Abstract
Lactoperoxidase (LPO) catalyzes the oxidation of numerous of organic and inorganic substrates by hydrogen peroxide. It has very vital activity in the innate immune system by decreasing or stopping the activation of the bacteria in milk and mucosal secretions. This study’s purpose was to investigate in vitro effect of some phenolic acids (ellagic, gallic, ferulic, caffeic, quercetin, p-coumaric, syringic, catechol and epicatechin) on the purified LPO. This enzyme was purified from milk by using different methods such as Amberlite CG-50 resin, CM-Sephadex C-50 ion-exchange and Sephadex G-100 gel filtration chromatography. LPO was purified 28.7-fold with a yield of 20.03%. We found phenolic acids have inhibition effects on bovine LPO enzyme to different concentrations. Our study showed lower concentrations of caffeic acid, ferulic acid and quercetin exhibited much higher inhibitory effect on enzyme, so these three of them were clearly a more potent inhibitor than the others were. All of compounds were non-competitive inhibitors.
Introduction
Lactoperoxidase (LPO) (donor: H2O2 oxidoreductase E.C.1.11.1.7) is a member of oxidoreductase family and has been vital role in protecting the lactating mammary gland and the intestinal tract of newborn babies against pathogenic bacteria or organisms. Numerous researchers showed LPO is a glycoprotein and an important enzyme in milk and catalyzes the oxidation of several different reactions by hydrogen peroxide of a large range of substrates such as the oxidation of endogenous thiocyanate (SCN−) to the antibacterial hypothiocyanate (OSCN−). LPO is present in so many gland secretions such as saliva, tears and milkCitation1–6. LPO consists of a single polypeptide chain containing 612 amino acid residues, and molecular weight of LPO for bovine milk is about 80 kDaCitation7,Citation8.
Phenolic acids are the most important groups of secondary metabolites and bioactive compounds. There is an increasing awareness and interest in the past few years owing to their many potential benefits. They are both powerful antioxidants and demonstrate anticarcinogenic, antibacterial, antiviral and anti-inflammatory actionsCitation9,Citation10.
Ellagic acid is strong antioxidant and has been found in so many fruit and vegetable, therefore it has a numerous applications in food, pharmaceutical and chemical industriesCitation11. This molecule has many different important vital activities like antiviral activities radical chemopreventive and scavenging effectsCitation12. Gallic acid (3,4,5-trihydroxybenzoic acid) is natural antioxidant and represents a big family of plant secondary polyphenolic metabolites. Its derivatives are widely spread and are found in white tea, blackberry, hazelnut, gallnuts, etc.Citation13. Ferulic acid (3-hydroxy-4-methoxycinnamic acid-hydroxycinnamic acid) is a phenolic compound in most of the plants. It is used a bioactive ingredient of many foods. It is a natural antioxidant and has chemopreventive effects. Furthermore, it represses experimental carcinogenesis in the tongue, skin and colon of experimental animals, forestomach and lungsCitation14. Caffeic acid (3,4-dihydroxycinnamic) has a strong antioxidant effect on different systems. Caffeic acid and its derivatives have been showed very important effects on different cancer cell proliferationCitation15. Quercetin is a one of the flavonoid family and found in numerous plant, including, leaves, vegetables, grains, red wine and tea. Researchers have been reported that quercetin has many vital biologic properties to protection from the various illness such as different types of cancer, osteoporosis, aging and cardiovascular problemsCitation16. p-Coumaric acid is a natural hydroxycinnamic phenolic acid that exists in wine in an esterified form with tartaric acid, the tartaric p-coumaroyl ester. It can also exist as glucose heterosideCitation17.
Syringic acid is a naturally occurring o-methylated trihydroxybenzoic acid, a type of chemical compound. It can be found in the açaí palm (Euterpe oleracea) and its oil (1.073 mg/kg)Citation18, in Ardisia elliptica. Catechol was first isolated in 1839 by H. Reinsch by distilling catechin from catechu, the juice of Mimosa catechu (Acacia catechu L.). Catechol occurs in free form naturally in kino and in beechwood tar; its sulphonic acid has been detected in the urine of horse and humansCitation19. Catechol is produced industrially by direct hydroxylation of phenol with hydrogen peroxideCitation20. It is a common building block in organic synthesisCitation21. Epicatechin is a natural phenol antioxidant plant secondary metabolite. The term of catechin is also commonly used to refer to the related family of flavonoids and the subgroupCitation22.
