Abstract
Various triheterocyclic compounds containing benzimidazole, thiophene, and 1,2,4-triazole rings (3–6) were synthesized and screened for their antioxidant activities. The structures of the synthesized compounds (2–6) were judged by 1H NMR, 13C NMR, elemental analysis, and LC-MS spectral data. Antioxidant activities of the synthesized compounds (2–6) were determined with CUPric Reducing Antioxidant Capacity (CUPRAC), ABTS (2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)/persulfate, and DPPH (1,1-diphenyl-2-picrylhydrazyl) assays. Most of the compounds showed a significant antioxidant activity and especially, compound 5c showed very good SC50 value for DPPH method and compound 5h exhibited very high scavenging activity to ABTS method.
Introduction
Free radicals are mainly generated by metabolic processes in the human body, and some radicals have essential roles in normal cell processes such as neural signal transductionCitation1. On the other hand, excessive free radical incursion, causing very important diseases, can damage all components of the cell including DNA, proteins, and lipidsCitation2. Antioxidants are chemicals that can react with free radicals and terminate their chain reactions, by which damage to essential biomolecules are prevented. As oxidative stress plays an important role in many diseases, such as cancer, Parkinson, Alzheimer, heart failure, and stroke, the use of antioxidants is intensively studied in medicinal chemistry, particularly as a means for the treatment of these widespread diseasesCitation3,Citation4.
Benzimidazole and its derivatives are well known in medicinal chemistry due to their diverse biological properties like antibacterial, antifungal, antitubercular, anti-inflammatory, anticancer, antihypertensive, antiviral, antidiabetic, anticoagulants, and antioxidant activitiesCitation5–8. In addition to these, various compounds containing benzimidazole ring are well known as drugs such as Albendazole, Mebendazole, Thiabendazole (antihelmintic drugs)Citation4, Omeprazole, Pantoprazole, Lansoprazole (antiulcerative drugs)Citation4, Domperidone (antidopaminergic drug)Citation7, Pimozide (antipsychotic drug)Citation8, and Rifaximin (anticancer drug)Citation9. Moreover, heterocyclic compounds including thiophene ring has been incorporated into a wide variety of therapeutically important agents due to their biological activitiesCitation10–13. Various compounds containing the thiophene ring are well known as drugs such as Ticlopidine (antiplatelet), Raltitrexed (antitumoral), Tenoxicam (anti-inflammatory), Cefalotin, Ticarcillin, Cefoxitin (antibacterial), Tioconazole, Sertaconazole (antifungal), Olanzapine, Tiagabine, Clotiazepam (action on the central nervous system), and Dorzolamide (diuretics)Citation14. On the other hand, benzimidazoles bearing open-chain thiosemicarbazide and mercapto-1,2,4-triazole moities are also more potent antioxidant compoundsCitation15. In the view of the above observations, it was thought to be interesting to synthesize and screen some new trisubstituted benzimidazole derivatives for antioxidant activities.
Experimental
Chemistry
All the chemicals were supplied from Merck (Darmstadt, Germany), Aldrich and Fluka (Buchs SG, Switzerland). The melting points were determined on capillary tubes on a Büchi oil heated melting point apparatus (Essen, Germany) and uncorrected. 1H NMR and 13C NMR spectra were performed on Varian-Mercury 400 MHz spectrophotometer (Varian, Darmstadt, Germany) in DMSO-d6 using TMS as internal. Splitting patterns were designated as follows: s: singlet; d: doublet; m: multiplet. The elemental compositions were determined on a Carlo Erba 1106 CHN analyzer (Heraeus, Hanau, Germany); the experimental values were in agreement (±0.4%) with calculated ones. The IR spectra were recorded on a Perkin-Elmer 100 FT-IR spectrophotometer as KBr pellets. The mass spectra was recorded on Thermo Scientific Quantum Access max LC-MS spectrophotometer (San Jose, CA). All reactions were monitored by TLC using precoated aluminum sheets (silica gel 60 F 2.54 0.2 mm thickness).
Synthesis of 2-(2-thiophen-2-ylmethyl)-1H-benzimidazole (2)
Compound 1 (0.013 mol) was added to the solution of o-phenylenediamine (0.01 mol) in methanol (40 mL). Then, the mixture was stirred for 1 h at room temperature. Then, it was refluxed for 2 h. The end of the reaction was monitored by TLC (ethyl acetate/hexane = 3:1), and then the mixture was cooled to room temperature. The product was precipitated by addition of water. It was filtrated, dried, and recrystallized from ethanol.
Yield: 91%; m.p. 180–181 °C (182–183 °CCitation16); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 4.37 (s, 2H, CH2), 6.95–6.99 (m, 2H, Ar–H), 7.10–7.12 (m, 2H, Ar–H), 7.37 (d, 1H, J = 4.4, Ar–H), 7.47 (m, 2H, Ar–H), 12.30 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 29.77 (CH2), 121.88 (2C), 125.44, 126.60 (2C), 127.39 (2C), 139.85 (3C) (Ar–C), 153.17 (C=N); Anal Calcd for C12H10N2S: C, 67.26; H, 4.70; N, 13.07. Found: C, 67.22; H, 4.67; N,13.03. LC-MS: 215.31 [M + 1].
