Abstract
A new affinity gel was synthesized for the purification of xanthine oxidase (XO, EC 1.2.3.22) from bovine milk. The gel was prepared on a Sepharose 4B matrix on which a spacer arm based on l-tyrosine was covalently attached via CNBr activation, followed by reaction with the XO inhibitor p-aminobenzamidine. The elution conditions of affinity gel were determined at different pH values and ionic strengths. Maximum elution of XO was achieved at pH 9.0 and ionic strength around 0.4. The overall purification for XO was 1645-fold with 20.49% yield. SDS-PAGE of the enzyme indicates a single band with an apparent MW of 150 kDa. The gel provides a simple, rapid and effective useful for the purification of XO. Heat stability was determined on purified XO activity. Xanthine oxidase was preserved up to 70% with activity exposure of 60 °C and incubated for 60 min. These results indicated that the enzyme was heat stable.
Introduction
Xanthine oxidoreductase (XOR) which is a member of the molybdenum hydroxylase family of proteins contains binding sites for molybdopterin, iron and flavin cofactorsCitation1–3. XOR exists in two interconvertible forms xanthine oxidase and xanthine dehydrogenase (XD; EC 1.1.3.204). XD uses NAD+ as an electron acceptor and produces NADH; XO primarily produces ROS such as superoxide and hydrogen peroxide by preferentially using molecular oxygen as an electron acceptorCitation2. Xanthine oxidase is related to gout and hyperuricemia. In addition, XO also plays an important role in ischemic and other types of tissue and vascular injuries, inflammatory diseases and chronic heart failureCitation4–10.
XOR was first purified from this source over 80 years agoCitation11,Citation12. XO is an enzyme which is purified from milk in abundance and is cheapCitation11. In the literature, there are a number of purification procedures for the XOCitation13–16. In previous studies, numerous chromatographic step and the organic solvents were used during the enzyme purificationCitation16–19. However, the most commonly used purification method is the affinity chromatography, which gives high yields of the enzyme from a wide variety of sources. Affinity chromatography takes advantage of the purification of many proteins by using specific ligands or chemical groups with the affinity for the protein purification. Inhibitor of XO is frequently used as ligands in these methods, as benzamidine derivates are specific inhibitors of xanthine oxidaseCitation12. In the literature, there are a number of chromatographic methods used for the purification of xanthine oxidaseCitation12,Citation17–Citation21. Many researchers have used chromatographic methods except affinity chromatography. Affinity chromatography is indisputable superiority of other chromatographic techniques. The present report describes the successful purification of XO from bovine milk using a novel affinity gel. The affinity gel was prepared on the Sepharose 4B matrix with a spacer arm incorporating l-tyrosine, to which p-aminobenzamidine was covalently attached for affinity gel purification of XO.
Unpurified XO present in cream and ice-cream mix is different from many other enzymes. Other studies indicated that XO was stable in high temperature but unstable in extreme pH, high ionic strength and organic solventsCitation22–24. However to our knowledge, no study is available on the heat stability for purified xanthine oxidase enzyme activity. We aimed to determine heat stability for purified XO.
Experimental
Materials
The chemicals used in our study were obtained from Sigma-Aldrich (Taufkirchen, Germany). All other chemicals used were of analytical grade and obtained from either Sigma-Aldrich or Merck (Darmstadt, Germany).
Preparation of affinity gel
Four grams of CNBr was added to 20 mL Sepharose 4B and with 4 M NaOH the mixture's pH was kept at 11. The reaction was stopped by filtering the gel on a Buchner funnel and washing with cold 0.1 M NaHCO3 (pH 10) buffer. l-Tyrosine by using saturated l-tyrosine solution in the same buffer was coupled to Sepharose-4B-l-tyrosine activated with CNBr. The reaction was completed by stirring with a magnet for 90 min. In order to remove excess of l-tyrosine from the Sepharose-4B-l-tyrosine gel, the mixture was washed with distilled water. The affinity gel was obtained by diazotization of p-aminobenzamidine and coupling of this compound to the Sepharose-4B-l-tyrosine. The pH was adjusted to 9.5 with 1.0 M NaOH and, after gentle stirring for 3 h at room temperature; the coupled red Sepharose derivative was washed with 1 L of water and then 200 mL of 0.05 M Tris–sulfate (pH 7.5) buffer.
