Abstract
Context: Mammary and placental 17β-hydroxysteroid dehydrogenase type 1 (17βHSD1).
Objective: To assess the impact of testosterone, tibolone, and black cohosh on purified mammary and placental 17βHSD1.
Materials and methods: 17βHSD1 was purified from human mammary gland and placenta by column chromatography, its activity was monitored by a radioactive activity assay, and the degree of purification was determined by gel electrophoresis. Photometric cofactor transformation analysis was performed to assess 17βHSD1 activity without or in presence of testosterone, tibolone and black cohosh.
Results: 17βHSD1 from both sources displayed a comparable basal activity. Testosterone and tibolone metabolites inhibited purified mammary and placental 17βHSD1 activity to a different extent, whereas black cohosh had no impact.
Discussion: Studies on purified enzymes reveal the individual action of drugs on local regulatory mechanisms thus helping to develop more targeted therapeutic intervention.
Conclusion: Testosterone, tibolone and black cohosh display a beneficial effect on local mammary estrogen metabolism by not affecting or decreasing local estradiol exposure.
Introduction
With conventional postmenopausal hormone therapy (HT), especially combined estrogen plus progestin therapy, being associated with an increased breast cancer (BC) riskCitation1, alternative therapy options for vasomotor symptom relief are warranted. Tibolone (OrgOD14) is a unique single-agent, tissue-selective agent for symptomatic postmenopausal women. Its metabolites have estrogenic, progestagenic, and androgenic propertiesCitation2. Both, its 3α-OH metabolite (Org4094), and 3β-OH metabolite (Org30126) bind to estrogen receptor (ER) alpha, while its Δ-metabolite (OM38) binds to the androgen- (AR), and progesterone receptor (PR)Citation3. The binding affinity of OM38 to the AR is high displaying an agonistic activity comparable to testosteroneCitation4, making it a treatment option for postmenopausal women also suffering from loss of libidoCitation5. Loss of libido may be associated with decreased serum androgens, e.g. due to agingCitation6, or bilateral oophorectomy, respectivelyCitation7,Citation8. From 2006 until 2012, a testosterone patch was approved by the European Medicines Agency for women with hypoactive sexual desire disorder following hysterectomy and bilateral oophorectomy. Symptomatic menopausal women who do not tolerate or refuse a conventional HT may also be offered a phytotherapy such as black cohosh (Cimicifuga racemosa; CR)Citation9. Authors have speculated that black cohosh may work through estrogen-like compoundsCitation10. However, today black cohosh is thought to reduce hot flushes by modulating the central dopaminergic and serotoninergic metabolismCitation11, and not via steroid hormone receptorsCitation12.
The impact of tiboloneCitation2, testosteroneCitation13, and black cohoshCitation14 on the breast is still a subject of controversy. Exogenous hormones may affect the breast by modulating the local endocrine milieu. Normal and malignant breast cells and tissue, respectively, have been shown to possess the enzymes necessary for local estrogen biosynthesis: aromatase, which converts androgens to estrogens; steroid sulfatase (STS), which hydrolyzes estrone sulfate (E1S) to estrone (E1); and 17β-hydroxysteroid dehydrogenase type 1 (17βHSD1), which reduces E1 to estradiol (E2)Citation15. Thus, independently from serum hormone levels, locally produced E2 may have an impact on mammary carcinogenesisCitation16. For tiboloneCitation17–22, testosteroneCitation23, and an isopropanolic extract of black cohosh (iCR)Citation24 an inhibitory effect on STS activity was found in vitro and ex vivo, respectively.
In the present study, we focused on 17βHSD1 which is one of at least 14 different 17βHSD types identified so farCitation25. In the past, conclusions regarding 17βHSD1 activity were drawn from 17βHSD1 mRNA expressionCitation26, or cell culture studiesCitation27–30, respectively. However, mRNA expression does not necessarily reflect enzyme activityCitation31, and additional regulatory mechanisms of cellular hormone metabolism may alter results in cell culture. Thus, ideally, enzymes’ characteristics are studied using purified enzymes.
The aim of this pilot study was to purify the enzyme 17βHSD1 from human mammary gland and to test for the impact of tibolone metabolites, testosterone, and black cohosh (iCR) on 17βHSD1 activity. Human placenta was used as a technical control.
Methods
Chemicals, reagents, and steroids
OrgOD14, Org4094, Org30126, and OM38 were provided by NV Organon (Oss, the Netherlands). The isopropanolic-aqueous extract of CR (iCR) (Nr. 010720) was provided by Schaper & Brümmer GmbH & Co KG, Salzgitter, Germany. The concentration of the extract was 100 mg/ml in relation to the dry residue and the alcohol concentration was 40% (v/v). Isolated enzymes were incubated with iCR at a concentration of 10 ng/ml and 1 ng/ml, respectivelyCitation24. The radioactive labelled steroid [3H]-E1 (specific activity, 57.50 Ci/mmol) was purchased from Perkin Elmer Life and Analytical Sciences (Boston, MA). Unlabelled testosterone (T), E2 and E1 were obtained from Sigma Aldrich. Unless stated otherwise, all chemicals were purchased from Sigma Aldrich (Deisenhofen, Germany).