Synthesis of methyl [2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl] acetate (3)
To a solution of compound 2 (0.01 mol) in acetone (30 mL), dry K2CO3 (0.025 mol) was added and stirred for 15 min at room temperature. Then, methyl bromoacetate (0.011 mol) was added. The mixture was stirred for 10 h. After the reaction was completed (monitored by TLC, ethyl acetate:hexane = 3:1), the product was precipitated by addition of water. It was filtrated, dried, and recrystallized from ethanol:water (1:1).
Yield: 90%; m.p. 166–167 °C; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 3.56 (s, 3H, OCH3), 4.48 (s, 2H, CH2), 5.21 (s, 2H, NCH2), 6.92–6.97 (m, 2H, Ar–H), 7.17–7.20 (m, 2H, Ar–H), 7.37 (d, J = 6.0, 1H, Ar–H), 7.42 (d, J = 9.2, 1H, Ar–H), 7.58 (d, J = 9.2, 1H, Ar–H); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 28.02 (CH2), 44.86 (CH2), 52.69 (OCH3), 110.63, 119.17, 122.18, 122.61, 125.82, 126.88, 127.12, 136.09, 138.85, 142.46 (Ar–C), 153.44 (C=N), 167.70 (C=O); Anal Calcd for C15H14N2O2S: C, 62.92; H, 4.93; N, 9.78. Found: C, 62.89; H, 4.90; N, 9.73. LC-MS: 287.13 [M + 1].
Synthesis of 2-[2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl]acetohydrazide (4)
Hydrazine monohydrate (0.025 mol) was added to the solution of compound 3 (0.01 mol) in ethanol (25 mL). Then, it was refluxed for 6 hours. The end of the reaction was monitored by TLC (ethyl acetate:hexane = 4:1). After cooling the mixture to room temperature, a white solid was appeared. This crude product was filtrated, dried, and recrystallized from ethanol.
Yield: 84%; m.p. 202–204 °C; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 4.37 (s, 2H, CH2), 4.49 (s, 2H, NH2), 4.82 (s, 2H, NCH2), 6.95–7.00 (m, 2H, Ar–H), 7.14–7.21 (m, 2H, Ar–H), 7.35–7.43 (m, 2H, Ar–H), 7.56 (d, J = 8.4, 1H, Ar–H), 9.52 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 28.15 (CH2), 44.92 (NCH2), 110.50, 119.13, 122.02, 122.41, 125.66, 126.82, 127.23, 136.01, 139.02, 142.53 (Ar–C), 153.76 (C=N), 166.33 (C=O); Anal Calcd for C14H14N4OS: C, 58.72; H, 4.93; N, 19.57. Found: C, 58.76; H, 4.98; N, 19.55. LC-MS: 287.20 [M+1].
Synthesis of compounds 5a–h
A mixture of compound 4 (0.01 mol) and corresponding isothiocyanate (0.01 mol) in ethanol (15 mL) was refluxed for 3 h. The end of the reaction was monitored by TLC (ethyl acetate:hexane = 3:1). Then, the mixture was cooled to room temperature and the product was observed by addition of water. It was filtrated off, dried, and recrystallized from ethanol–water (3:1).
N-methyl-2-{[2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl] acetyl} hydrazine carbothioamide (5a). Yield: 89%; m.p. 216–217 °C; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 2.90 (s, 3H, CH3), 4.40 (s, 2H, CH2), 4.94 (s, 2H, NCH2), 6.93–6.98 (m, 2H, Ar–H), 7.14–7.22 (m, 2H, Ar–H), 7.34–7.44 (m, 2H, Ar–H), 7.57 (d, J = 7.6, 1H, Ar–H), 8.06 (s, 1H, NH), 9.31 (s, 1H, NH), 10.25 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 28.13 (CH2), 31.39 (CH3), 44.88 (NCH2), 110.62, 119.15, 121.95, 122.45, 125.67, 126.77, 127.74, 136.05, 138.93, 142.50 (Ar–C), 153.87 (C=N), 163.45 (C=O), 170.19 (C=S); Anal Calcd for C14H14N4OS: C, 53.46; H, 4.77 N, 19.48. Found: C, 53.42; H, 4.71 N, 19.40. LC-MS: 360.27 [M + 1].
N-ethyl-2-{[2-(thiophen-2-yl-methyl)-1H-benzimidazol-1-yl] acetyl} hydrazine carbothioamide (5b). Yield: 82%; m.p. 221–222 °C; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 1.05 (t, J = 6.0, 3H, CH3), 3.48 (q, J = 6.0, 2H, CH2), 4.45 (s, 2H, CH2), 4.95 (s, 2H, NCH2), 6.92–6.98 (m, 2H, Ar–H), 7.14–7.21 (m, 2H, Ar–H), 7.34–7.96 (m, 2H, Ar–H), 7.57 (d, J = 7.2, 1H, Ar–H), 8.04 (s, 1H, NH), 9.22 (s, 1H, NH), 10.24 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 14.81 (CH3), 28.09, 38.96 (CH2), 44.88 (NCH2), 110.68, 119.07, 122.16, 122.50, 125.69, 126.83, 127.22, 136.00, 138.84, 142.30 (Ar–C), 153.68 (C=N), 163.86 (C=O), 169.49 (C=S); Anal Calcd for C16H17N5OS2: C, 54.67; H, 5.13; N, 18.75. Found: C, 54.65; H, 5.11; N, 18.73. LC-MS: 374.18 [M + 1].
