Abstract
A new affinity gel was synthesized for the purification of carbonic anhydrase (CA, EC 4.2.1.1) isozymes from erythrocytes. The gel was prepared on a Sepharose 4B matrix on which a spacer arm based on ethylenediamine was covalently attached via CNBr activation, followed by reaction with the CA inhibitor 4-isothiocyanato-benzenesulfonamide. The derivatized gel incorporated thioureido-benzenesulfonamide moieties as CA ligand. The binding capacity of the new affinity gel was determined at different temperatures, pH values, ionic strengths and elution buffers. The maximum binding of various CAs was achieved at 25 °C with pH 8.5 and ionic strength around 0.4. The overall purifications for human (h) hCA I and hCA II were 672- and 580-fold, and with 62 and 43% yields, respectively. SDS–polyacrylamide gel electrophoresis showed single bands for each purified isozymes, corresponding to a molecular weight of approx. 29 kDa. This is an easily obtainable, efficient and robust affinity gel, useful for the purification of many other α-CAs.
Introduction
Carbonic anhydrases (CA, EC 4.2.1.1) are a family of metalloenzymes that catalyzes the reversible hydration of carbon dioxide to form bicarbonate with generation of protonsCitation1. The widespread abundance of several CA isoforms in plants, animals and microorganisms suggests that this simple conversion of a membrane-permeable gas into a membrane-impermeable ionic product is vital to many important biological functionsCitation2. Sixteen CA isozymes have been described up to now in mammals, named CA I through CA XV, with CA II and CA IX being the most active catalysts of carbon dioxide hydrationCitation1,Citation2. Some of these isozymes are cytosolic (CA I, CA II, CA III, CA VII and CA XIII), others are membrane bound (CA IV, CA IX, CA XII and CA XIV), two are mitochondrial (CA VA and CA VB) and one is secreted in saliva (CA VI)Citation3. These isozymes are involved in the pH homeostasis, ion transport, water and electrolyte balance, bone resorption, calcification and tumorigenesisCitation4,Citation5. CA isozymes are associated to many diseases such as glaucoma, obesity, cancer, epilepsy and osteoporosisCitation6,Citation7. CA I is the most abundant isozymes with a relatively low enzyme activity. CA II is present in lower amounts, but it is referred to as the “high activity” isoenzyme because of its higher specific activity compared to CA ICitation8. CA I is sensitive to sulfonamide inhibitors but CA II has even higher affinity for this class of inhibitorsCitation9–11. Over the years, CA has been purified from a number of sources. One of the cheapest and most readily available sources of CA is mammalian bloodCitation12,Citation13. There are several CA purification procedures known in the literatureCitation9,Citation12,Citation14,Citation15. However, the most commonly used purification method is the affinity chromatography, which gives high yields of the enzyme from a wide variety of sourcesCitation16–19.
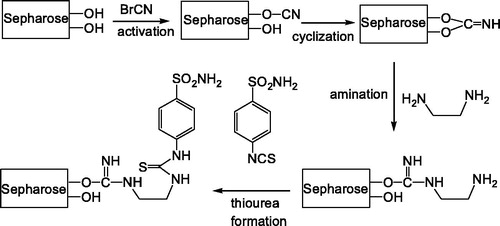
Affinity chromatography takes advantage of the purification of many proteins by using specific ligands or chemical groups with affinity for the protein to be purified. Aromatic and heteroaromatic sulfonamides are frequently used as ligands in these methods, as they are specific and strong inhibitors of many CAsCitation19–21. A large number of affinity gels, using a variety of matrices, spacer arms and ligands, have been described in the literatureCitation19–25. The present report describes the successful purification of CA from erythrocytes using a novel affinity gel. The affinity gel was prepared on the Sepharose 4B matrix with a spacer arm incorporating ethylenediamine, to which 4-isothiocyanato-benzenesulfonamide was covalently attached, leading to the corresponding thioureido-benzenesulfonamide, as a new ligand for affinity gel purification of CAs.
Materials and methods
Materials
All chemicals were of analytical grade and obtained from Sigma-Aldrich (Taufkirchen, Germany) and Merck (Darmstadt, Germany). Human blood samples were obtained from the Balikesir University Faculty of Medicine.