Human placenta and mammary gland
Placenta tissue was obtained from one healthy pregnant woman immediately after term delivery at the Muenster University Hospital. The placenta was without any obvious pathological alterations, as determined by the responsible physician. Placenta was perfused with 1.5 l of isotonic saline to remove blood prior to immediate freezing in liquid nitrogen. Normal breast tissue was obtained from one premenopausal woman undergoing reduction mammoplasty for cosmetic reasons at a private clinic for plastic surgery in Muenster. A mammogram was routinely performed prior to surgery to exclude pathological alterations. Breast tissue was frozen in liquid nitrogen immediately after tissue removal. Both, placenta and breast tissue were stored at −80 °C prior to enzyme purification. If not used for this study, all tissues obtained would have been disposed as biological waste and not further analyzed. Study approval was obtained from the local ethics committee (reg. no. 1IXGreb) and all women gave written informed consent to their tissue being analyzed. The study was performed according to the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
17βHSD1 enzyme activity
17βHSD1 activity was measured in two different assays. During the purification procedure, enzyme enrichment was determined using a radioactive activity assay (RAA), while enzyme kinetics was studied by a non-radioactive photometric assay (PA). For the RAA, a modified protocol was used assessing the enzymatic conversion of [3H]-E1 to [3H]-E2 by thin layer chromatography (TLC)Citation32,Citation33. Briefly, a total volume of 400 µl assay solution consisted of (i) 200 µl purified enzyme diluted in assay buffer (0.2 M Tris-HCl, 0.1 M Na2HPO4 pH 6.9) or assay buffer alone (blank), (ii) 100 µl substrate solution (260 pMol [3H]-E1 (specific activity 57.5 Ci/mmol, Sigma) diluted in assay buffer, and (iii) 100 µl NADH co-substrate solution (1 mg/ml in assay buffer). Accurately 1 mg/ml stock solutions of E1 and E2 in 95% ethanol (Serva) were used as hormone markers. The assay solution was incubated for 15 min at 37 °C in a water bath prior to adding 100 µl non-radioactive marker hormones, and stopping the enzymatic reaction by adding 2 ml toluene, respectively. Samples were subsequently mixed by inverting the tubes 100-times and centrifuged at 1500 g for 10 min. Total reduction was determined by mixing an aliquot of 100 µl of the organic phase with 100 µl of assay buffer and 3 ml of scintillation cocktail prior to scintillation counting. The remaining organic phase was evaporated under a nitrogen stream, and reconstituted in Folch solution (2:1 chloroform:methanol). Accurately 50 µl of the solution were subjected to TLC on Silica gel GF 254 (Sigma Aldrich, Deisenhofen, Germany) using 13% ethanol in toluene as solvent. After visualization with 254 nm ultraviolet (UV) light, the zones on the plate corresponding to E1 and E2 as well as the non-fluorescent zones were cut apart and separately eluted with 3 ml ethanol and 6 ml scintillation fluid. Aliquots were taken from each fraction from the TLC plate for counting [3H] activity. A Wallac 1409 (β-counter) liquid scintillation counter was used for the radioactivity measurements, and Riafluor Perkin Elmer Life and Analytical Sciences (Boston, MA) was used as scintillation fluid. Enzyme activity was expressed as E2 [fmol] × protein [mg]−1 × min−1. The amount of total protein was determined according to BradfordCitation34. For the PA, the conversion of the co-substrate NADH to NAD+ in the presence of the substrate E1 was used to determine E2 formationCitation35. Therefore, 60 µl enzyme solution in assay buffer (0.2 M Tris-HCl, 0.1 M Na2HPO4, pH 6.9) were mixed with 30 µl substrate (381 µg/ml E1 in 95% ethanol), and 60 µl NADH (1 mg/ml) in assay buffer in the presence or absence of the following substancesCitation23,Citation31 (): T (10−6 M), Org4094 (10−6 M), Org30126 (10−6 M), OM38 (10−6 M), or iCR (10 ng/ml or 1 ng/ml), respectively. Enzyme inhibition experiments were performed at least twice with similar results. The volume of the test substances did not exceed 5% of the total volume. Extinction of NADH was determined photometrically at 366 nm and 37 °C. Enzyme activity was expressed as E2 [fmol] × protein [mg]−1 × min−1.
Figure 1. Chemical structures of testosterone and tibolone metabolites modified from van de Ven et al. [Citation20].
![Figure 1. Chemical structures of testosterone and tibolone metabolites modified from van de Ven et al. [Citation20].](/cms/asset/4b6726e7-3515-4165-a4de-df69c9f3685d/ienz_a_943205_f0001_b.jpg)
Purification of 17βHSD1
The multistep protocol for the isolation of 17βHSD1 from human placenta (100 g) and mammary gland (80 g) is presented in .
Figure 2. Schematic overview of the purification procedure of 17βHSD1 from human placenta (section Purification of 17βHSD1).
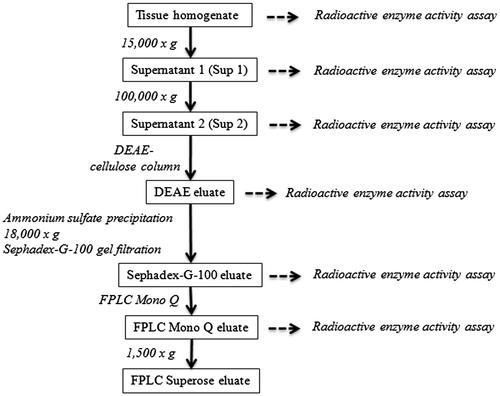
Tissue homogenization and separation of the microsome fraction
All purification steps were performed at 0–4 °C. Using a scalpel, blood vessels were removed macroscopically from tissue samples. The tissue was minced, and 5 ml buffer B/g wet weight was added (buffer B: 10 mM Tris-HCl pH 7.5, 0.5 mM EDTA, 1 mM dithioerythritol (DTE), 0.02% sodium azide, 125 µl/l protease inhibitor cocktail (Roche)). The samples were then homogenized three times for 30 s using a Heidolph Diax 100 homogenizer. The homogenate was subsequently centrifuged at 15 000 g using a Beckman J-21 B centrifuge (Krefeld, Germany). The pellet (P1) containing cellular debris and intact cells was discarded, and the supernatant (Sup1) was ultra-centrifuged at 100 000 g using a Beckman 45 Ti rotor (Krefeld, Germany) for 1 h. The resulting pellet (P2) containing cell organelles was also discarded. The supernatant (Sup2) was lyophilized, reconstituted in 1/10 volume of buffer C (buffer C: 10 mM Tris-HCl pH 7.5, 0.05% β-mercaptoethanol, 0.02% sodium azide) to be used as the starting material for the following chromatographic enzyme purification steps.