Enzyme purification
Fresh bovine milk was cooled down to 4 °C overnight without added preservative. EDTA and toluene were then added to give final concentrations of 2 mM and 3% (v/v), respectively. The milk was churned with a blender at maximum speed for 30 min at room temperature. This sample was brought to 38% saturation by addition of solid ammonium sulphateCitation19,Citation25. The suspension was centrifuged at 15 000 rpm for 30 min and the precipitate formed was discarded. The supernatant was brought to 50% saturation with solid ammonium sulphate. The precipitate formed was collected by centrifugation at 15 000 rpm for 60 min and dissolved 0.1 M Tris–HCl (pH 7.6) buffer. The pooled precipitate obtained from bovine milk by using ammonium sulfate precipitation was subjected to affinity chromatography. The sample was applied to the Sepharose-4B-l-tyrosine-p-aminobenzamidine affinity column equilibrated with 0.1 M glycine/0.1 M Na2SO4 (pH 9.0) buffer. The sample was applied to the affinity gel was washed with 0.1 M glycine (pH 9.0) buffer. XO was then eluted with 25 mM benzamidine in 0.1 M glycine/0.1 M Na2SO4 (pH 9.0) buffer fractions of 1. 5 mL were collected and their absorbance measured at 280 nm.
Activity measurements
Xanthine oxidase activity was determined by the modified method of Massey et al.Citation20 The conversion of xanthine uric acid was followed by monitoring the change in absorbance at 292 nm, using UV-Visible Spectrophotometer (ɛ292 = 9.5 mM−1 cm−1). The reaction mixture contained 50 mM Tris–HCl (pH 7.6) buffer and 0.15 mM xanthine at 37 °C. The assay was initiated by the addition of the enzyme. One unit of enzyme activity was defined as the amount of enzyme that converts 1 μmol of xanthine to uric acid per min under defined conditionsCitation18,Citation20.
Heat inactivation
Thermal inactivation was carried out at temperature ranging from 45 to 60 °C and was incubated at time ranging from 15 to 60 min. The xanthine oxidase samples were incubated at different temperatures and predetermined intervals of time immediately chilled to 37 °C and assayed xanthine oxidase activity. All measurements were repeated three times.
Total protein determination
After elution step enzyme was determined spectrophotometrically at 280 nm and protein during the purification steps was determined spectrophotometrically at 595 nm according to the Bradford method, using bovine serum albumin as the standardCitation26.
SDS polyacrylamide gel electrophoresis
The purity of enzyme XO from using ammonium sulfate precipitation and affinity chromatography was assessed by SDS polyacrylamide gel electrophoresis according to the method of LaemmliCitation27. The electrophoretic pattern was photographed with a system to produce an image of the gelCitation25.
Result and discussion
In our study, a new gel for the purification of the XO enzyme was synthesized. Bovine milk purified by ammonium sulphate precipitation followed by affinity chromatography specifically designed for XO. The affinity gel has been synthesized in order to reduce the number of the purification steps of xanthine oxidase. Benzamidine also inhibited bovine milk XO but only at a bit high concentrationsCitation19. Thus, we were interested in reassessing the effects of benzamidine derivatives on XO activity and use of affinity matrix for the purification of XO. We found that p-aminobenzamidine was a competitive inhibitor of XO. In addition, we found that the apparent affinity p-aminobenzamidine for XO increased with increasing pH.
Xanthine oxidase was extracted from fresh bovine milk without added preservative using toluene. Filtration of the churned milk through filter paper could be fractionated directly with ammonium sulphate. The entire enzyme was successfully collected in a narrow range of ammonium sulphate concentration (38–50% saturation) at this step 37.2% yield purification was achieved (). The precipitate formed was collected and dissolved 0.1 M Tris–HCl (pH 7.6). The dissolved sample prior to that it was loaded onto the novel affinity column prepared from Sepharose 4B-l-tyrosine-p-aminobenzamidine.