Methods
Preparation of affinity gel
A total of 4 g CNBr was added to 20 mL Sepharose 4B and with 4 M NaOH the mixture’s pH was kept at 11. Mixture was transferred to a Buchner funnel and washed with 250 mL cold 0.1 M NaHCO3 (pH: 10) buffer solution and was transferred to a beaker in the same buffer. Five millilitres of ethylene diamine was added to the suspension and the mixture was stirred with magnetic stirrer for 2 h. Then the suspension was kept at 4 °C for 16 h. At the end of this process, the gel was washed with water. Washing was repeated with 100 mL of 0.1 M NaHCO3 (pH: 10) buffer. Ethylenediamine attached gel was taken up in 40 mL of the same buffer. 50 mg of 4-isothiocyanato-benzenesulfonamide were dissolved in 5 mL DMSO and added to the suspension. The mixture was stirred with magnetic stirrer for 3 h. The suspension is then transferred to the Buchner funnel and washed with 1 L distilled water and 200 mL of 0.05 M Tris-SO4 (pH: 7.5) buffer and stored in the same buffer.
Purification of carbonic anhydrase from human erythrocytes
Human blood samples were obtained from the Balikesir University Faculty of Medicine, using bottles containing anticoagulant acid-citrate-dextrose (ACD). The blood samples were centrifuged at 5000 rpm for 20 min and the plasma and buffy coat were removed. After washing the packed red cells two times with NaCl (0.9%), the erythrocytes were hemolyzed with cold water. The ghosts and intact cells were removed by centrifugation at 15 000 rpm for 40 min at 4 °C. The hemolysate was applied to the Sepharose 4B-ethylene diamine-4-thioureido-benzenesulfonamide affinity column equilibrated with 25 mM Tris/0.1 M Na2SO4 (pH: 8.5) buffer. The affinity gel was washed with 25 mM Tris/22 mM Na2SO4 (pH 8.5) buffer, and CA isozymes were eluted under different elution conditions.
Protein determination
After elution step enzyme was determined spectrophotometrically at 280 nm and protein during the purification steps was determined spectrophotometrically at 595 nm according to the Bradford method, using bovine serum albumin as the standardCitation26.
Enzyme assay
Carbonic anhydrase activity was measured by the Maren methodCitation27, which is based on the determination of the time required for solution pH to decrease from 10.0 to 7.4 due to hydration of CO2.
SDS polyacrylamide gel electrophoresis
SDS polyacrylamide gel electrophoresis was performed after purification of the enzymes. It was carried out in 10 and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to the Laemmli procedure. A 10 µL sample was applied to the electrophoresis medium. Gels were stained for 1.5 h in 0.1% Coommassie Brilliant Blue R-250 in 50% methanol and 10% acetic acid, then destained with several changes of the same solvent without the dyeCitation28.
Results and discussion
In this study, a new affinity gel was prepared for the purification of CA isozymes from a variety of sources. Sepharose 4B was selected as a matrix due to its long operational life, stability to mechanical stress and possession of favorable flow rates. These features are particularly important during routine purifications for large scale production of proteins. Several analogs of sulfonamides have been demonstrated to possess good binding affinities for CAs and they are also frequently used on affinity gels for the purification of these proteinsCitation29.
In the literature there are several types of methods that have been used with different matrices and different spacer arms that undergo activation methods. It is known that affinity gels prepared with these activation methods can undergo partial deterioration in the course of the reactionCitation19,Citation25,Citation30. Affinity gel was prepared with Sepharose 4B, ethylenediamine and 4-isothiocyanato-benzenesulfonamide (Scheme 1). Ethylenediamine was covalently attached to the matrix after activation of sepharose with cyanogen bromide (Scheme 1). This leads initially to the formation of an iminocarbonate which is easily derivatized with primary amines in mild conditions, leading to the aminoethyl-arm derivatized sepharose. This intermediate was reacted with 4-isothiocyanato-benzenesulfonamide leading to the thioureido-benzenesulfonamide derivatized gel (Scheme 1).