DEAE-cellulose anion exchange chromatography and Sephadex G-100 gel filtration
In an initial purification step, a DEAE-cellulose column (Pharmacia, Freiburg, Germany) was equilibrated with buffer C, and the sample was loaded for 1 h at a flow rate of 0.5 ml/min using an Abimed Gilson Minipulse pump (Middleton, WI). Subsequently, bound protein was eluted using a linear concentration gradient of 0.11 M to 1 M NaCl in buffer C, collecting 1 ml fractions for 180 min. To concentrate the peak fractions for the subsequent gel filtration step, an ammonium sulfate precipitation was performed by slowly adding (NH4)2SO4 to a final concentration of 65% using a magnetic stirrer. The precipitate was subjected to centrifugation at 18 000 g, the supernatant was discarded, and the pellet was reconstituted in 5–10 ml buffer C. 150 µl of this protein fraction was added to a Sephadex G-100 gel filtration column (Sigma, Deisenhofen, Germany), and equilibrated with buffer C. The sample was separated by the column using buffer C at a flow rate of 0.25 ml/min. The eluted proteins were collected in 0.5 ml fractions.
FPLC-anion exchange chromatography and gel filtration
Further purification of 17βHSD1 was achieved by separating the peak fractions of the Sephadex G-100 gel filtration by FPLC anion exchange chromatography on a Pharmacia LCC-500 FPLC (Freiburg, Germany) apparatus equipped with a Mono Q FPLC 8525260 anion exchange column (Pharmacia, Freiburg, Germany). 6 × 2 ml of the Sephadex G100 peak fractions were sequentially loaded onto the equilibrated Mono Q column in buffer C at a flow rate of 0.5 ml/min. After loading, the column was washed with 15 ml buffer C. Elution of the protein fractions was achieved by a concentration gradient of 0 M to 1 M NaCl. At a flow rate of 1 ml/min, a gradient of buffer C to buffer D (buffer D: buffer C + 1 M NaCl) was applied for 15 min, followed by 10 min of buffer D. The eluate was collected in 0.5 ml fractions. The final purification step consisted of an FPLC gel filtration. For this purpose, the volume of the Mono Q peak fractions was reduced by a 2 h centrifugation at 1500 g using Centricon Ultracel YM-10 Centrifugal Filter Devices (Amicon, Sigma Aldrich, Deisenhofen, Germany) with a cut-off of 10 kilo Dalton (kDa). This step resulted in a 100-fold volume reduction. Accurately 50 µl of the concentrated Mono Q fractions were subsequently separated by FPLC gel filtration on a Superose 12 10/300 GL (Amersham Bioscience, GE Healthcare, Muenchen, Germany) column using buffer C at a flow rate of 0.1 ml/min. The eluate was collected in 0.2 ml fractions.
SDS-PAGE and silver staining
To determine the purity of the chromatographic eluate fractions, samples were separated by SDS-PAGE followed by silver staining. 20–30 µg of protein/lane were boiled in SDS-sample buffer containing β-mercaptoethanol for 10 min prior to separation on 11.5% polyacrylamide gels at 25 mA for about 1 h. Molecular weights were estimated by comparison with low range molecular weight marker (Amersham). Silver staining was performed using a modification of the procedure of Heukeshoven and DernickCitation36. Gels were fixed with 10% trichloroacetic acid for 30 min and subsequently washed 3 × 15 min with ddH2O. The gels were incubated in solution 1 (30% ethanol, 68 g/l sodium acetate × 3 H2O, 2.5 g/l Na2S2O3 × 5 H2O, 0.5% glutaraldehyde) for 15 min, followed by 2 × washing with ddH2O for 10 min each. The gels were subsequently incubated in silver staining solution (8% AgNO3, 18.4 mM NaOH, 0.5% NH3) for 15 min, washed with ddH2O for 7 min and treated with developer solution (60 mg/l citric acid, 10% ethanol, 0.5 ml formalin) until protein bands became visible. The reaction was stopped using 1% acetic acid.
Statistical analysis
The means and standard deviations of all variables were calculated. Multiple comparisons between groups were performed using R-package “multcomp”, version 1.3.2Citation37. A p value <0.05 was considered to be significant.
Results
Purification of the enzyme 17βHSD1 from placenta
During the purification procedure (), samples were subjected in fractions to the respective column, analyzed for protein contentCitation34, and reunited into three to four eluate pools with comparable protein content (). Each pool was analyzed for 17βHSD1 enzyme activity, and the pool with highest activity was subjected to the next column separation step (, ). Overall, a 61.5-fold enrichment of enzyme activity was achieved, resulting in a mean specific 17βHSD1 activity of 32.61 fmol E2/mg of protein/min (, ). Aliquots of the fractions obtained during the purification steps were analyzed for purity by SDS-PAGE and subsequently silver staining was done (). While the homogenate and Sup1 displayed bands distributed over a broad molecular weight range, a 70 kDa protein was isolated from the DEAE, Sephadex G-100, and Mono-Q eluates that were further purified by Superose chromatography. The molecular weight corresponds to the dimeric, enzymatically active form of the enzymeCitation35. Thus, according to its specific activity this protein was identified to be the enzyme 17βHSD1. Since it was already successfully purified after the Mono-Q purification step, and in order to avoid further protein loss for the kinetic studies, the Superose column eluate was not further analysed for 17βHSD1 activity.