N-phenyl-2-{[2-(thiophen-2-yl-methyl)-1H-benzimidazol-1-yl] acetyl} hydrazine carbothioamide (5c). Yield: 92%; m.p. 193–195 °C; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 4.49 (s, 2H, CH2), 5.77 (s, 2H, NCH2), 6.95–7.00 (s, 2H, Ar–H), 7.16–7.23 (s, 3H, Ar–H), 7.33–7.51 (s, 6H, Ar–H), 7.59 (d, J = 6.8, 1H, Ar–H), 9.69 (s, 2H, NH), 10.52 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 28.15 (CH2), 45.92 (NCH2), 110.68, 119.13, 119.36, 122.14, 122.48, 125.69, 126.82 (2C), 127.29 (2C), 128.72, 129.47, 136.06, 138.91, 139.90, 142.45 (Ar–C), 156.82 (C=N), 164.28 (C=O), 168.46 (C=S); Anal Calcd for C21H19N5OS2: C, 59.83; H, 4.54; N, 16.61. Found: C, 59.80; H, 4.51; N, 16.56. LC-MS: 422.22 [M + 1].
N-(4-Fluorobenzyl)-2-{[2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl] acetyl} hydrazine carbothioamide (5d). Yield: 87%; m.p. 199–200 °C; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 4.58 (s, 2H, CH2), 5.05 (s, 2H, NCH2), 6.88–7.62 (m, 11H, Ar–H), 9.73 (s, 2H, NH), 10.42 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 28.13 (CH2), 45.86 (NCH2), 110.67, 115.94, 116.18, 119.12, 119.36, 122.13, 122.49, 125.68, 126.97, 129.19, 131.49, 132.89, 134.19, 139.83, 140.50, 144.21 (Ar–C), 155.48 (C=N), 164.61 (C=O), 169.39 (C=S); Anal Calcd For C21H18FN5OS2: C, 57.39; H, 4.13; N, 15.93. Found: C, 57.41; H, 4.10; N, 15.87. LC-MS: 440.27 [M + 1].
N-(4-bromobenzyl)-2-{[2-(thiophen-2-yl-methyl)-1H-benzimidazol-1-yl] acetyl} hydrazine carbothioamide (5e). Yield: 87%; m.p. 195–196 °C; NMR (DMSO-d6, 400 MHz) δ (ppm): 4.57 (s, 2H, CH2), 5.00 (s, 2H, NCH2), 6.95–7.60 (m, 11H, Ar–H), 9.79 (s, 2H, NH), 10.56 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 28.16 (CH2), 45.02 (NCH2), 110.66, 119.14, 122.12, 122.48, 125.68, 126.78, 126.81, 126.86, 127.15, 127.28, 131.55, 132.23, 135.26, 138.32, 141.27, 143.81 (Ar–C), 153.70 (C=N), 165.68 (C=O), 171.19 (C=S); Anal Calcd for C21H18BrN5OS2: C, 50.40; H, 3.63; N, 13.99. Found: C, 50.36; H, 3.58; N, 13.89. LC-MS: 502.23, 500.13 [M + 1].
N-(3-iodobenzyl)-2-{[2-(thiophen-2-yl-methyl)-1H-benzimidazol-1-yl] acetyl} hydrazine carbothioamide (5f). Yield: 86%; m.p. 197 °C; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 4.47 (s, 2H, CH2), 5.00 (s, 2H, NCH2), 6.95–7.58 (m, 11H, Ar–H), 9.83 (s, 2H, NH), 10.53 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 28.15 (CH2), 44.92 (NCH2), 95.17 (C–I), 110.66, 119.16, 122.11, 122.47, 125.68, 126.71, 126.80 (2C), 127.20, 127.28, 130.62, 136.06, 138.92, 140.87, 142.51 (Ar–C), 153.71 (C=N), 164.70 (C=O), 171.09 (C=S); Anal Calcd for C21H18IN5OS2: C, 46.07; H, 3.31; N, 12.79. Found: C, 46.01; H, 3.25; N, 12.71. LC-MS: 548.22 [M + 1].
N-(4-methylbenzyl)-2-{[2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl] acetyl} hydrazine carbothioamide (5g). Yield: 82%; m.p. 202 °C; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 2.27 (s, 3H, CH3), 4.48 (s, 2H, CH2), 5.00 (s, 2H, NCH2), 6.95–7.60 (m, 11H, Ar–H), 9.62 (s, 2H, NH), 10.48 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 21.01 (CH3), 28.16 (CH2), 44.59 (NCH2), 110.66, 117.45, 119.14, 122.10, 122.46, 125.67, 126.76 (2C), 126.81 (2C), 127.17, 129.17, 129.84, 136.79, 138.92, 142.49 (Ar–C), 153.73 (C=N), 165.86 (C=O), 169.46 (C=S); Anal Calcd for C22H21N5OS2: C, 60.66; H, 4.86; N, 16.08. Found: C, 60.62; H, 4.84; N, 16.05. LC-MS: 436.23 [M + 1].