In this work, we have purified LPO enzyme from bovine milk. First, we determined the inhibition effects some natural antioxidant phenols including ellagic acid, gallic acid, ferulic acid, caffeic acid, quercetin, p-coumaric acid, syringic acid, catechol and epicatechin on LPO. Previously, the inhibition kinetics of these molecules has not been reported. In this study, we reported LPO activity and kinetic constant (Ki) for these molecules in vitro.
Materials and methods
Purification of LPO
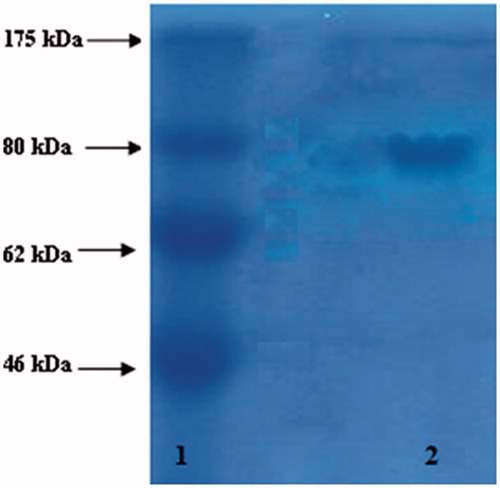
All of the purification steps made accordingly by Sisecioglu et al.Citation23–26. Fractions of purified enzyme were lyophilized and SDS-PAGE gel electrophoreses used for the checking purity of enzymeCitation27 (). Protein concentration was measured according to the method of Lowry using bovine serum albumin as a standardCitation28.
Figure 1. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of purified LPO. Column 1: standard proteins: MBP (maltose-binding protein β-galactosidase, 175 kDa), MBP (maltose binding protein)-paramyosin (fusion of MBP and paramyosin, 80 kDa), maltose-binding protein and chitin binding domain (62 kDa), CBD-Mxe intein-2CBD (fusion of the chitin binding domain and the Mxe intein followed by two chitin-binding domains, 46 kDa). Column 2: purified LPO from bovine milk (80 kDa) (LPO: lactoperoxidase).
Determination of LPO activity
Shindler and Bardsley’sCitation29 method was used with a slight change for checking of activity of LPO. The 2.8 ml of 1 mM ABTS in 0.1 M phosphate buffer, pH 6.0, was mixed with 0.1 ml of enzyme sample in 1 mM phosphate buffer, pH 6.0, and 0.1 ml of 3.2 mM H2O2 solution. The absorbance was measured at 412 nm as a function of time in every 15 s during 3 min. The one unit of activity is defined as the amount of enzyme catalysing the oxidation of 1 μmol of ABTS min−1 at 298°K (molar absorption coefficient 32 400 M−1 cm−1)Citation30,Citation31.
The inhibition effects of phenolic acids on LPO activity
To determine the effects of molecules on LPO, enzyme activities were measured in the presence of ellagic acid (11–99 µM), gallic acid (59–176 µM), ferulic acid (2–39 µM), caffeic acid (19–41 µM), quercetin (5–49 µM), p-coumaric acid (20–142 µM), syringic acid (17–118 µM), catechol (36–55 µM) and epicatechin (34–103 µM) at cuvette concentrations. Control cuvette activity in the absence of inhibitor was taken as 100%. For each inhibitor, an activity (%)-[Inhibitor] graphs were drawn. For determination of the Ki constant, three different inhibitor concentration (11, 33 and 55 µM ellagic acid), (78, 118 and 157 µM gallic acid), (2, 10 and 20 µM ferulic acid), (19, 26 and 33 µM caffeic acid), (5, 15 and 25 µM quercetin), (41, 81 and 122 µM p-coumaric acid), (51, 84 and 118 µM syringic acid), (42, 46 and 49 µM catechol) and (46, 57 and 69 µM epicatechin) were used. In these studies, six different ABTS concentrations were used as substrate (0.167–0.5 mM). Ki constant obtained from the Lineweaver–Burk graph (1/V-1/[S]), and all inhibition type was found for all inhibitor (; ). Analysis of data obtained was made by t-test and they are given as X ± SD.