Figure 3. Purification of 17βHSD1 from human placenta. (A) Protein content of DEAE anion exchange chromatography fractions. Numbers 1–4 designate pooled fractions which were assayed for 17βHSD1 activity by RAA. (B) Pool D1 displayed the highest enzymatic activity. Error bars = SD. (C) Protein content of Sephadex-G-100 gel filtration chromatography fractions. DEAE pool 1 was concentrated by (NH4)2SO4-precipitation prior to gel filtration. Numbers 1–4 designate pooled fractions which were assayed for 17βHSD1 activity by RAA. (D) Pool G2 displayed the highest enzymatic activity. Error bars = SD. (E) Protein content of Mono Q FPLC anion exchange chromatography fractions. Numbers 1–3 designate pooled fractions which were assayed for 17βHSD1 activity by RAA. (F) Pooled fractions M1-3 show comparable degrees of activity. Error bars = SD. (G) Protein content of Superose FPLC gel filtration chromatography fractions. Pooled fraction M1 was cleared by centrifugation prior to gel filtration. The boxed fractions were used for further inhibitor studies (). (H) Overview of enzyme activity enrichment during the purification procedure as measured by RAA. Abbreviations: Homo = homogenate, Sup = supernatant, D1 = DEAE, G2 = Sephadex-G100, M1 = Mono-Q. Error bars = SD. (I) Silver stained SDS polyacrylamide gel of aliquots taken during the purification procedure. A single band of the expected molecular mass is obtained after the DEAE chromatography and the following purification steps, demonstrating purity of the preparation.
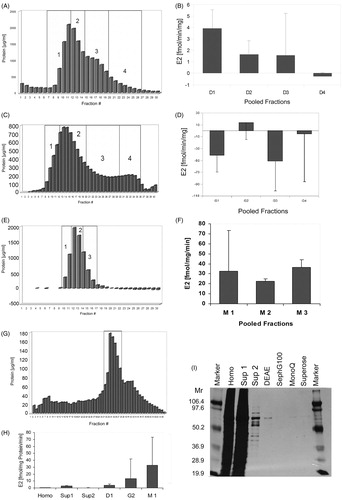
Table 1. Purification of 17βHSD1 from placenta. Purification was carried out as described in 2.4, starting with 50 g fresh human term placenta. Throughout purification, enzyme activity was assayed as described in 2.3 by following the oxidation of NADH at 366 nm using estrone as substrate. Abbreviations: E2 = estradiol.
Placental 17βHSD1 inhibitor studies
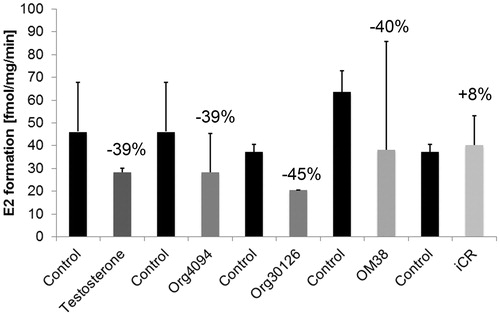
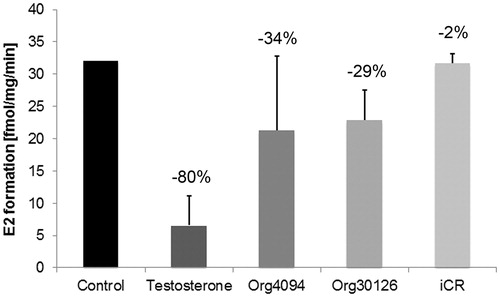
Placental 17βHSD1 inhibitor studies were carried out using the substances testosterone (10−6 M), Org4094 (10−6 M), Org30126 (10−6 M) and OM38 (10−6 M), and iCR (10 ng/ml), respectively. Prior to co-incubation with the respective substance, basal 17βHSD1 enzyme activity was measured for control purposes. By photometry, the extent of inhibition was evaluated per min for a maximum of 9 min. The protocol was repeated twice for each substance. Comparisons between the inhibitory substances were made indirectly by comparing the percentaged decrease of 17βHSD1 enzyme activity (). Org4094, Org30126, OM38 and testosterone displayed a comparable non-significant inhibitory potency (p > 0.05). However, iCR did not have an effect on placental 17βHSD1 activity.
Figure 4. Inhibitory effect of testosterone (T) and tibolone (Tib) metabolites on purified 17βHSD1 from placenta (n = 2). 60 µl aliquots of pooled Superose fractions containing purified 17βHSD1 () were photometrically assayed for enzyme activity prior to and after addition of T, Tib metabolites Org4094, Org30126 and OM38 (10−6 M each) and iCR (10 ng/ml), respectively. Incubation time was ≤ 9 min. While iCR had no inhibitory effect, T and Tib metabolites displayed a non-significant inhibitory activity (p > 0.05). Error bars = SD. Numbers above columns indicate percentage of inhibition relative to untreated control.
Purification of the enzyme 17βHSD1 from normal mammary gland
Purification of 17βHSD1 from human breast tissue (), and purity check by SDS-PAGE and subsequent silver staining () were performed accordingly (section Purification of the enzyme 17βHSD1 from placenta). However, there were slight changes to the protocol due to the high fat content of breast tissue: in the homogenate, 17βHSD1 activity analysis was impossible, and SDS-PAGE was only performed for Sup2, DEAE, and Sephadex G-100 eluates, respectively. Again, a 70 kDa protein was isolated and identified to be the enzyme 17βHSD1 (, ). Further purification steps were not necessary.