N-(4-nitrobenzyl)-2-{[2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl] acetyl} hydrazine carbothioamide (5h). Yield: 78%; m.p. 210–211 °C; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 4.51 (s, 2H, CH2), 5.10 (s, 2H, NCH2), 6.91–7.07 (m, 2H, Ar–H), 7.16–7.24 (m, 2H, Ar–H), 7.40 (d, J = 7.6, 1H, Ar–H), 7.59 (d, J = 8.0, 2H, Ar–H), 7.88 (d, J = 8.0, 2H, Ar–H), 8.22 (d, J = 8.0, 2H, Ar–H), 10.13 (s, 2H, NH), 10.75 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 28.15 (CH2), 44.95 (NCH2), 110.69, 119.11 (2C), 122.20, 122.56, 124.17 (2C), 125.73, 126.86 (2C), 127.29 (2C), 136.07, 138.82 (2C), 142.34 (Ar–C), 153.69 (C=N), 165.79 (C=O), 170.39 (C=S); Anal Calcd for C21H18N6O3S2: C, 54.06; H, 3.89; N, 18.01. Found: C, 54.03; H, 3.85; N, 17.96. LC-MS: 467.23 [M + 1].
Synthesis of compounds 6a–g
To a solution of compounds 5a–g (0.01 mol) in ethanol (5 mL), 2N NaOH (10 mL) was added. Then, the mixture was refluxed for 4 h. The end of the reaction was monitored by TLC (ethyl acetate/hexane = 3:1). Then, the resulting solution was cooled to room temperature and acidified to pH 5–6 with 37% HCl. The precipitated product was filtrated off, washed with water, and recrystallized from ethanol.
Synthesis of compound 6h
To a solution of compound 5h (0.01 mol) in ethanol (5 mL), 2N NaHCO3 (10 mL) was added. Then, the mixture was refluxed for 12 h. After the completion of the reaction (TLC), the above purification methods were applied to give pure product 5h.
4-Methyl-5-{[2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl]methyl}-4H-1,2,4-triazole-3-thione (6a). Yield: 82%; m.p. 248–250 °C; IR (cm−1, KBr): 2567 (SH), 1616, 1574 (C=N); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 4.49 (s, 2H, CH2), 5.64 (s, 2H, NCH2), 6.88–6.96 (m, 2H, Ar–H), 7.17 (d, J = 7.2, 2H, Ar–H), 7.33 (d, J = 4.4, 1H, Ar–H), 7.50 (d, J = 7.4, 1H, Ar–H), 7.60 (d, J = 4.4, 1H, Ar–H), 13.52 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 27.92 (CH2), 30.35 (CH3), 39.17 (NCH2), 110.84, 119.26, 122.82, 122.68, 125.66, 126.75, 127.09, 135.88, 138.96, 142.56 (Ar–C), 148.74, 153.66 (C=N), 167.96 (C=S); Anal Calcd for C16H15N5S2: C, 56.28; H, 4.43; N, 20.51. Found: C, 56.24; H, 4.40; N, 20.46. LC-MS: 342.29 [M + 1].
4-Ethyl-5-{[2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl]methyl}-4H-1,2,4-triazole-3-thione (6b). Yield: 77%; m.p. 256–258 °C; IR (cm−1, KBr): 2536 (SH), 1632, 1581 (C=N); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 1.04 (t, 3H, CH3), 3.97 (q, 2H, CH2), 4.51 (s, 2H, CH2), 5.69 (s, 2H, NCH2), 6.89 (t, J = 4.0, 1H, Ar–H), 7.16–7.22 (m, 3H, Ar–H), 7.34 (d, J = 5.2, 1H, Ar–H), 7.48 (d, J = 7.2, 1H, Ar–H), 7.60 (d, J = 7.2, 1H, Ar–H), 13.57 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 13.45 (CH3), 28.01 (CH2), 39.01 (NCH2), 110.78, 119.29, 122.36, 122.80, 125.74, 126.79, 127.08, 135.79, 138.77, 142.46 (Ar–C), 148.08, 153.55 (C=N), 167.48 (C=S); Anal Calcd for C17H17N5S2: C, 57.44; H, 4.82; N, 19.70. Found: C, 57.40; H, 4.77; N, 19.64. LC-MS: 356.48 [M + 1].
4-Phenyl-5-{[2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl]methyl}-4H-1,2,4-triazole-3-thione (6c). Yield: 87%; m.p. 172–173 °C; IR (cm−1, KBr): 3137 (NH), 1645, 1563 (C=N), 1321 (C=S); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 4.28 (s, 2H, CH2), 5.36 (s, 2H, NCH2), 6.84–7.60 (m, 12H, Ar-H), 13.85 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 27.88 (CH2), 38.30 (NCH2), 110.73, 115.94, 116.17, 117.00, 118.98, 119.06, 119.35, 122.22, 122.57, 122.98, 125.78, 127.12, 130.92, 135.66, 138.62, 142.50 (Ar–C), 148.04, 153.25 (C=N), 169.27 (C=S); Anal Calcd for C21H17N5S2: C, 62.51; H, 4.25; N, 17.36. Found: C, 62.44; H, 4.20; N, 17.31. LC-MS: 404.69 [M + 1].