Figure 2. Lineweaver–Burk graph in five different substrate (ABTS) concentrations and in three different (A) ferulic acid and (B) quercetin concentrations for the determination of Ki.
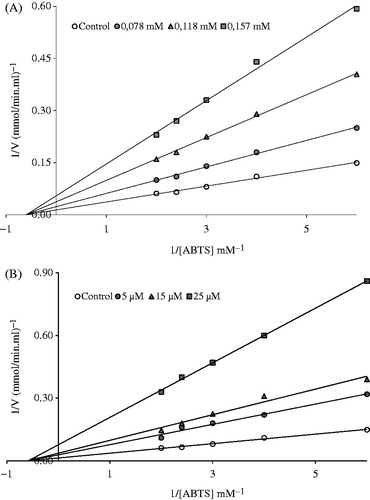
Table 1. Chemical structures and Ki values of phenolics compounds of LPO.
Results
CM Sephadex C-50 ion-exchange chromatography column used for purification of LPO and elution of each fraction were checked the Rz (A412/A280 nm). Fractions with Rz values 0.8 or higher were collected. The enzyme obtained from ion exchange chromatography was applied to a Sephadex G-100 gel filtration chromatography. Again, we collected 0.8 or higher values of Rz as shown (), specific activity was counted for the purified enzyme and crude extract solution, so yield of 0.18 mg (Rz: 0.8) and yielding a purification of 20.03-fold from 1 L bovine milk. Ki constant for these compounds as inhibitors of LPO were determined. Lineweaver–Burk graphs were used for the calculation of Ki values ().
Table 2. Purification steps of LPO from bovine milk.
Discussion
Milk is vital liquid secreted from the mammary glands of females of all mammal species. This mysterious liquid is playing crucial role for support their offsprings’ nutritional, immune and protective needs. Milk and its products play an important role in nutrition and have precisely benefits for humans. Milk is a rich source of many different substances such as proteins, enzyme, fat, minerals and vitaminsCitation32. Human milk is very important in terms of enzyme content, for example, lactoferrin, lysozyme, glycomacropeptide and LPO. Human milk has low LPO activity compared with cow’s milk. The both LPO variety show the same property and weightCitation32,Citation33. This system is the most significant microbial inhibitor in raw milk. LPO is naturally present in raw milk and together with thiocyanate and peroxide constitutes the LPO systemCitation30,Citation31,Citation33.
There are a few studies existing in the literature on the inhibitory effects of different compounds on LPO enzyme. Sisecioglu et al. Citation25 investigated the effects of propofol, a phenolic compound, on the LPO from bovine milk. They found that propofol is a competitive inhibitor with IC50 and Ki values 15.97 µM and 3.72 µM, respectivelyCitation25. Similarly, the competitive inhibitor effect of melatonin and serotonin on LPO activity was determined by same groupCitation26.
Haeme peroxidases are a class of enzyme that catalyse the organic and inorganic compounds by using hydrogen peroxides or alkyl hydroperoxides. At the same time, peroxidases are able to catalyse the oxidative coupling reactions of a broad range of phenolic compoundsCitation34,Citation35. LPO has a haeme group that is very tightly bound to the polypeptide chain. Sisecioglu et al.Citation36 discussed effects of norepinephrine on LPO in an article. It was reported that norepinephrine has phenolic hydroxyl group and it can bind the haeme group of LPO by this groupCitation36. Another study Monzani et al. reported that LPO undergoes inactivation during the oxidation of phenolsCitation37.