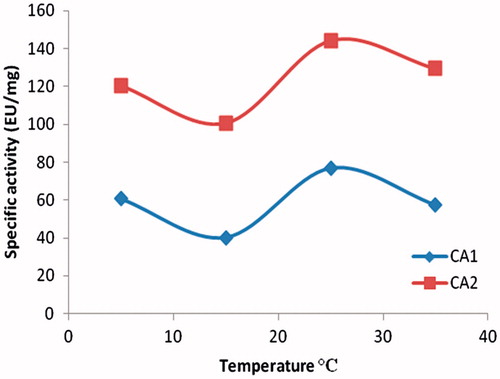
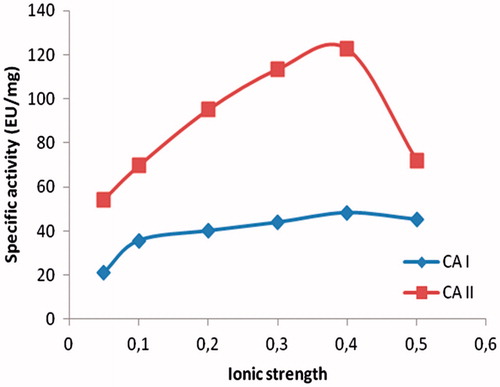
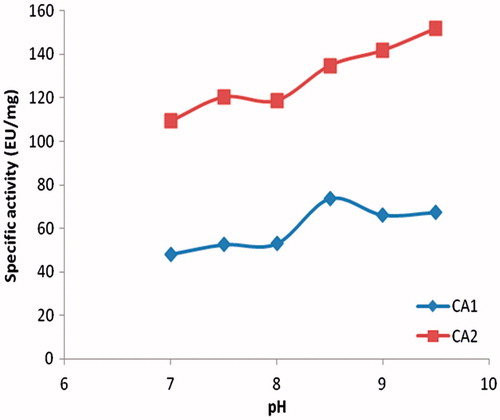
The hemolysate was applied to the Sepharose 4B-ethylene diamine-4-thioureido-benzenesulfonamide affinity column equilibrated with 25 mM Tris/0.1 M Na2SO4 (pH: 8.5) buffer. The affinity gel was washed with 25 mM Tris/22 mM Na2SO4 (pH: 8.5) buffer, and CA isozymes were eluted under different elution conditions. The eluates were characterized by protein determination at 280 nm and assaying CO2 hydratase activity for hCA I and hCA II. Specific activities for hCA I and hCA II were calculated by using hemolysate and purified enzyme solution. As a result, hCA I and hCA II were purified up to 672.72- and 580.68-fold with a recovery ratio of 62 and 43% compared to homogenate, respectively (). These values are better than some of the reported affinity gels and equivalent to othersCitation19–25. These values of the absorbance showed that some proteins, bound to the affinity material, have been removed from the column by the washing solutions. At the end of the last step, highly purified enzymes were obtained exhibiting a single band on SDS-PAGE. The human isozymes migrated as single bands in all cases, with apparently identical molecular masses (). We used only one step for purification, Sepharose 4B-ethylene diamine-4-thioureido-benzenesulfonamide affinity chromatography by modification of elution conditions. hCA I and hCA II were purified using the affinity gel with different elution buffers (). The most suitable elution buffers were 1 M NaCl/50 mM Na2HPO4 (pH: 6.3) for hCA I and 0.1 M CH3COONa/0.5 M NaClO4 (pH: 5.6) for hCA II (). The binding capacities of the affinity gel for the hCA I and hCA II isozymes were determined at different temperatures () and ionic strengths (). For this aim, buffers were prepared with different compounds at different concentrations for elution. For elution of hCA I and hCA II the buffers pH were 6.3 and 5.6, respectively. The binding capacities of the affinity gel for the hCA I and hCA II isozymes were determined at different temperatures in the range from 5 °C to 35 °C. For determination of optimum ionic strength, enzyme activity was determined using different concentrations of NaSO4, in the range from 0.1 to 0.5. The enzyme was seen to exhibit the highest activity at 25 °C and maximum binding was achieved at ionic strength around 0.4. In order to determine the optimum pH for equilibration and washing buffers, 25 mM Tris/0.1 M Na2SO4 and 25 mM Tris/22 mM Na2SO4 buffers were used in the pH range of 7.0–9.5 (). Maximum binding was determined for hCA I and hCA II at pH: 8.5 and 9.5, respectively. These results showed differences with other studies in the literatureCitation19–25,Citation30.