4-(4-Fluorobenzyl)-5-{[2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl]methyl}-4H-1,2,4-triazole-3-thione (6d). Yield: 80%; m.p. 254–255 °C; IR (cm−1, KBr): 3119 (NH), 1618, 1583 (C=N), 1316 (C=S); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 4.18 (s, 2H, CH2), 5.35 (s, 2H, NCH2), 6.81 (d, J = 6.4, 1H, Ar–H), 6.89 (d, J = 6.4, 1H, Ar–H), 7.12–7.16 (m, 2H, Ar–H), 7.30–7.35 (m, 3H, Ar–H), 7.49–7.55 (m, 4H, Ar–H), 13.85 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 27.80 (CH2), 39.36 (NCH2), 110.77, 119.17, 122.22, 122.63, 125.75, 126.69, 127.12, 128.48 (2C), 129.98 (2C), 130.26, 133.37, 136.66, 138.65, 142.37 (Ar–C), 147.95, 153.08 (C=N), 169.08 (C=S); Anal Calcd for C21H16FN5S2: C, 59.84; H, 3.83; N, 16.61. Found: C, 59.79; H, 3.80; N, 16.55. LC-MS: 404.69 [M + 1].
4-(4-Bromobenzyl)-5-{[2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl]methyl}-4H-1,2,4-triazole-3-thione (6e). Yield: 79%; m.p. 227–228 °C; IR (cm−1, KBr): 3179 (NH), 1628, 1571 (C=N), 1315 (C=S); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 4.40 (s, 2H, CH2), 5.37 (s, 2H, NCH2), 6.83–7.70 (m, 11H, Ar–H), 13.86 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 27.90 (CH2), 39.35 (NCH2), 110.74, 119.14, 122.19, 123.66, 125.77, 126.73, 127.15, 130.66 (2C), 132.22 (2C), 132.62, 132.96, 135.72, 138.64, 142.31 (Ar–C), 147.89, 153.10 (C=N), 168.96 (C=S); Anal Calcd for C21H16BrN5S2: C, 52.28; H, 3.34; N, 14.52. Found: C, 52.21; H, 3.30; N, 14.46. LC-MS: 483.14 [M + 1].
4-(3-Iodobenzyl)-5-{[2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl]methyl}-4H-1,2,4-triazole-3-thione (6f). Yield: 85%; m.p. 250 °C (decomp.); IR (cm−1, KBr): 3098 (NH), 1659, 1586 (C=N), 1317 (C=S); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 4.27 (s, 2H, CH2), 5.38 (s, 2H, NCH2), 6.84–7.81 (m, 11H, Ar–H), 13.88 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 27.91 (CH2), 40.19 (NCH2), 95.27 (C-I), 110.76, 119.12, 122.26, 122.63, 125.82, 126.71, 127.13, 128.05, 131.57, 134.36, 135.61, 136.71, 138.62, 138.93, 142.09 (Ar-C), 147.83, 153.02 (C=N), 169.02 (C=S); Anal Calcd for C21H16IN5S2: C, 47.64; H, 3.05; N, 13.23. Found: C, 47.60; H, 3.00; N, 13.18. LC-MS: 530.19 [M + 1].
4-(4-Methylbenzyl)-5-{[2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl]methyl}-4H-1,2,4-triazole-3-thione (6g). Yield: 79%; m.p. 243–245 °C; IR (cm−1, KBr): 3106 (NH), 1630, 1573 (C=N), 1297 (C=S); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 2.35 (s, 3H, CH3), 4.18 (s, 2H, CH2), 5.33 (s, 2H, NCH2), 6.81–7.54 (s, 11H, Ar-H), 13.81 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 21.24 (CH3), 27.78 (CH2), 40.61 (NCH2), 110.77, 119.15, 122.16, 122.56, 125.75, 126.70, 127.09, 128.18 (2C), 130.44 (2C), 130.76, 135.66, 138.61, 139.99, 142.37 (Ar–C), 148.04, 153.07 (C=N), 169.13 (C=S); Anal Calcd for C22H19N5S2: C, 63.28; H, 4.59; N, 16.77. Found: C, 63.23; H, 4.52; N, 16.74. LC-MS: 418.41 [M + 1].
4-(4-Nitrobenzyl)-5-{[2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl]methyl}-4H-1,2,4-triazole-3-thione (6h). Yield: 69%; m.p. 253 °C (decomp.); IR (cm−1, KBr): 3159 (NH), 1627, 1598 (C=N), 1326 (C=S); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 4.35 (s, 2H, CH2), 5.47 (s, 2H, NCH2), 6.84–7.68 (s, 11H, Ar–H), 14.02 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 27.97 (CH2), 39.43 (NCH2), 110.80, 119.09, 122.16, 122.45, 125.00, 125.78, 126.74, 127.09 (2C), 130.15 (2C), 135.67, 138.63, 138.75, 142.21, 147.79 (Ar–C), 148.13, 153.15 (C=N), 168.12 (C=S); Anal Calcd for C21H16N6O2S2: C, 56.23; H, 3.60; N, 18.74. Found: C, 56.18; H, 3.54; N, 18.68. LC-MS: 449.31 [M + 1].
Antioxidant activity
In this study, the antioxidant activities of the synthesized compounds were determined using CUPric reducing antioxidant capacity assay, radical scavenging activities of the synthesized compounds ABTS and DPPH systems. Antioxidant activity can be considered as an index of pharmacological usefulnessCitation17. Oxidants play role in many diseases. It is therefore not a complete surprise that many registered drugs have an antioxidant action which may contribute to their pharmacological activityCitation18. Catechin, Ascorbic acid, and Trolox® (Sigma–Aldrich, St. Louis, MO) were used as reference antioxidants.