Table 1. Summary of the purification of bovine milk xanthine oxidase.
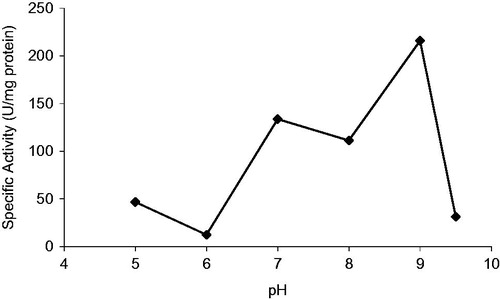
The sample was applied to the Sepharose-4B-l-tyrosine-p-aminobenzamidine affinity column equilibrated with 0.1 M glycine/0.1 M Na2SO4 (pH 9.0) buffer. The affinity gel was washed with 0.1 M glycine (pH 9.0) buffer, and XO was eluted at different pH buffers and different Na2SO4 concentrations in 25 mM benzamidine. Fractions of 1.5 mL were collected and their absorbance was measured at 280 nm. We explained optimum condition for XO purification. The eluates were characterized by protein determination at 280 nm and assaying XO activity. Specific activity for XO was calculated by using ammonium sulphate precipitation and purified enzyme solution. As a result, XO was purified up to 1645-fold with a recovery ratio of 20.49% (). These values are better than some of the reported affinity gels, and equivalent to othersCitation12,Citation16–19. Highly purified enzymes were obtained exhibiting a single band on SDS-PAGE. The XO migrated as single bands in all cases, with apparently identical molecular masses (). This result was similar to other studies in the literatureCitation28–30. We used only one step for purification, i.e. Sepharose-4B-l-tyrosine-p-aminobenzamidine affinity chromatography by modification of the elution conditions. XO purified using the affinity gel with different elution buffers with different pH and different Na2SO4 concentration in 25 mM benzamidine ( and ). The most suitable elution buffer was pH 9.0 ( and ) and 0.1 M Na2SO4 molarities () for XO. shows the typical elution pattern of the enzyme activity on affinity column. The enzyme activity and total protein concentration were determined from all fractions collected from each purification step. The fractions with the highest xanthine oxidase activity and the lowest protein contents were pooled in 6 and 7 tubes, respectively.
Figure 1. SDS-PAGE of bovine milk xanthine oxidase. The pooled fractions from ammonium sulfate precipitation and affinity chromatography (Sepharose-4B, l-tyrosine, p-aminobenzamidine) were analyzed by SDS-PAGE (12% and 3%) and revealed by Coomassie Blue staining. Experimental conditions were as described in the method. Lane 1 proteins in ammonium sulfate precipitation. Lane 2 contained 3 µg of various molecular mass standards. Fifteen micrograms of purified xanthine oxidase (lane 3) migrated with a mobility corresponding to an apparent Mr 150 kDa.
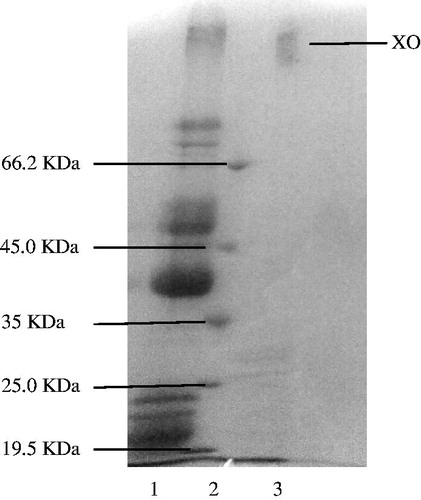
Figure 2. (A) Purification of bovine milk XO by affinity chromatography with 0.1 M glycine/0.1 M Na2SO4 (pH 9.0) buffer in 25 mM benzamidine elution solution. This material was eluted by increasing the ammonium sulfate concentration. Protein concentration was determined by measuring an absorbance of 280 nm and XO activities of fractions were assayed activity using xanthine substrate. 1 unit = 1 µmol min−1 per mL. Abbreviation: U, units. (B) Purification of bovine milk XO by affinity chromatography with 0.1 M glycine/0.1 M Na2SO4 (pH 8.0) buffer in 25 mM benzamidine elution solution. (C) Purification of bovine milk XO by affinity chromatography with 0.1 M glycine/0.1 M Na2SO4 (pH 7.0) buffer in 25 mM benzamidine elution solution. (D) Purification of bovine milk XO by affinity chromatography with 0.1 M glycine/0.1 M Na2SO4 (pH 6.0) in 25 mM benzamidine elution solution. (E) Purification of bovine milk XO by affinity chromatography with 0.1 M glycine/0.1 M Na2SO4 (pH 5.0) in 25 mM benzamidine elution solution.
