yang L, Wu WT, et al. De novo design, synthesis and biological evaluation of 1,4-dihydroquinolin-4-ones and 1,2,3,4-tetrahydroquinazolin-4-ones as potent kinesin spindle protein (KSP) inhibitors. Bioorg Med Chem 2011;19:5612–27
- Chou LC, Tsai MT, Hsu MH, et al. Design, synthesis, and preclinical evaluation of new 5,6- (or 6,7-) disubstituted-2-(fluorophenyl)quinolin-4-one derivatives as potent antitumor agents. J Med Chem 2010;53:8047–58
- Semenza G. HIF-1 and human disease: one highly involved factor. Genes Dev 2000;14:1983–91
- Pacchiano F, Carta F, McDonald PC, et al. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem 2011;54:1896–902
- Takacova M, Holotnakova T, Barathova M, et al. Src induces expression of carbonic anhydrase IX via hypoxia-inducible factor 1. Oncol Rep 2010;23:869–74
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Dayan F, Roux D, Brahimi-Horn M, et al. The oxygen sensor factor-inhibiting hypoxia-inducible factor-1 controls expression of distinct genes through the bifunctional transcriptional character of hypoxia-inducible factor-1alpha. Cancer Res 2006;66:3688–98
- Svastová E, Hulíková A, Rafajová M, et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett 2004;577:439–45
- Chiche J, Ilc K, Laferrière J, et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 2009;69:358–68
- Cecchi A, Hulikova A, Pastorek J, et al. Carbonic anhydrase inhibitors. Design of fluorescent sulfonamides as probes of tumor-associated carbonic anhydrase IX that inhibit isozyme IX-mediated acidification of hypoxic tumors. J Med Chem 2005;48:4834–41
- Thiry A, Dogné JM, Masereel B, et al. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol Sci 2006;27:566–73
- Winum JY, Rami M, Scozzafava A, et al. Carbonic anhydrase IX: a new druggable target for the design of antitumor agents. Med Res Rev 2008;28:445–63
- Maresca A, Temperini C, Vu H, Pham NB. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J Am Chem Soc 2009;131:3057–62
- Maresca A, Temperini C, Pochet L, et al. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem 2010;53:335–44
- Parkkila S, Innocenti A, Kallio H, et al. The protein tyrosine kinase inhibitors imatinib and nilotinib strongly inhibit several mammalian alpha-carbonic anhydrase isoforms. Bioorg Med Chem Lett 2009;19:4102–6
- Dubois L, Lieuwes NG, Maresca A, et al. Imaging of CA IX with fluorescent labelled sulfonamides distinguishes hypoxic and (re)-oxygenated cells in a xenograft tumour model. Radiother Oncol 2009;92:423–8
- Gangjee J, Shi SF, Queener RL. Synthesis and biological activities of conformationally restricted, tricyclic nonclassical antifolates as inhibitors of dihydrofolate reductases. J Med Chem 1997;40:1930–6
- Alqasoumi SI, Al-Taweel AM, Alafeefy AM, et al. Discovering some novel tetrahydroquinoline derivatives bearing the biologically active sulfonamide moiety as a new class of antitumor agents. Eur J Med Chem 2010;45:1849–53
- Monks D, Scudiero P, Skehan P, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 1991;83:757–66
- Barenbrock SJ, Matthias K. Screening enzyme-inhibitory activity in several ascidian species from Orkney Islands using protein tyrosine kinase (PTK) bioassay-guided fractionation. J Biotechnol 2005;117:225–32