CUPric reducing antioxidant capacity assay
The cupric reducing antioxidant capacity (CUPRAC) of the synthesized compounds was determined according to the method of Apak et al.Citation19 To a test tube 1 mL each of 10 mM Cu(II) chloride (Sigma Chemical Co), 7.5 mM neocuprine (Sigma Chemical Co), and NH4Ac (Fluka Chemical Co., Switzerland) buffer (1 M, pH 7.0) solutions were added. About 5 µL of compound solution in ethanol and 1.095 µL of water were added to the initial mixture so as to make the final volume 4.1 mL. The tubes were stoppered, and after 30 min, the absorbance at 450 nm was recorded against a reagent blank containing no compound. Trolox® (Sigma Chemical Co) was also tested under the same conditions as a standard antioxidant compound. The standard curve was linear between 8 and 0.25 mg/mL Trolox® (r2 = 0.999). CUPRAC values were expressed as milligrams of Trolox® equivalent of 1 mg synthesized compound.
DPPH-free radical scavenging assay
The DPPH radical scavenging activity of the synthesized compounds was measured using the method of Brand-WilliamsCitation20. Briefly, 0.1 mM DPPH (Aldrich-Germany) was prepared in methanol. At 1 mL of this solution was added 0.5 mL of the sythesized compound ethanol solution, at different concentrations. Test compounds were allowed to react with stable free radical, in the dark for 50 min, at room temperature. After incubation period, the decrease in absorbance at 517 nm was measured, using a UV–Visible spectrophotometer (1601UV-Shimadzu). Radical scavenging activity was measured by using Trolox®, Catechin, and Ascorbic acid as standards and all values are expressed as SC50 (microgram of compound per milliliter), the concentration of the samples that causes 50% scavenging of DPPH radical. The DPPH radical stock solution was prepared fresh daily.
ABTS/persulfate assay
The scavenging activity of the synthesized compounds was determined by ABTS radical cation decolorization assay protocol, as developed by Re et al.Citation21 ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] was dissolved in water to a 7 mM concentration. ABTS (Sigma Chemical Co) radical cation (ABTS•+) was produced by reacting ABTS stock solution with 2.45 mM potassium persulfate (Sigma Chemical Co) (final concentration) and allowing the mixture to stand in the dark for 16–18 h at room temperature. Before usage, the ABTS solution was diluted to get an absorbance of 0.700 ± 0.020 at 734 nm with PBS at pH 7.4 (phosphate-buffered saline prepared by mixing 5 mM of NaH2PO4, 5 mM of Na2HPO4, and 153.84 mM of NaCl in 1 L of distilled water). About 200 µL of the compound solution in ethanol was added to 1.8 mL of the resulting blue-green ABTS radical solution. The mixture was incubated in the dark at room temperature for 5 min. After incubation, the decrease of absorbance at 734 nm was measured by using a UV–Visible spectrophotometer (1601UV-Shimadzu). All determinations were carried out three times. ABTS radical scavenging activity was measured by using Trolox®, Catechin, and Ascorbic acid as standards, and the percentage scavenging was calculated from the formula:
Result and discussion
Chemistry
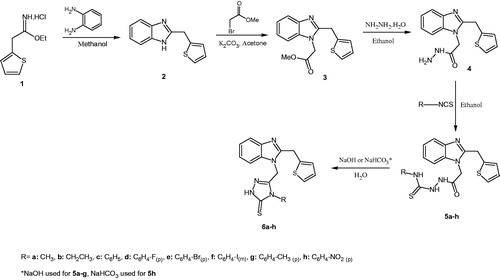
The reaction sequence to achieve the target compounds is depicted in Scheme 1. The starting compound 2-(2-thiophen-2-ylmethyl)-1H-benzimidazole (2) was prepared according to the previously reported procedureCitation22. The reaction of compound 2 with methyl bromoacetate in the presence of dry potassium carbonate afforded methyl [2-(thiophen-2-ylmethyl)-1H-benzimidazol-1-yl] acetate (3). Treatment of compound 3 with hydrazine hydrate in ethanol gave 2-[2-(thiophen-2-yl-methyl)-1H-benzimidazol-1-yl]acetohydrazide (4)Citation23–25. The carbothioamide derivatives (5a–h) were prepared with the reaction of compound 4 and corresponding isothiocyanate derivatives in ethanol. Finally, intramolecular cyclization reaction of compounds 5a–h in 2N NaOH or 2N NaHCO3 resulted in the target 1, 2, 4-triazol-3-thione derivatives (6a–h).
Spectral investigation of all compounds is in accordance with proposed structures. NH and NH2 signals were controlled by D2O addition to DMSO-d6 solution of compounds. In 1H NMR spectra, NH signals were observed at 12.30 ppm, 9.52 ppm and between 13.52 and 14.02 ppm for compounds 2, 4 and 6a–h, respectively, and three NH signals were shown between 8.04 and 10.75 ppm for compounds 5a–h. Furthermore, no NH signal was observed and the formation of new OCH3 and NCH2 signals for compound 3, proved the alkylation reaction. In 1H NMR spectra of compound 4, OCH3 signal disappeared and new NH and NH2 signals were formed at 9.52 ppm and 4.82 ppm, respectively. Two new NH signals were formed between 8.04 and 10.75 ppm and NH2 signal disappeared in 1H NMR spectra of compounds 5a–h. 1H NMR spectra of compounds 6a–h revealed the absence of the NH signals originated from carbothioamide derivatives, instead of that, new NH signal coming from triazol ring was recorded. 13C NMR spectra of all compounds showed benzimidazole C=N signals at about 153 ppm. The C=S and triazole C=N signals were observed at about 167 and 143 ppm. In addition, all the compounds have suitable molecular ion peak and elemental analysis result.