Phenolic acids are very important compounds for life, because they have important role and functions. These molecules include a lot of member. Numerous investigations were reported effects of these molecules on enzyme and different proteins; for instance, caffeine has anti-inflammatory and neuroprotective properties. Investigation of Anwar and friends showed caffeine has therapeutic role on cholinergic systemCitation38. Ferulic acid, caffeic acid, quercetin, gallic acid, syringic acid, catechol, p-coumaric acid and ellagic acid were showed inhibition effects on human carbonic anhydraseCitation39. These two researches exhibited, phenolic acids have beneficial or harmful effects on life. Therefore, we have to care when used it. LPO is very important protein owing to the fact that LPO find in the milk of all mammals and all of the mammal babies drink it. Our investigation showed, all phenolic compounds inhibited LPO. Ki values for all molecules were in the range of 2.83–28.29 µM. Gallic acid was a weak inhibitor (Ki of 28.29 µM) similar to the ellagic acid (Ki of 14.74 µM). The most effective inhibitor detected in this study in was caffeic acid (Ki of 2.83 µM). Furthermore, ferulic acid (Ki of 4.79 µM) and quercetin (Ki of 5.99 µM) were the other two best inhibitors ().
Conclusion
Although numerous benefits of phenolic acids and their effects on health are well known in the literatures, all of phenolic compounds used showed non-competitive type of inhibition effects on the purified enzyme in this study. Especially caffeic acid, ferulic acid and quercetin had greater inhibition on LPO than other phenolic acids. As it is known, LPO is very vital activity for innate immune system because of removing bacteria from milk and mucosal secretions. If enzyme activity is reduced, this means that immune system is weakened. But this is particularly undesirable since this affects the immune system of infants.
Declaration of interest
The authors declared that there is no conflict of interest.
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no RGP-VPP-254.
References
- Dumonte C, Rousst B. Identification, purification, and characterization of a non-heme lactoperoxidase in bovine milk. J Biol Chem 1983;258:14166–72
- Shin K, Tomita M, Lonnerdal B. Identification of lactoperoxidase in mature human milk. J Nutr Biochem 2000;11:94–102
- Morin DE, Rowan LL, Hurley WL. Comparative-study of proteins, peroxidase-activity and N-acetyl-beta-d-glucosaminidase activity in llama milk. Small Ruminant Res 1995;17:255–61
- Reiter B, Perrandin JP. Peroxidase in chemistry and biology. Vol. 1. Boca Raton (FL): CRC Press; 1991
- Spiegeleer PD, Sermon J, Vanoirbeek K, et al. Role of porins in sensitivity of Escherichia coli to antibacterial activity of the lactoperoxidase enzyme system. Appl Environ Microb 2005;71:3512–8
- De-Wit JN, Van-Hooydonk ACM. Structure, functions and applications of lactoperoxidase in natural antimicrobial systems. Neth Milk Dairy J 1996;50:227–44
- Cals M, Maillierat P, Birignon G, et al. Primary structure of bovine lactoperoxidase, a 4th member of a mammalian heme peroxidesa family. Eur J Biochem 1991;198:733–9
- Paul KG, Ohlsson PI. The lactoperoxidase system: chemistry and biological significance. Vol. 27. New York: Marcel Dekker Inc.; 1985
- Robbiris RJ. Phenolic acids in foods: an overview of analytical methodology. J Agric Food Chem 2003;51:2866–87
- Matilla P, Hellström J. Phenolic acids in potatoes, vegetables and some of their products. J Food Comp Anal 2007;20:152–60
- Mullen W, Yokota T, Lean MEJ, Crozier A. Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC-MS. Phytochemistry 2003;64:617–24
- Huang W, Niu H, Li Z, et al. Ellagic acid from acorn fringe by enzymatic hydrolysis and combined effects of operational variables and enzymes on yield of the production. Biores Technol 2008;99:1518–25
- Lu Z, Nie G, Belton PS, et al. Structure activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem Int 2006;48:263–74