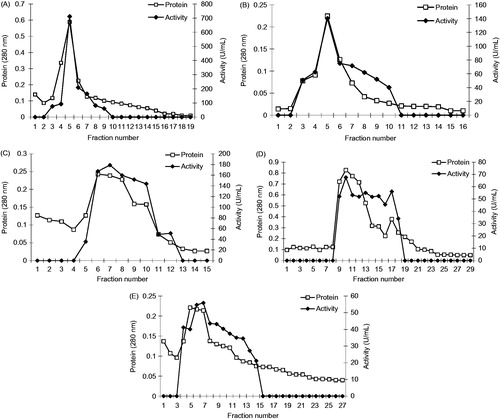
Figure 3. (A) Purification of bovine milk XO by affinity chromatography with 0.1 M glycine/1.0 M Na2SO4 (pH 9.0) in 25 mM benzamidine elution solution. (B) Purification of bovine milk XO by affinity chromatography with 0.1 M glycine/0.5 M Na2SO4 (pH 9.0) in 25 mM benzamidine elution solution. (C) Purification of bovine milk XO by affinity chromatography with 0.1 M glycine/0.1 M Na2SO4 (pH 9.0) in 25 mM benzamidine elution solution.
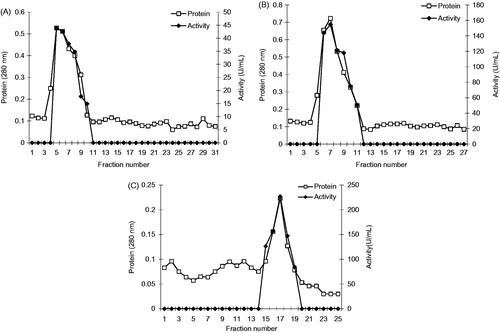
Figure 5. Purification of bovine milk XO by affinity chromatography. Fractions from the ammonium sulfate extraction were pooled as described. This material was eluted by increasing the ammonium sulfate concentration. Protein concentration was determined by measuring an absorbance of 280 nm and XO activities of fractions were assayed activity using xanthine substrate. 1 unit = 1 µmol min−1 per mL. Abbreviation: U, units.
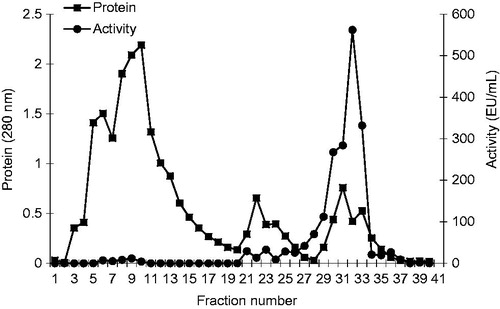
The partial purification of milk XO is ammonium sulphate precipitation and affinity chromatography on Sepharose 4B-folate gel but this method was applicable to an early stage in the enzyme purificationCitation17. XO was originally carried out from mouse mammary gland with overall purification of approximately 74-fold. The procedure included heat treatment, ammonium sulphate precipitation and affinity chromatography on benzamidine-SepharoseCitation18. Rat liver XO was purified in 199-fold by using heat treatment, ammonium sulphate precipitation and affinity chromatography on benzamidine-SepharoseCitation12. Ozer et Al.Citation19 purified the bovine milk xanthine oxidase in 328-fold by using toluene and heat, ammonium sulphate fractionation, and DEAE–Sepharose (fast flow) column chromatography. Xanthine oxidase from rat liver purification procedure is homogenate, heat treatment, ammonium sulphate precipitation, HTP (hydroxyapatite) and Q-Sepharose fast flow column. The purification is 1167-fold with 19% yieldCitation20. In previous studies the data is of quite high purification foldCitation12,Citation16–19. In our study, affinity chromatography on Sepharose 4B-l-tyrosine-p-aminobenzamidine provides a rapid and effective means of isolating xanthine oxidase in sufficient quantities for biochemical analyses. The Vmax and KM of the purified enzyme were determined 0.58 EU and 17.0 mM by a substrate, with xanthine, respectively.