Figure 1. SDS-PAGE pattern. Molecular mass standards: β-galactosidase (116 kDa), bovine serum albumin (66.2 kDa), egg albumin (45.0 kDa), lactate dehydrogenase (35 kDa), REase Bsp981 (Escherichia coli) (25 kDa).
Figure 2. Effect of different buffers for elution of hCA I and hCA II. 1. 50 mM Na2HPO4/1 M NaCl – 50 mM Na2HPO4/0.2 M KSCN; 2. 0.1 M KI/0.1 M Tris-SO4 – 50 mM Na2HPO4/0.2 M KSCN; 3. 0.1 M KI/0.1 M Tris-SO4 – 0.1 M NaCH3COO/0.5 M NaCIO4; 4. 50 mM Na2HPO4/1 M NaCl – 0.4 M NaN3/0.1 M Tris-SO4; 5. KI/Tris-SO4 – 0.4 M NaN3/0.1 M Tris-SO4; 6. 50 mM Na2HPO4/1 M NaCl – 0.1 M NaCH3COO/0.5 M NaCIO4.
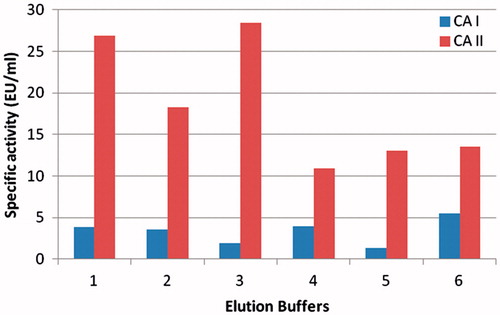
Table 1. Purification scheme of hCAI and hCAII from human erythrocytes by Sepharose 4B-ethylene diamine-4-isothiocyanato-benzenesulfonamide affinity chromatography. Experimental analysis were performed at +4 °C. CA enzyme activity was determined colorimetrically using CO2-hydration method of Maren.
hCA II has generally a higher affinity for sulfonamides than hCA ICitation31. 4-isothiocyanato-benzenesulfonamide exhibited quite effective inhibition to hCA II, but rather different from hCA I. Ki value for hCA I and hCA II were 5 µM and 0.185 µM for 4-isothiocyanato-benzenesulfonamide, respectivelyCitation32. Ki values for p-amino benzenesulfonamide were found to be 28 µM for hCA ICitation33 and 10.1 µM for hCA IICitation34. 4-isothiocyanato-benzenesulfonamide was more effective inhibitor than p-amino benzenesulfonamide for hCA I and hCA II. So, we used this compound for a ligand to synthesize the affinity gel. The purification fold and yield of Sepharose 4B-L Tyrosine-p-aminobenzene sulfonamide gel for hCA I was better than hCAIICitation30. However, Sepharose 4B-ethylene diamine-4-thioureido-benzenesulfonamide gel purification fold and yield for hCA II was better than for hCAI. In addition, the binding capacities of the affinity gel for the hCA I and hCAII were better than other studiesCitation19–25,Citation30. Thus, Sepharose 4B-ethylene diamine-4-thioureido-benzenesulfonamide affinity gel is shown to be favourable for the purification of hCA I and hCA II in active form. These results mean that the procedure used in the purification is good enough to be used in further studies and also has an advantage of taking very short experimental period.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This work has been supported by Balikesir University Research Project (2012/16).
