Abstract
Overproduction of reactive oxygen species results in oxidative stress that can cause fatal damage to vital cell structures. It is known that the use of antioxidants could be beneficial in the prevention or delay of numerous diseases associated with oxidative stress. Melatonin (MLT) is known as a powerful free-radical scavenger and antioxidant. It was found that indole ring of MLT can be employed by bioisosteric replacement by other aromatic rings. Quinoline derivatives constitute an important class of compounds for new drug development. Owing to quinoline and hydrazones appealing physiological properties and are mostly found in numerous biologically active compounds a series of quinoline-2-carbaldehyde hydrazone derivatives were synthesized as bioisosteric analogues of MLT, characterized and in vitro antioxidant activity was investigated by evaluating their reducing effect against oxidation of a redox-sensitive fluorescent probe. Cytotoxicity potential of all compounds was investigated both by lactate dehydrogenase leakage assay and by MTT assay.
Introduction
The balance between free radicals and the antioxidant system of the body has a vital importance in maintaining the health status of organismCitation1–3. Reactive oxygen species (ROS) and reactive nitrogen species are well known as both harmful and beneficial species. Oxidative stress that gives increase to generation of mainly ROS has been connected with an extensive range of diseases including those of cardiovascular, inflammatory, neurodegenerative, and autoimmune originCitation4,Citation5.
In recent years, various biological activities of melatonin (MLT) have been defined resulting in much attention in the development of synthetic derivatives possessing antioxidant activityCitation6–9. These derivatives have structural resemblance to MLT, being derivatives of either substituted or bioisosteric moieties of the indole ringCitation10,Citation11.
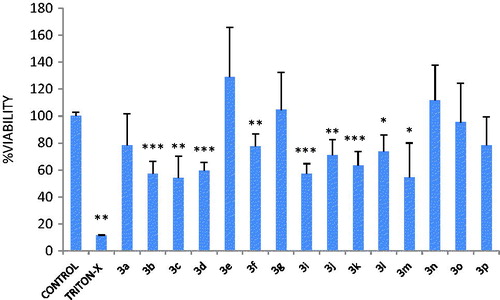
In our ongoing study, we have synthesized various series of indole derivatives, with some modifications on MLT ()Citation4,Citation6–9. The synthesized bioisosteric MLT analogues have been evaluated for their potential radical scavenging activity and protective effect against oxidative damageCitation4,Citation6–9. A review on the structure activity relationship of several of these derivatives has been previously published by SuzenCitation12. Most of the compounds exhibited noteworthy antioxidant activity in DPPH scavenging, superoxide dismutase, and lipid peroxidation assays. To determine the structural parameters of the antioxidant activity substitutions on the second position of the indole ring and of the alkyl chain of the acyl group have been underlined. Substitution of the indole ring of MLT with a naphthalene or quinolone ring leads to compounds of similar behaviorCitation13. Quinoline and their derivatives play important roles in the synthesis of natural products and as therapeutic agents, and constitute an important class of compounds for new drug development. They have been synthesized by many researchers as model compoundsCitation14 and found to exhibit different pharmacological activities covering anti-cancer, anti-mycobacterial, anti-microbial, anti-convulsant, anti-inflammatory, and cardiovascular activitiesCitation15–19.
Figure 1. (a) Melatonin, (b) melatonin analogue indole-3-carboxaldehyde hydrazones that have higher antioxidant activity than MLTCitation4,Citation6–9.
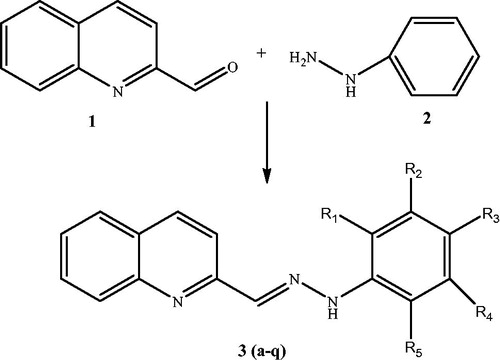
It has been experimentally established that some quinolone derivatives can function as free-radical scavengersCitation20–22. Synthetic quinoline derivatives such as 2-chloroquinoline-3-carboxaldehydesCitation14, spiro-substituted 4-hydroxypyranoquinolinonesCitation21, and pyrimido quinoline derivativesCitation23 have shown significant antioxidant activity. In this study, quinoline derivatives were synthesized in order to investigate whether the quinoline is a substantial heterocyclic bioisoster of the MLT indole moiety. A series of quinoline-2-carboxaldehydes hydrazones (3a–q) were synthesized ( and ), characterized, and their antioxidant activity was investigated in vitro by evaluating their reducing effect against oxidation of a redox-sensitive fluorescent probe, 2′,7′-dichlorofluorescin-diacetate (DCFH-DA), in the presence of cumene hydroperoxide. The results were compared with MLT, as the parent compound. The cytotoxicity of all compounds was investigated in two assays in Chinese hamster ovary (CHO-K1) cells; the lactate dehydrogenase (LDH) leakage assay and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Seventeen compounds were characterized on the basis of 1H and 13C NMR, Mass, and FT-IR spectra, and elemental analysis. Compounds 3a–dCitation24 were published previously.