Figure 5. Purification of 17βHSD1 from human breast tissue. (A) Protein content of DEAE anion exchange chromatography fractions. Numbers 1–3 designate pooled fractions which were assayed for 17βHSD1 activity by RAA. (B) Error bars = SD. (C) Protein content of Sephadex-G-100 gel filtration chromatography fractions. DEAE pool 1 was concentrated by (NH4)2SO4-precipitation prior to gel filtration. Numbers 1–3 designate pooled fractions, which were assayed for 17βHSD1 activity by RAA. (D) Error bars = SD. (E) Overview of enzyme activity enrichment during the purification procedure as measured by RAA. Abbreviations: Sup = supernatant, D1 = DEAE, G2 = Sephadex-G100. Error bars = SD, N = 2. (F) Silver stained SDS polyacrylamide gel of aliquots taken during the purification procedure. A single band of the expected molecular mass is obtained after the DEAE chromatography and the following purification steps, demonstrating purity of the preparation.
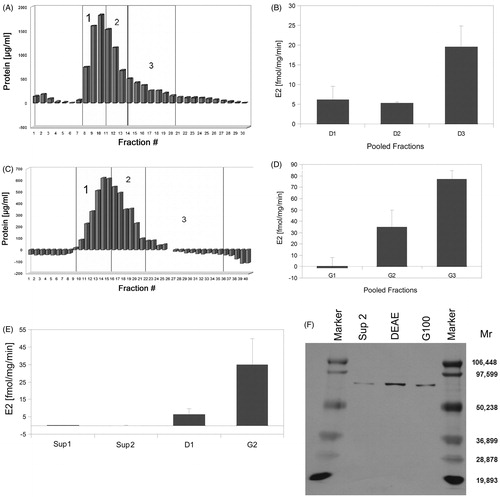
Table 2. Purification of 17βHSD1 from human mammary gland. Purification was carried out as described in 2.4, starting with 80 g fresh human breast tissue from mammoplasty. Throughout purification, enzyme activity was assayed as described in 2.3 by following the oxidation of NADH at 366 nm using estrone as substrate. Abbreviations: E2 = estradiol.
Mammary 17βHSD1 inhibitor studies
Mammary 17βHSD1 inhibitor studies were carried out accordingly (section Placental 17βHSD1 inhibitor studies). Due to the small amount of purified enzyme, the kinetic studies were performed with testosterone, Org4094, Org30126 at 10−6 M each, and iCR at 1 ng/ml each, respectively, but not with OM38. Overall, testosterone was a stronger though not significant 17βHSD1 inhibitor in comparison to Org4094 and Org30126, respectively (p > 0.05) (). Similarly to our findings in placenta, iCR did not have an effect on mammary 17βHSD1 activity.
Figure 6. Inhibitory effect of testosterone (T) and tibolone (Tib) metabolites on purified 17βHSD1 from human breast tissue (n = 2). Sephadex fractions containing purified 17βHSD1 () were photometrically assayed for enzyme activity prior to and after addition of T, Tib metabolites Org4094, and Org30126 (10−6 M each), and iCR (1 ng/ml), respectively. Incubation time was ≤9 min. While iCR had no inhibitory effect, T displayed a non-significant stronger inhibitory activity compared to Tib metabolites (p > 0.05). Error bars = SD. Numbers above columns indicate percentage of inhibition relative to untreated control.
Comparison of enzyme activities between placental and normal mammary gland
When comparing basal 17βHSD1 activity in placenta and human breast tissue, E2 formation differed between single purification steps. However, fully isolated 17βHSD1 activity was similar (). For complete 17βHSD1 isolation more purifications steps were necessary in placenta than in breast tissue as proven by SDS gel electrophoresis and silver staining ( and ). In placenta, the reaction equilibrium for 17βHSD1 was already reached after 6 min whereas in breast tissue it took 9 min (). When comparing the extent of 17βHSD1 inhibition we found a significant treatment effect when comparing placenta and mammary gland (p = 0.029). There was a stronger inhibitory effect on mammary 17βHSD1 for testosterone, while Org30126 was a more potent inhibitor of placental 17βHSD1 activity. Org4094 displayed similar effects on placental and mammary 17βHSD1. ICR did not reveal any inhibitory effect on 17βHSD1.
Figure 7. Comparative overview of 17βHSD1 activity from human placenta and breast tissue at different purification steps as determined by photometric assay. Abbreviations: S1 = supernatant, G2 = Sephadex-G100. Times indicate duration of photometric measurement. Error bars = SD.
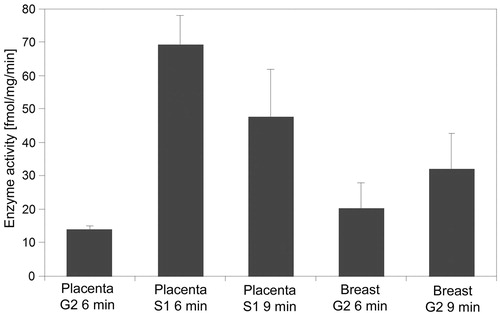
Table 3. Estradiol formation (fmol E2/mg of protein/min) in placenta and mammary gland depending on the 17βHSD1 purification step. Abbreviations: E2 = estradiol.
Discussion
In this pilot study, we have investigated the impact of testosterone, tibolone metabolites, and an isopropanolic extract of black cohosh on human placental and mammary 17βHSD1 activity. To avoid interactions with accessory proteins and complex cellular regulatory mechanisms, 17βHSD1 had to be purified to homogeneity using a multistep protocol. We purified the enzymes from a native tissue source to avoid a potential influence of recombinant protein expression on posttranslational modifications of 17βHSD1Citation38–40. We found that (1) more steps were required to obtain purified 17βHSD1 from placenta compared to breast tissue, (2) 17βHSD1 from both tissues displayed a comparable basal activity, with a slightly higher enzyme activity of the enzyme isolated from breast, (3) testosterone and tibolone metabolites inhibited purified 17βHSD1 activity in human placenta and breast tissue to a significantly different extent, whereas (4) iCR had no impact on 17βHSD1 activity.