According to the IR spectroscopic data of compounds 6a–h, N-alkyl substituted triazoles (6a, b) were found in their thioenol form (SH) and N-phenyl substituted triazoles (6c–h) were found in their thiocarbonyl form (C=S) in solid state. IR spectral data of these compounds proved this situation. SH signal was observed only for compounds 6a, b at 2567, 2536 cm−1 and C=S was observed only for compounds 6c–h between 1297 and 1326 cm−1. Also, NH signal was shown for compounds 6c–h between 3098 and 3179 cm−1. According to the 1H NMR and 13C NMR spectral data, compounds 6a–h were found only in their thiocarbonyl from (C=S). In the 1H NMR spectra of these compounds, NH peaks were observed as a singlet between 13.52 and 14.02 ppm, and so their thiocarbonyl form was proved. 13C NMR spectrum of 6a–h showed signals at 147.83–148.74 and 167.48–169.27 ppm due to C=N and C=S, respectivelyCitation26–28.
CUPRAC antioxidant activity assay
The CUPRAC method of antioxidant capacity measurement, introduced by the analytical chemistry laboratory of Istanbul University to world literatureCitation19, is based on the absorbance measurement of the CUPRAC chromophore, Cu(I)-neocuproine (Nc) chelate, formed as a result of the redox reaction of antioxidants with the CUPRAC reagent, bis(neocuproine) copper(II) cation [Cu(II)-Nc], where absorbance is recorded at the maximal light absorption wavelength of 450 nm. The orange–yellow color is due to the Cu(I)-Nc charge-transfer complex formedCitation29. The maximum antioxidant capacity in the CUPRAC method was observed for the compound 5c (). The compounds 5b, 5h, and 6f showed very good antioxidant capacity. On the other hand, the compounds 4, 6a, 6b, 6c, 6d, and 6g showed good activity, while compounds 2 and 3 showed very little activity.
Table 1. Antioxidant activity values to CUPRAC method and SC50 values to DPPH method of synthesized compounds.
DPPH scavenging assay
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical has been used widely in the model system to investigate the scavenging activities of several synthesized and natural compoundsCitation30. The DPPH method is based on the fact that the free radical is purple in color, and that the purple color of DPPH decays in the presence of an antioxidant. The color changed from purple to yellow after reduction, which can be quantified by its decrease of absorbance at wavelength 517 nm. The results were expressed as SC50 (µg/mL, ). The compound 5c had the smallest SC50 value than other synthesized compounds. Also, the compound 5c had the best anti-oxidant activity to CUPRAC method. The compounds 5b, 5d, 5e, and 5h showed good SC50 values. On the other hand, the compounds 5a, 5f, 5g, 6e, 6h, and 6g showed weak activity, while compounds 2 and 3 showed no activity.
ABTS scavenging assay
The pre-formed radical monocation of 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) is generated by oxidation of ABTS with potassium persulfate and is reduced in the presence of such hydrogen-donating antioxidants. Initially, each compound was tested at the final concentration of 6.25 µg/mL in the assay mixture. Various dilutions of basic solutions were made and tested to determine by the decolorization of the ABTS and assessed as an extinguishing of the absorbance at 734 nm. The compound 5h showed very good scavenging activity at the final concentration of 6.25 µg/mL (). The compounds 4, 5a, 5b, 5c, 5d, 6a, 6b, 6c, and 6f exhibited good scavenging activity at the final concentration of 6.25 µg/mL. On the other hand, the compounds 5g, 6e, 6g, and 6h showed weak scavenging activity, while compounds 2 and 3 showed no activity at the same concentration.
Table 2. ABTS radical scavenging activity values of the synthesized compounds.