Table 1. Quinoline-2 carboxaldehyde hydrazones.
Materials and methods
In this study, a series of new quinoline-2-carbaldehyde hydrazones (3a–q) was developed () as bioisosteric analogues of MLT. The target hydrazones derived from quinoline-2-carboxaldehyde 1 and the appropriate hydrazine derivatives 2 () using simple reaction strategies that have been adoptedCitation25. Phenyl hydrazine derivatives and quinoline-2-carboxaldehyde were heated in the presence of ethanol. The chemical reagents used in synthesis were purchased from Sigma (Steinheim, Germany) and Aldrich (St. Louis, MO). Uncorrected melting points were determined with a Büchi SMP-20 apparatus (Sigma, Steinheim, Germany). The 1H and 13C NMR spectra were recorded with a Varian 400 MHz using TMS as an internal standard and DMSO-d6 as a solvent. ESI mass spectra were determined on a Waters micromass ZQ spectrometer (Waters, Milford, MA), FT-IR spectra were recorded on a Jasco 420Fourier instrument (Jasco, Portland, OR). Elemental analyses were performed using CHNS-932 (LECO, St. Joseph, MI). All spectral analysis was performed at Ankara University, Faculty of Pharmacy, Central Laboratory.
General procedure for the synthesis of compounds 3a–q
Quinoline-2-carboxaldehyde 1 (0.1 mmol) was reacted with phenyl hydrazine or its derivatives (0.13 mmol) in 10 ml of EtOH, in the presence of 0.5 g CH3COONa on the hot water bath. Upon cooling, the precipitate was collected and washed with cold EtOH. The crude product was purified by column chromatography using chloroform: i-propanol (100:1) as an eluent to give 3a–r. The final compounds were obtained as a mixture of E/Z stereoisomers.
2-[2-(2′,4′-difluorophenyl)hydrazinylidene]methyl}quinoline (3e): Yield 57.8%, m.p. 156–157 °C; 1H-NMR 7.04 (1H, m, H-6′), 7.27 (1H, m, H-3′), 7.55 (1H, m, H-5′), 7.63 (1H, m, H-8), 7.73 (1H, m, H-5), 7.94 (2H, t, H-6, H-7), 8.13 (1H, d, H-3), 8.26 (1H, s, CH), 8.30 (1H, d, H-4), 10.81 (1H, s, NH); EIMS m/z, 284 (M + H, %100). Anal. Calcd. for C16H11F2N3: C, 67.84%; H, 3.91%; N, 14.83%; found: C, 67.51%; H, 4.31%; N, 14.93%.
2-[2-(2′,5′-difluorophenyl)hydrazinylidene]methyl}quinoline (3f): Yield 73.5%, m.p. 183–184 °C; 1H-NMR 6.61 (1H, m, H-4′), 7.22 (1H, m, H-6′), 7.40 (1H, m, H-3′), 7.56 (1H, m, H-8), 7.73 (1H, m, H-5), 7.95 (2H, t, H-6, H-7), 8.20 (1H, d, H-3), 8.31 (1H, s, CH), 8.32 (1H, d, H-4), 11.02 (1H, s, NH); EIMS m/z, 284 (M + H, %100). Anal. Calcd. for C16H11F2N3: C, 67.84%; H, 3.91%; N, 14.83%; found: C, 68.24%; H, 4.14%; N, 14.87%.