Previous purification procedures of tissue 17βHSD have resulted in an about 500-fold enzyme enrichmentCitation41. In our study, we could achieve about 300-fold 17βHSD1 enrichment. Considering that the enzyme activity in the tissue homogenate in these studies was low and thus difficult to determine precisely, our data are in a comparable range.
Upon isolating 17βHSD1 from human placenta and mammary gland, several noteworthy differences arose. First, E2 formation in mammary Sup1 and Sup2 was lower compared to the corresponding placental fractions. Since electrophoresis did not reveal a relevant difference between mammary and placental Sup2 cytosolic protein content we suppose the higher breast fat content to have prevented efficient separation of E2. Secondly, in placenta, E2 formation was lower in Sup2 than in Sup1, although enzyme enrichment was expected. Our experimental conditions may account for that finding, e.g. the existence of membrane-bound 17βHSD2 catalyzing the conversion of E2 to E1, the absence of cofactors increasing the enzymatic activity, and/or a reduced stability of the purified enzyme at low protein concentrations. Results were similar for the mammary 17βHSD1 purification procedure. However, due to the great variation of results, it was impossible to fully assess the relevance of this finding. Finally, mammary 17βHSD1 isolation was already complete after Sephadex-G-100 gel filtration, while two additional purification steps were necessary for placental 17βHSD1 isolation. Differences in the intracellular protein composition of the tissues analyzed may account for this observation that also indicates an easier separation of contaminating proteins from 17βHSD1 in breast tissue than in placenta. This hypothesis is supported by electrophoresis in our study, showing a considerably reduced amount of proteins in mammary Sup2 compared to placenta, but also by others having demonstrated the absence of a variety of placental proteins in normal breast tissueCitation42.
Our study revealed a non-significant inhibitory effect of testosterone on 17βHSD1 activity in both, placenta and breast tissue. However, the extent of enzyme inhibition was tissue-dependent, and mammary 17βHSD1 seemed to be more sensitive to co-incubated testosterone although this difference was not significant. Apart from 17βHSD1, previous studies found an inhibitory effect of testosterone on mammary aromataseCitation43, and STS activityCitation23, respectively. These in vitro findings support the hypothesis of an anti-estrogenic effect of testosterone on the mammary gland, which has previously been postulatedCitation44–46. However, long-term randomized placebo-controlled trials with testosterone in women are missing.
Similar to testosterone, our study demonstrated a non-significant inhibitory effect of tibolone metabolites on purified 17βHSD1. In placenta, Org30126 was the strongest 17βHSD1 inhibitor. In breast tissue, Org30126 and Org4094 displayed a comparable inhibitory effect on 17βHSD1. Unfortunately, due to only small amounts of purified mammary 17βHSD1 we were not able to perform analogous experiments for mammary 17βHSD1. However, in postmenopausal non-human primates, treatment with tibolone for two years has been shown to reduce local E2 formation in those subjects with low breast fat content suggesting breast composition being a relevant factor for the extent of local estrogen formationCitation33. Furthermore, in contrast to testosterone, tibolone’s safety on the mammary gland has been studied before in humans. In healthy postmenopausal women, tibolone has been shown to significantly reduce BC riskCitation2 while risk of BC recurrence was significantly increased in BC survivorsCitation47. The latter possibly relates to complex inter- and intra-cellular regulatory circuits blunting tibolone’s estrogen weakening effect observed in cell culture and healthy breast tissue.
In contrast to testosterone and tibolone metabolites, iCR did not affect purified 17βHSD1 activity at all. Similarly, black cohosh does not affect aromataseCitation48 but inhibits STS activity in vitroCitation24. However, in malignant MDA-MB123 cells 17βHSD1 activity was inhibited by black cohosh extracts at high concentrationCitation48. These contradictory findings might be due to black cohosh’s inhibitory action rather on STS than on 17βHSD1 activity, to its incubation in supra-physiological concentration, or to the presence of a 17βHSD1 isoform in MDA-MB-123 cells resistant to inhibition by black cohosh, respectively. The absent (17βHSD1, aromatase) or even beneficial effect (STS) of black cohosh on local estrogen metabolism is in agreement with previous studies in humans. Black cohosh has not been shown to increase BC risk in postmenopausal women. In contrast, there was a trend for BC risk reduction in healthy womenCitation49, and a reduced BC recurrence risk in breast BC survivorsCitation50. Furthermore, black cohosh does not increase mammographic breast density or breast cell proliferation in postmenopausal womenCitation51.
Our study has some limitations. First, due to small amounts of purified enzyme we were not able to perform analogous assays for each condition, and statistical analysis has to be interpreted with caution. Secondly, we had to use two different assays to measure 17βHSD1 activity. A modified radioactive activity assay (RAA)Citation32,Citation33 was utilized to demonstrate enzyme enrichment during the purification procedure. In contrast, enzyme kinetics were studied by a more convenient photometry-based method, which allows for continuous measurements without requiring huge amounts of enzyme. For comparison reasons, some aspects need to be considered. While the rate of E2 formation is measured directly by the RAA, E2 assessment is carried out indirectly via NADH oxidation by the photometric assay (PA). During the enzyme purification procedure the RAA was superior to the PA, since, at least during the early purification steps, the sample was contaminated by additional enzymes and substrates, which would have affected the assay result. During the purification procedure, the sample volume decreased continuously, ultimately limiting the sample volume available for activity assays. Since the amount of protein needed to perform the RAA is about 3-fold higher than for the PA, we used the latter for the kinetic measurements also allowing continuous measurements on a single enzyme sample. However, RAA and PA were equivalent when the purified enzyme activity was assessed. Finally, direct comparison of kinetic data between different studies may partially be hampered by differences in enzyme- and co-enzyme concentrationsCitation52, buffers, and pH used throughout the assay, as well as the time interval after which the assay reaction is stoppedCitation53.