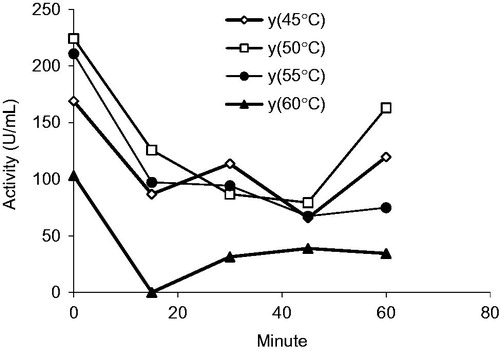
There were no data for XO heat stability but there were data for XO in cream and ice-cream mixCitation31. The applications of the enzyme for industrial purposes depend on its stability under harsh conditions such as high temperatureCitation31,Citation32. The heat inactivation profiles of purified XO from milk are shown in . The enzyme was incubated at different temperatures and different time, pH 7.6 the residual enzyme activity was measured using xanthine as substrate. This behavior is more pronounced between 45 °C and 60 °C. For data curves all the temperature inactivation was initially fast when incubated at 15 min. It accelerated as heating progressed, followed by a more gradual decrease in activity on continued exposure. The enzyme activity decreased due to a slight heat denaturation of the enzyme with increasing temperature and inactivation time. XO is relatively heat stable, preserving up to 70% activity after 60 min of heating at 60 °C. This indicated that the enzyme was not denatured at higher temperatures (). We determined heat stability of purified XO and it was determined to be highly heat stable.
Conclusion
Xanthine oxidase usage has a very wide area. So, purification of XO is commercially important. The purification of XO, in from bovine milk have been achieved by means of a simple, rapid and effective procedure. This is important, because, due to the broad and complementary substrate specificity of this enzyme, they are essential when investigating the metabolic fate of any new therapeutic agent or chemical present in the environment. Besides the simplicity of the method described herein, it should emphasize the advantage of time and raw material saving without compromising the final specific activity of this enzyme. The purified XO preparations show the absorption spectrum, specific activity and the SDS-electrophoretic behavior characteristic of highly purified enzyme. To our knowledge, no study is available on the heat stability for purified XO enzyme activity; hence it was determined in this study.
Declaration of interest
This work was supported by Balikesir University Research Project (2009/17). This work was carried out in the Balikesir University Research Center of Applied Sciences (BURCAS).