2-[2-(3′,5′-difluorophenyl)hydrazinylidene]methyl}quinoline (3g): Yield 59.0%, m.p. 218–220 °C; 1H-NMR 6.58 (1H, m, H-4′), 6.79 (2H, dd, H-2′; H-6′), 7.56 (1H, m, H-8), 7.73 (1H, m, H-5), 7.95 (2H, m, H-6, H-7), 8.03 (1H, s, CH), 8.17 (1H, d, H-3), 8.31 (1H, d, H-4), 11.22 (1H, s, NH); EIMS m/z, 284 (M + H, %100). Anal. Calcd. for C16H11F2N3: C, 67.84%; H, 3.91%; N, 14.83%; found: C, 68.08%; H, 3.88%; N, 14.87%.
2-[2-(2′-chlorophenyl)hydrazinylidene]methyl}quinoline (3h): Yield 33.0%, m.p. 136–138 °C; 1H-NMR 6.86 (1H, m, H-5′), 7.30 (1H, t, H-4′), 7.36 (1H, dd, H-8), 7.55 (1H, m, H-6′), 7.72 (2H, m, H-5, H-3′), 7.96 (2H, dd, H-6, H-7), 8.15 (1H, d, H-3), 8.31 (1H, d, H-4), 8.47 (1H, s, CH), 10.49 (1H, s, NH); 13C-NMR 106.99, 115.46, 117.62, 121.72, 127.02, 127.7, 128.27, 128.99, 129.16, 130.27, 133.23, 136.65, 141.33 (=CH), 142.26, 147.90, 155.62; EIMS m/z, 282 (M + H, %100); 284.4 (M + H + 2). Anal. Calcd. for C16H12ClN3• 0.1H2O: C, 67.77%; H, 4.34%; N, 14.82%; found: C, 67.42%; H, 4.24%; N, 14.73%.
2-[2-(3′-chlorophenyl)hydrazinylidene]methyl}quinoline (3i): Yield 78.1%, m.p. 214–215 °C; 1H-NMR 6.84 (1H, dd, H-4′), 7.09 (1H, dd, H-6′), 7.25 (1H, s, H-2′), 7.30 (1H, t, H-5′), 7.57 (1H, t, H-8), 7.75 (1H, t, H-5), 7.97 (2H, t, H-6, H-7), 8.06 (1H, s, CH), 8.16 (1H, d, H-3), 8.33 (1H, d, H-4), 11.08 (1H, s, NH); 13C-NMR 111.94, 112.44, 118.04, 119.93, 127.11, 128.04, 128.60, 129.18, 130.49, 131.61, 134.70, 136.88, 138.96 (=CH), 146.68, 148.13, 155.38; EIMS m/z, 282 (M + H, %100); 284.3 (M + H + 2). Anal. Calcd. for C16H12ClN3• 0.1H2O: C, 67.77%; H, 4.34%; N, 14.82%; found: C, 67.41%; H, 4.43%; N, 14.72%.
2-[2-(4′-chlorophenyl)hydrazinylidene]methyl}quinoline (3j): Yield 60.3%, m.p. 205–207 °C; 1H-NMR 7.20 (2H, d, H-3′, H-5′), 7.33 (2H, d, H-2′, H-6′), 7.56 (1H, t, H-8), 7.74 (1H, t, H-5), 7.96 (2H, t, H-6, H-7), 8.04 (1H, s, CH), 8.13 (1H, d, H-3), 8.32 (1H, d, H-4), 11.04 (1H, s, NH); 13C-NMR 114.64, 117.93, 123.80, 127.02, 127.97, 128.58, 129.14, 129.77, 130.47, 136.78, 138.29 (=CH), 144.14, 148.15, 155.54; EIMS m/z, 282 (M + H, %100); 284.3 (M + H + 2). Orh 1: 281.74. Anal. Calcd. for C16H12ClN3• 0.5H2O: C, 66.09%; H, 4.51%; N, 14.45%; found: C, 65.67%; H, 4.83%; N, 14.35%.
2-[2-(2′,5′-dichlorophenyl)hydrazinylidene]methyl}quinoline (3k): Yield 37.7%, m.p. 181–183 °C; 1H-NMR 6.92 (1H, dd, H-4′), 7.43 (1H, d, H-3′), 7.60 (1H, m, H-8), 7.68 (1H, d, H-6′), 7.77 (1H, m, H-5), 8.00 (2H, t, H-6, H-7), 8.21 (1H, d, H-3), 8.36 (1H, d, H-4), 8.51 (1H, s, CH), 10.67 (1H, s, NH); 13C-NMR 114.12, 115.73, 118.00, 120.44, 127.18, 127.99, 128.39, 129.13, 130.32, 131.47, 133.39, 136.83 (=CH), 142.46, 147.88, 154.92; EIMS m/z, 316 (M + , %100); 318 (M + 2); 320 (M + 4). Orh 7: Anal. Calcd. for C16H11Cl2N3: C, 60.77%; H, 3.51%; N, 13.29%; found: C, 60.50%; H, 3.69%; N, 13.31%.