Conclusions
The enzyme 17βHSD1 was successfully purified from human placenta and mammary gland. Basal activity was comparable, with a slightly higher enzyme activity of mammary 17βHSD1. Testosterone and tibolone metabolites inhibited purified 17βHSD1 activity in human placenta and breast tissue to a different though not significant extent. For testosterone, the inhibitory effect was stronger on mammary 17βHSD1, whereas Org4094, and Org30126 were stronger inhibitors of placental 17βHSD1. Black cohosh (iCR) had no impact on 17βHSD1 activity. Our data emphasize the role of local steroid metabolism for the action of both, endogenous molecules and exogenous therapeutic drugs. Therefore, comparisons of studies on purified enzymes with in vitro studies is mandatory to elucidate regulatory mechanisms which in turn improves the understanding of carcinogenesis.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Chlebowski RT, Anderson GL. Changing concepts: menopausal hormone therapy and breast cancer. J Natl Cancer Inst 2012;104:517–27
- Formoso G, Perrone E, Maltoni S, et al. Short and long term effects of tibolone in postmenopausal women. Cochrane Database Syst Rev 2012;2:CD008536
- de Gooyer ME, Deckers GH, Schoonen WG, et al. Receptor profiling and endocrine interactions of tibolone. Steroids 2003;68:21–30
- Notelovitz M. Postmenopausal tibolone therapy: biologic principles and applied clinical practice. MedGenMed: Medscape General Medicine 2007;9:2
- Ziaei S, Moghasemi M, Faghihzadeh S. Comparative effects of conventional hormone replacement therapy and tibolone on climacteric symptoms and sexual dysfunction in postmenopausal women. Climacteric 2010;13:147–56
- Labrie F, Belanger A, Cusan L, et al. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab 1997;82:2396–402
- Graziottin A, Koochaki PE, Rodenberg CA, Dennerstein L. The prevalence of hypoactive sexual desire disorder in surgically menopausal women: an epidemiological study of women in four European countries. J Sex Med 2009;6:2143–53
- Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Muhlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab 2000;85:645–51
- Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev 2012;9:CD007244
- Jarry H, Harnischfeger G, Duker E. The endocrine effects of constituents of Cimicifuga racemosa. In vitro binding of constituents to estrogen receptors. Planta medica 1985:316–19
- Burdette JE, Liu J, Chen SN, et al. Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J Agric Food Chem 2003;51:5661–70
- Gaube F, Wolfl S, Pusch L, et al. Gene expression profiling reveals effects of Cimicifuga racemosa (L.) NUTT. (black cohosh) on the estrogen receptor positive human breast cancer cell line MCF-7. BMC Pharmacol 2007;7:11
- Kenemans P, van der Mooren MJ. Androgens and breast cancer risk. Gynecol Endocrinol 2012;28:46–9
- Einbond LS, Soffritti M, Degli ED, et al. Chemopreventive potential of black cohosh on breast cancer in Sprague-Dawley rats. Anticancer Res 2012;32:21–30
- Subramanian A, Salhab M, Mokbel K. Oestrogen producing enzymes and mammary carcinogenesis: a review. Breast Cancer Res Treat 2008;111:191–202
- Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol 2006;102:89–96
- Desreux J, Kloosterboer H, Noel A, et al. Effects of tibolone on sulfatase pathway of estrogens metabolism and on growth of MCF-7 human breast tumors implanted in ovariectomized nude mice. Gynecol Obstet Invest 2007;63:31–8
- Raobaikady B, Day JM, Purohit A, et al. The nature of inhibition of steroid sulphatase activity by tibolone and its metabolites. J Steroid Biochem Mol Biol 2005;94:229–37
- Kloosterboer HJ. Tissue-selective effects of tibolone on the breast. Maturitas 2004 24;49:S5–15
- van de Ven J, Donker GH, Sprong M, et al. Effect of tibolone (Org OD14) and its metabolites on aromatase and estrone sulfatase activity in human breast adipose stromal cells and in MCF-7 and T47D breast cancer cells. J Steroid Biochem Mol Biol 2002;81:237–47
- Purohit A, Malini B, Hooymans C, Newman SP. Inhibition of oestrone sulphatase activity by tibolone and its metabolites. Horm Metab Res 2002;34:1–6
- Chetrite G, Kloosterboer HJ, Pasqualini JR. Effect of tibolone (Org OD14) and its metabolites on estrone sulphatase activity in MCF-7 and T-47D mammary cancer cells. Anticancer Res 1997;17:135–40
- Gotte M, Kalkhake K, Ploeger S, et al. Effect of testosterone on E1S-sulfatase activity in non-malignant and cancerous breast cells in vitro. J Steroid Biochem Mol Biol 2009;117:168–75
- Stute P, Nisslein T, Gotte M, et al. Effects of black cohosh on estrogen biosynthesis in normal breast tissue in vitro. Maturitas 2007 20;57:382–91
- Peltoketo H, Luu-The V, Simard J, Adamski J. 17beta-hydroxysteroid dehydrogenase (HSD)/17-ketosteroid reductase (KSR) family; nomenclature and main characteristics of the 17HSD/KSR enzymes. J Mol Endocrinol 1999;23:1–11
- Miettinen M, Mustonen M, Poutanen M, et al. 17Beta-hydroxysteroid dehydrogenases in normal human mammary epithelial cells and breast tissue. Breast Cancer Res Treat 1999;57:175–82