- Anselmi C, Bernardi F, Centini M, et al. Interaction of ferulic acid derivatives with human erythrocytes monitored by pulse field gradient NMR diffusion and NMR relaxation studies. Chem Phys Lipids 2005;134:109–17
- Rajendra PN, Karthikeyan A, Karthikeyan S, Reddy BV. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol Cell Biochem 2011;349:11–19
- Boots A, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 2008;585:325–37
- Salameh D, Brandam C, Medawar W, et al. Highlight on the problems generated by p-coumaric acid analysis in wine fermentations. Food Chem 2008;107:1661–7
- Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.). J Agric Food Chem 2008;25:4631–6
- Zheng LT, Rhu GM, Kwon BM, et al. Anti-inflammatory effects of catechols in lipopolysaccharide-stimulated microglia cells: inhibition of microglial neurotoxicity. Eur J Pharmacol 2008;588:106–13
- Ullmann’s Encyclopedia of Industrial Chemistry. 6th ed. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co.; 2003:638
- Almeida WLC, Vitor DN, Pereira MRG, et al. Redox properties of ruthenium complex with catechol are involved in toxicity to glial cells. J Chil Chem Soc 2007;52:1240–3
- Hou WC, Lin RD, Chen CT, Lee MH. Monoamine oxidase B (MAO-B) inhibition by active principles from Uncaria rhynchophylla. J Ethnopharmacol 2005;100:216–20
- Cals M, Maillierat P, Birignon G, et al. Primary structure of bovine lactoperoxidase, a fourth member of a mammalian heme peroxidase family. Eur J Biochem 1991;198:733–9
- Khmelnistky YL, Levashov AV, Klyachko NL, Martinek K. Engineering biocatalytic systems in organic media with low water content. Enzyme Microb Technol 1988;10:710–24
- Sisecioglu M, Cankaya M, Gulcin I, Ozdemir H. The inhibitory effect of propofol on lactoperoxidase. Protein Peptide Lett 2009;16:46–9
- Şişecioğlu M, Çankaya M, Gülçin İ, Özdemir M. Interactions of melatonin and serotonin to lactoperoxidase enzyme. J Enzyme Inhib Med Chem 2010;25:779–83
- Laemmli DK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5
- Segel IH. Biochemical calculations. New York: John Wiley and Sons, Inc.; 1968:403
- Shindler JS, Bardsley WG. Steady-state kinetics of lactoperoxidase with ABTS as chromogen. Biochem Biophys Res Commun 1975;67:1307–12
- Ozdemir H, Uguz MT. In vitro effects of some anaesthetic drugs on lactoperoxidase enzyme Activity. J Enzyme Inhib Med Chem 2005;20:491–5
- Ozdemir H, Aygul I, Kufrevioglu OI. Purification of lactoperoxidase from bovine milk and investigation of the kinetic properties. Prep Biochem Biotech 2001;31:125–34
- Darewicz M, Dziuba B, Minkiewicz P, Dziuba J. The preventive potential of milk and colostrum proteins and protein fragments. Food Rev Int 2011;27:357–88
- Haddain MS, Ibrahim SA, Robinson RK. Preservation of raw milk by activation of the natural lactoperoxidase systems. Food Control 1996;7:149–52
- Hamid H, Rehman KU. Potential applications of peroxidases. Food Chem 2009;15:1177–86
- Chanwun T, Muhamad N, Chirapongsatonkul N, Churngchow N. Hevea brasiliensis cell suspension peroxidase: purification, characterization and application for dye decolorization. AMB Express 2013;3:14. doi:10.1186/2191-0855-3-14
- Sisecioglu M, Gülçin İ, Cankaya M, et al. The effects of norepinephrine on lactoperoxidase enzyme (LPO). Sci Res Essays 2010;5:1351–6
- Monzani E, Gatti AL, Profumo A, et al. Oxidation of phenolic compounds by lactoperoxidase. Evidence for the presence of a low-potential compound II during catalytic turnover. Biochemistry 1997;36:1918–26
- Anwar J, Spanevello RM, Thome G, et al. Effects of caffeic acid on behavioral parameters and on the activity of acetylcholinesterase in different tissues from adult rats. Pharm Biochem Behav 2012;103:386–94
- Sarikaya SBO, Gulcin I, Supuran CT. Carbonic anhydrase inhibitors: inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem Biol Drug Des 2010;75:515–20