2-[2-(3′,4′-dichlorophenyl)hydrazinylidene]methyl}quinoline (3l): Yield 57.6%, m.p. 219–221 °C; 1H-NMR 7.07 (1H, dd, H-6′), 7.36 (1H, d, H-2′), 7.45 (1H, d, H-5′), 7.54 (1H, t, H-8), 7.71 (1H, t, H-5), 7.93 (2H, t, H-6, H-7), 8.02 (1H, s, CH), 8.13 (1H, d, H-3), 8.29 (1H, d, H-4), 11.11 (1H, s, NH); 13C-NMR 113.52, 114.11, 118.08, 121.32, 127.20, 128.09, 128.60, 129.21, 130.51, 131.76, 132.49, 136.90, 139.61(=CH), 145.29, 148.12, 155.19; EIMS m/z, 316 (M+, %100); 318 (M + 2); 320 (M + 4). Anal. Calcd. for C16H11Cl2N3: C, 60.77%; H, 3.51%; N, 13.29%; found: C, 60.47%; H, 3.66%; N, 13.24%.
2-[2-(3′,5′-dichlorophenyl)hydrazinylidene]methyl}quinoline (3m): Yield 49.1%, m.p. 242–244 °C; 1H-NMR 6.94 (1H, t, H-4′), 7.12 (2H, d, H-2′, H-6′), 7.56 (1H, t, H-8), 7.73 (1H, t, H-5), 7.95 (2H, dd, H-6, H-7), 8.03 (1H, s, CH), 8.16 (1H, d, H-3), 8.31 (1H, d, H-4), 11.19 (1H, s, NH); 13C-NMR 111.43, 118.21, 119.16, 127.33, 128.17, 128.63, 129.26, 130.56, 135.52, 137.02, 140.39 (=CH), 147.51, 148.10, 155.02; EIMS m/z, 316 (M+, %100); 318 (M + 2); 320 (M + 4). Anal. Calcd. for C16H11Cl2N3• 0,5H2O: C, 59.10%; H, 3.72%; N, 12.92%; found: C, 58.79%; H, 3.79%; N, 12.99%.
2-[2-(2′-bromophenyl)hydrazinylidene]methyl}quinoline (3n): Yield 35.3%, m.p. 144–146 °C; 1H-NMR 6.81 (1H, t, H-4′), 7.34 (1H, t, H-5′), 7.52 (1H, dd, H-3′), 7.57 (1H, d, H-6′), 7.56 (1H, d, H-8), 7.73 (1H, t, H-5), 7.96 (2H, dd, H-6, H-7), 8.15 (1H, d, H-3), 8.32 (1H, d, H-4), 8.48 (1H, s, CH), 10.26 (1H, s, NH); 13C-NMR 107.04, 115.66, 117.82, 121.82, 126.98, 127.87, 128.37, 129.07, 129.16, 130.27, 133.23, 136.65, 141.03 (=CH), 142.36, 147.90, 155.32; EIMS m/z, 326 (M+, %100); 328 (M + 2). Anal. Calcd. For C16H12BrN3• 0.25H2O : C, 58.11%; H, 3.81%; N, 12.71%; found: C, 57.86%; H, 3.95%; N, 12.65%.
2-[2-(3′-bromophenyl)hydrazinylidene]methyl}quinoline (3o): Yield 54.3%, m.p. 218–220 °C; 1H-NMR 7.00 (1H, d, H-6′), 7.13 (1H, d, H-4′), 7.24 (1H, t, H-5′), 7.38 (1H, s, H-2′), 7.57 (1H, t, H-8), 7.75 (1H, t, H-5), 8.00 (2H, q, H-6, H-7), 8.05 (1H, s, CH), 8.15 (1H, d, H-3), 8.33 (1H, d, H-4), 11.07 (1H, s, NH); 13C-NMR 112.06, 115.037, 117.80, 122.59, 123.02, 126.89, 127.81, 128.37, 128.96, 130.27, 131.69, 136.65, 138.77 (=CH), 146.55, 147.91, 155.13; EIMS m/z, 326 (M+, %100); 328 (M + 2). Anal. Calcd. for C16H12BrN3: C, 58.91%; H, 3.71%; N, 12.88%; found: C, 58.53%; H, 3.71%; N, 12.79%.