Conclusion
In conclusion, we have synthesized some new triheterocyclic compounds containing benzimidazole, thiophene, and 1,2,4-triazole rings. Most of the carbothioamide derivatives (5) displayed radical scavenging activity in all two methods. Compound 5c also showed the highest antioxidant activity with SC50 value 9.89 ± 0.07 µg/mL to DPPH method. Besides, nitrogen containing carbothioamide derivative (5h) was found more efficient than widely used reference antioxidants ascorbic acid and Trolox® for ABTS assay. Also, 1,2,4-triazole derivatives (6a, 6b, 6c, 6f, and 6d) showed very good ABTS scavenging activity. The results could be inspiration for further investigation of potential antioxidant activity within triheterocyclic compounds.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Dayem AA, Choi HY, Kim JH, Cho SG. Role of oxidative stress in stem, cancer, and cancer stem cells. Cancers 2010;2:859–84
- Gey KF. Ten-year retrospective on the antioxidant hypothesis of arteriosclerosis: threshold plasma levels of antioxidant micronutrients related to minimum cardiovascular risk. J Nutr Biochem 1995;6:206–36
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol 1997;88:291–5
- Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr 1996;16:33–50
- Preston PN. Synthesis, reactions, and spectroscopic properties of benzimidazoles. Chem Rev 1974;74:279–314
- Kazimierczuk Z, Andrzejewska M, Kaustova J, Klimesova V. Synthesis and antimycobacterial activity of 2-substituted halogenobenzimidazoles. Eur J Med Chem 2005;40:203–8
- Sharma D, Narasimhan B, Kumar P, Jalbout A. Synthesis and QSAR evaluation of 2-(substituted phenyl)-1H-benzimidazoles and [2-(substituted phenyl)-benzimidazol-1-yl]-pyridin-3-yl-methanones. Eur J Med Chem 2009;44:1119–27
- Yenisehirli A, Naseri E. Omeprazole, ansoprazole and pantoprazole had no effect on blood pressure and electrocardiogram of anesthetized rat. Pharmacol Res 2008;58:65–71
- Rivkin A, Gim S. Rifaximin: new therapeutic indication and future directions. Clin Therap 2011;33:812–27
- Iqbal CMA, Satyendra D, Apurba T, et al. Synthesis and antimicrobial screening of some novel substituted thiophenes. J Drug Med 2012;4:112–18
- Ferreira ICF, Calhelha RC, Estevinho LM, Queiroz MJR. Screening of antimicrobial activity of diarylamines in the 2,3,5-trimethylbenzo[b]thiophene series: a structure–activity evaluation study. Bioorg Med Chem Lett 2004;14:5831–3
- Gadad AK, Kumar H, Shishoo CJ, et al. Synthesis of some 2-aminoacetylamino-3-carbethoxy/anilido-4,5,6,7-tetrahydrobenzo[b]thiophenes for local anesthetic activity. Ind J Chem Soc 1994;33:298–301
- Janosik T, Bergman J. Progress in heterocyclic chemistry. Vol. 19. Oxford: Elsevier; 2008:112– 34
- Ponticello GS, Freedman MB, Habecker CN, et al. Thienothiopyran-2-sulfonamides: a novel class of water-soluble carbonic anhydrase inhibitors. J Med Chem 1987;30:591–7
- Kus C, Kılcigil GA, Ekel BC, Iscan M. Synthesis and antioxidant properties of some novel benzimidazole derivatives on lipid peroxidation in the rat liver. Arch Pharm Res 2004;27:156–63
- Cui W, Kargbo RB, Sajjadi-Hashemi Z, et al. Efficient one-pot synthesis of 2-substituted benzimidazoles from triacyloxyborane intermediates. Synlett 2012;23:247–50
- Esteves M, Siquet C, Gaspar A, et al. Antioxidant versus cytotoxic properties of hydroxycinnamic acid derivatives – a new paradigm in phenolic research. Arch Pharm Chem Life Sci 2008;341:164–73
- Bast A, Haenen GRMM, Bruynzeel AME, Van der Vijgh WJF. Protection by flavonoids against anthracycline cardiotoxicity: from chemistry to clinical trials. Cardiovasc Toxicol 2007;7:154–9
- Apak R, Güçlü K, Özyürek M, Karademir SE. A novel total antioxidant capacity index for dietary polyphenols, vitamins C and E, using their cupric ion reducing capability in the presence of Neocuproine: CUPRAC method. J Agric Food Chem 2004;52:7970–81
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 1995;28:25–30
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26:1231–7
- Pinner A. Die Imidoäther und ihre Derivate, 1 Auflage; Oppenheim: Berlin; 1892
- Menteşe E, Karaali N, Yılmaz F, et al. Microwave-assisted synthesis and biological evaluation of some benzimidazole derivatives containing a 1,2,4-triazol Ring. Arch Pharm Chem Life Sci 2013;346:556–61
- Yilmaz F, Menteşe E, Karaali N, Kahveci B. Microwave-assisted synthesis of some 5(6)-nitro-1H-benzimidazoles and their hydrazide derivatives. Bull Chem Soc Ethiop 2013;27:265–71
- Kahveci B, Sosan N, Mentese E, Yilmaz F. Microwave-assisted synthesis of some novel benzimidazole compounds containing oxadıazole moiety. Rev Roum Chim 2013;58:511–15
- Demirbas N, Karaoğlu ŞA, Demirbas A, Sancak K. Synthesis and antimicrobial activities of some new 1-(5-phenylamino-[1,3,4]thiadiazol-2-yl)methyl-5-oxo [1,2,4]triazole and 1-(4-phenyl-5-thioxo-[1,2,4]triazol-3-yl)methyl-5-oxo-[1,2,4]triazole derivatives. Eur J Med Chem 2004;29:793–804
- Bekircan O, Menteşe E, Ülker S, Küçük C. Synthesis of some new 1, 2, 4-triazole derivatives starting from 3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1, 2, 4-triazol with anti-lipase and anti-urease activities. Arch Pharm Chem Life Sci 2014;347:387–97
- Foroumadi A, Soltani F, Moshafi MA, Ashraf-Askari R. Synthesis and in vitro antibactarial activity of some N-(5-aryl-1,3,4-thiadiazole-2-yl)piperazinyl quinolone derivatives. Il Farmaco 2003;58:1023–8
- Apak R, Gorinstein S, Böhm V, et al. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC technical report). Pure Appl Chem 2013;85:957–98
- Can Z, Dincer B, Sahin H, et al. Polyphenol oxidase activity and antioxidant properties of Yomra apple (Malus communis L.) from Turkey. J Enzyme Inhib Med Chem 2014. [Epub ahead of print]. doi: 10.3109/14756366.2013.858144