References
- Cincinelli A, Martellini T, Innocenti A, et al. Purification and inhibition studies with anions and sulfonamides of an alpha-carbonic anhydrase from the Antarctic seal Leptonychotes weddellii. Bioorg Med Chem 2011;19:1847–51
- Supuran CT. Carbonic anhydrases: an overview. Curr Pharm Des 2008;14:603–14
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Innocenti A, Antel J, Wurl M, et al. Carbonic anhydrase inhibitors. Inhibition of isozymes I, II, IV, V and IX with complex fluorides, chlorides and cyanides. Bioorg Med Chem Lett 2005;15:1909–13
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–50
- Demirdağ R, Çomaklı V, Şentürk M, et al. Purification and characterization of carbonic anhydrase from sheep kidney and effects of sulfonamides on enzyme activity. Bioorg Med Chem 2013;21:1522–5
- Carradori S, De Monte C, D’Ascenzio M, et al. Salen and tetrahydrosalen derivatives act as effective inhibitors of the tumor-associated carbonic anhydrase XII-A new scaffold for designing isoform-selective inhibitors. Bioorg Med Chem Lett 2013;23:6759–63
- Lindskog S. Purification and properties of bovine erythrocyte carbonic anhydrase. Biochim Biophys Acta 1960;39:218–26
- Chandra M, Waheed A, Singh RK. Characterization of functionally active immobilized carbonic anhydrase purified from sheep blood lysates. Process Biochem 2013;48:231–41
- Fiore AD, Simone GD, Menchise V, et al. Carbonic anhydrase inhibitors: X-ray crystal structure of a benzenesulfonamide strong CA II and CA IX inhibitor bearing a pentafluorophenylaminothioureido tail in complex with isozyme II. Bioorg Med Chem Lett 2005;15:1937–42
- Keilin D, Mann T. Carbonic anhydrase. Purification and nature of the enzyme. Biochem J 1940;34:1163–76
- Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 1995;64:375–401
- Rickli EE, Ghazanfar SA, Gibbons BH, Edsall JT. Carbonic anhydrases from human erythrocytes. Preparation and properties of two enzymes. J Biol Chem 1964;239:1065–78
- Armstrong JM, Myers DV, Verpoorte JA, Edsall JT. Purification and properties of human erythrocyte carbonic anhydrases. J Biol Chem 1966;241:5137–49
- Zhu XL, Sly WS. Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney. J Biol Chem 1990;265:8795–801
- Sharma A, Bhattacharya A, Singh S. Purification and characterization of an extra cellular carbonic anhydrase from Pseudomonas fragi. Process Biochem 2009;44:1293–7
- Arslan O, Kufrevioğlu OI. Affinity to some inhibitors of human carbonic anhydrase-I and bovine human carbonic anhydrase. J Environ Sci Health, Part A 1996;31:2017–21
- Ozensoy O, Arslan O, Sinan ÖS. A new method for the purification of carbonic anhydrase isozymes by affinity chromatography. Biochemistry (Moscow) 2004;69:216–19
- Ores JC, Sala L, Cerveira GP, Susana Juliano Kalil. Purification of carbonic anhydrase from bovine erythrocytes and its application in the enzymic capture of carbon dioxide. Chemosphere 2012;88:255–9
- Murakami H, Sly WS. Purification and characterization of human salivary carbonic anhydrase. J Biol Chem 1987;262:1382–8
- Wistrand PJ, Knuuttila KG. Renal membrane-bound carbonic anhydrase. Purification and properties. Kidney Int 1989;35:851–9
- Ceyhun SB, Senturk M, Yerlikaya E, et al. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 2011;32:69–74
- Demirdağ R, Yerlikaya E, Aksakal E, et al. Influence of pesticides on the pH regulatory enzyme, carbonic anhydrase, from European Seabass liver and bovine erythrocytes. Environ Toxicol Pharmacol 2012;34:218–22
- Sentürk M, Gülçin I, Dastan A, et al. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–11
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 1976;72:248–51
- Maren TH. A simplified micromethod for the determination of carbonic anhydrase and its inhibitors. J Pharmacol Exp Ther 1960;130:26–9
- Laemmli DK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970;227:680–3
- Arslan O. Inhibition of bovine carbonic anhydrase by new sulfonamide compounds. Biochemistry (Moscow) 2001;66:982–3
- Arslan O, Nalbantoğlu B, Demir N, et al. A new method for the purification of carbonic anhydrase isozymes by affinity chromatography. Turk J Med Sci 1996;26:163–6
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–89
- Casini A, Scozzafava A, Mincione F, et al. Carbonic anhydrase inhibitors: water-soluble 4-sulfamoylphenylthioureas as topical intraocular pressure-lowering agents with long-lasting effects. J Med Chem 2000;43:4884–92
- Vullo D, Franchi M, Gallori E, et al. Carbonic anhydrase inhibitors: inhibition of the tumor-associated isozyme IX with aromatic and heterocyclic sulfonamides. Bioorg Med Chem Lett 2003;13:1005–9
- Iyer R, Barrese III AA, Parakh S, et al. Inhibition profiling of human carbonic anhydrase II by high-throughput screening of structurally diverse, biologically active compounds. J Biomol Screen 2006;11:782–91