- Ghersevich S, Poutanen M, Tapanainen J, Vihko R. Hormonal regulation of rat 17 beta-hydroxysteroid dehydrogenase type 1 in cultured rat granulosa cells: effects of recombinant follicle-stimulating hormone, estrogens, androgens, and epidermal growth factor. Endocrinology 1994;135:1963–71
- Lewintre EJ, Orava M, Vihko R. Regulation of 17 beta-hydroxysteroid dehydrogenase type 1 by epidermal growth factor and transforming growth factor-alpha in choriocarcinoma cells. Endocrinology 1994;135:2629–34
- Piao YS, Peltoketo H, Jouppila A, Vihko R. Retinoic acids increase 17 beta-hydroxysteroid dehydrogenase type 1 expression in JEG-3 and T47D cells, but the stimulation is potentiated by epidermal growth factor, 12-O-tetradecanoylphorbol-13-acetate, and cyclic adenosine 3′,5′-monophosphate only in JEG-3 cells. Endocrinology 1997;138:898–904
- Ghersevich S, Akinola L, Kaminski T, et al. Activin-A, but not inhibin, regulates 17beta-hydroxysteroid dehydrogenase type 1 activity and expression in cultured rat granulosa cells. J Steroid Biochem Mol Biol 2000;73:203–10
- Stute P, Gotte M, Kiesel L. Differential effect of hormone therapy on E1S-sulfatase activity in non-malignant and cancerous breast cells in vitro. Breast Cancer Res Treat 2008;108:363–74
- Soderqvist G, Olsson H, Wilking N, et al. Metabolism of estrone sulfate by normal breast tissue: influence of menopausal status and oral contraceptives. J Steroid Biochem Mol Biol 1994;48:221–4
- Stute P, Register TC, Blair RM, Cline JM. Effects of tibolone on estrogen biosynthesis in the mammary tissue of postmenopausal monkeys. Menopause 2006;13:232–40
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54
- Lin SX, Yang F, Jin JZ, et al. Subunit identity of the dimeric 17 beta-hydroxysteroid dehydrogenase from human placenta. J Biol Chem 1992 15;267:16182–7
- Heukeshoven J, Dernick R. Simplified method for silver staining of proteins in polyacrylamide gels and the mechanism of silver staining. Electrophoresis 1985;6:103–12
- Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical J 2008;3:346–63
- Sherbet DP, Guryev OL, Papari-Zareei M, et al. Biochemical factors governing the steady-state estrone/estradiol ratios catalyzed by human 17beta-hydroxysteroid dehydrogenases types 1 and 2 in HEK-293 cells. Endocrinology 2009;150:4154–62
- Khan N, Sharma KK, Andersson S, Auchus RJ. Human 17beta-hydroxysteroid dehydrogenases types 1, 2, and 3 catalyze bi-directional equilibrium reactions, rather than unidirectional metabolism, in HEK-293 cells. Arch Biochem Biophys 2004;429:50–9
- Geoghegan KF, Song X, Hoth LR, et al. Unexpected mucin-type O-glycosylation and host-specific N-glycosylation of human recombinant interleukin-17A expressed in a human kidney cell line. Protein Expr Purif 2013;87:27–34
- Lewis L. Human placental 17β-estradiol dehydrogenase: characterization and structural studies. Cancer Commission of Harvard University Publication No. 1448 1973
- Inaba N, Renk T, Daume E, Bohn H. Ectopic production of placenta-“specific” tissue proteins (PP5 and PP11) by malignant breast tumors. Arch Gynecol 1981;231:87–90
- de Gooyer ME, Oppers-Tiemissen HM, Leysen D, et al. Tibolone is not converted by human aromatase to 7alpha-methyl-17alpha-ethynylestradiol (7alpha-MEE): analyses with sensitive bioassays for estrogens and androgens and with LC-MSMS. Steroids 2003;68:235–43
- Dimitrakakis C, Zhou J, Wang J, et al. A physiologic role for testosterone in limiting estrogenic stimulation of the breast. Menopause 2003;10:292–8
- Zhou J, Ng S, Adesanya-Famuiya O, et al. Testosterone inhibits estrogen-induced mammary epithelial proliferation and suppresses estrogen receptor expression. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 2000;14:1725–30
- Hofling M, Lundstrom E, Azavedo E, et al. Testosterone addition during menopausal hormone therapy: effects on mammographic breast density. Climacteric 2007;10:155–63
- Kenemans P, Bundred NJ, Foidart JM, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol 2009;10:135–46
- Rice S, Amon A, Whitehead SA. Ethanolic extracts of black cohosh (Actaea racemosa) inhibit growth and oestradiol synthesis from oestrone sulphate in breast cancer cells. Maturitas 2007;56:359–67
- Obi N, Chang-Claude J, Berger J, et al. The use of herbal preparations to alleviate climacteric disorders and risk of postmenopausal breast cancer in a German case-control study. Cancer Epidemiol Biomarkers Prev 2009;18:2207–13
- Henneicke-von Zepelin HH, Meden H, Kostev K, et al. Isopropanolic black cohosh extract and recurrence-free survival after breast cancer. Int J Clin Pharmacol Ther 2007;45:143–54
- Hirschberg AL, Edlund M, Svane G, et al. An isopropanolic extract of black cohosh does not increase mammographic breast density or breast cell proliferation in postmenopausal women. Menopause 2007;14:89–96
- Bonney RC, Reed MJ, Davidson K, et al. The relationship between 17 beta-hydroxysteroid dehydrogenase activity and oestrogen concentrations in human breast tumours and in normal breast tissue. Clin Endocrinol 1983;19:727–39
- Tait GH, Newton CJ, Reed MJ, James VH. Multiple forms of 17 beta-hydroxysteroid oxidoreductase in human breast tissue. J Mol Endocrinol 1989;2:71–80