References
- Hille R. Structure and function of xanthine oxidoreductase. Eur J Inorg Chem 2006;2006:1913–26
- Nishino T, Okamoto K, Eger BT, et al. Mammalian xanthine oxidoreductase-mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS J 2008;275:3278–89
- Engerson TD, McKelvey TG, Rhyne DG, et al. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Invest 1987;79:1564–70
- Southern PA, Powis G. Free radicals in medicine II. Involvement in human disease. Mayo Clin Proc 1988;63:390–408
- Landmesser U, Drexler H. Allopurinol and endothelial function in heart failure: future or fantasy? Circulation 2002;106:173–5
- Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Phycol 2004;555:589–606
- Doehner W, Anker SD. Xanthine oxidase inhibition for chronic heart failure: is allopurinol the next therapeutic advance in heart failure? Heart 2005;91:707–9
- Kittleson MM, Hare JM. Xanthine oxidase inhibitors: an emerging class of drugs for heart failure. Eur Heart J 2005;26:1458–60
- Pacher P, Schulz R, Liaudet L, et al. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci 2005;26:302–10
- Ungvari Z, Gupte SA, Recchia FA, et al. Role of oxidativenitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol 2005;3:221–9
- Harrison R. Milk xanthine oxidase: properties and physiological roles. Int Dairy J 2006;16:546–54
- McManaman JL, Shellman V, Wright RM, et al. Purification of rat liver xanthine oxidase and xanthine dehydrogenase by affinity chromatography on benzamidine–sepharose. Arch Biochem Biophys 1996;332:135–41
- Abadeh S, Killacky J, Benboubetra M, et al. Purification and partial characterization of xanthine oxidase from human milk. Biochim Biophys Acta 1992;1117:25–32
- Kurosaki M, Zanotta S, Calzi ML, et al. Expression of xanthine oxidoreductase in mouse mammary epithelium during pregnancy and lactation: regulation of gene expression by glucocorticoids and prolactin. Biochem J 1996;319:801–10
- Stanton TB, Jensen NS. Purification and characterization of NADH oxidase from Serpulina (Treponema) hyodysenteriae. J Bacteriol 1993;175:2980–7
- Maia L, Mira L. Xanthine oxidase and aldehyde oxidase: a simple procedure for the simultaneous purification from rat liver. Arch Biochem Biophys 2002;400:48–53
- Nishino T, Nishino T, Tsushima K. Chromatography on Sepharose 4b/folate gel. FEBS Lett 1981;131:369–72
- McManaman JL, Neville MC, Wright RM. Mouse mammary gland xanthine oxidoreductase: purification, characterization, and regulation. Arch Biochem Biophys 1999;371:308–16
- Özer N, Muftuoglu M, Ataman D, et al. Simple, high-yield purification of xanthine oxidase from bovine milk. J Biochem Bioph Meth 1999;39:153–9
- Massey V, Brumby PE, Komai H. Studies on milk xanthine oxidase: some spectral and kinetic properties. J Biol Chem 1969;244:1682–91
- Xin Y, Yang H, Xia X, et al. Expression, purification and partial characterization of a xanthine oxidase (XOD) in Arthrobacter sp. Process Biochem 2012;47:1539–44
- Shaw AL, Hanson GR, McEwan AG. Cloning and sequence analysis of the dimethylsulfoxide reductase structural gene from Rhodobacter capsulatus. Biochim Biophys Acta 1996;1276:176–80
- Balladin DA, Narinesingh D, Stoute VA, et al. Immobilization of xanthine oxidase and its use in the quantitation of hypoxanthine in fish muscle tissue extracts using a flow injection method. Biochem Biotech 1997;62:317–28
- Waud WR, Rajagopalan KV. Preparation of bovine milk xanthine oxidase as a dehydrogenase form. Arch Biochem Biophys 1976;172:365–79
- Beyaztaş S, Arslan O. Affinity effects of some antibiotics on xanthine oxidase enzyme activities in vitro. Hacettepe J Biol Chem 2011;39:195–205
- Bradford MM. A rapid and sensitive method for the quantition of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5
- Nile SH, Kumar B, Park SW. In vitro evaluation of selected benzimidazole derivatives as an antioxidant and xanthine oxidase inhibitors. Chem Biol Drug Design 2013;82:290–5
- McManaman JL, Neville MC, Wrigh RM. Mouse mammary gland xanthine oxidoreductase: purification, characterization, and regulation. Arch Biochem Biophys 1999;371:308–16
- Liu S, Xing J, Zheng Z, et al. Ultrahigh performance liquid chromatography–triple quadrupole mass spectrometry inhibitors fishing assay: a novel method for simultaneously screening of xanthine oxidase inhibitor and superoxide anion scavenger in a single analysis. Anal Chim Acta 2012;715:64–70
- Balkishan NP, Sharma R, Rajput YS, et al. Activities and thermal stability of indigenous enzyme in cream and ice-cream mix. Milchwissenschaft 2010;65:190–2
- Harrison R. Milk xanthine oxidase: properties and physiological roles. Int Dairy J 2006;16:546–54