2-[2-(4′-bromophenyl)hydrazinylidene]methyl}quinoline (3p): Yield 55.2%, m.p. 210–212 °C; 1H-NMR 7.13 (2H, dd, H-5′, H-3′), 7.42 (2H, dd, H-2′, H-6′), 7.54 (1H, m, H-8), 7.72 (1H, m, H-5), 7.94 (2H, q, H-6, H-7), 8.01 (1H, s, CH), 8.11 (1H, d, H-3), 8.29 (1H, d, H-4), 11.02 (1H, s, NH); 13C-NMR 111.22, 114.89, 117.70, 126.81, 127.75, 128.36, 128.92, 130.25, 132.38, 136.56, 138.17 (=CH), 144.27, 147.92, 155.28; EIMS m/z, 326 (M+, %100); 328 (M + 2). Anal. Calcd. for C16H12BrN3• 0.25H2O: C, 58.11%; H, 3.81%; N, 12.71%; found: C, 57.79%; H, 3.75%; N, 12.61%.
2-[2-(2′,4′-dinitrophenyl)hydrazinylidene]methyl}quinoline (3q): Yield 11.9%, m.p. 255–257 °C; 1H-NMR 7.63 (1H, t, H-8), 7.78 (1H, t, H-5), 8.01 (2H, t, H-6, H-7), 8.22 (2H, t, H-4, H-3), 8.38 (1H, d, H-6′), 8.43 (1H, d, H-5′), 8.83 (2H, s, H-3′), 11.93 (1H, s, NH); EIMS m/z, 338 (M + H, %100). Anal. Calcd. for C16H11N5O4: C, 56.97%; H, 3.29%; N, 20.76%; found: C, 57.20%; H, 3.22%; N, 20.40%.
Biological activity studies
CHO-K1 cells were cultured in Ham’s F12 medium supplemented with 10% fetal bovine serum, 1% of a 100 U/ml penicillin–streptomycin solution, 2 mM (final concentration) l-glutamine, and 1 mM (final concentration) sodium pyruvate at 37 °C, in 5% CO2 atmosphere. This medium was used in all cell incubations.
Antioxidant activity on ROS-induced DCFH-DA oxidation
Cells were seeded at a density of 5 × 103 cells/well in black 96-well plates and incubated for 24 h at 37 °C in a humid atmosphere containing 5% CO2 for cell attachment. The medium in the wells was removed and then the cells were incubated with DCFH-DA (20 μM) containing medium for 1 h. Cells were washed with PBS to remove excess DCFH-DA. 10 µM-synthesized compounds and 1 µM cumene hydroperoxide were added into medium. The production of fluorescent DCF was evaluated by monitoring the fluoresence intensity at 488 nm excitation, and 530 nm emission wavelength for 60 minCitation26.
Cytotoxic effect via LDH assay
CHO cells were seeded at a density of 10 × 103 cells/well in 96-well plates and incubated for 24 h at 37 °C in a humid atmosphere containing 5% CO2 for cell attachment. Cytotoxic effects of the compounds at 10 µM concentration and for 24 h incubation were evaluated via the LDH activity assay according to the method of Hassoun et al.Citation27 with a minor modification. The activity of LDH in 100 μl of media was determined by direct calculation based on the decrease in absorbanceCitation28.
Cytotoxic effect via MTT assay
Cells were seeded at a density of 5 × 103 cells/well in 96-well plates and incubated at 37 °C in a humid atmosphere containing 5% CO2 for 24 h for cell attachment. The cells were treated with the newly synthesized compounds (10 µM) for 24 h. Control (medium only) and positive control (15 µM Triton X-100) were included in every experiment. Following the exposure period, the medium was removed, cells were washed with PBS, and then incubated with MTT at a final concentration of 0.5 mg/ml for 4 h at 37 °C. The medium was then removed and formed formazan crystals were dissolved in 150 µl of dimethyl sulfoxide. The absorbance was recorded at 550 nm on a microplate reader. Each experiment was repeated on three separate days. The ratio of the absorbance of treated samples to the absorbance of control (taken as %100) was expressed as % cell viability.
Results and discussion
Effects of synthesized hydrazone derivatives on cellular ROS
For the estimation of ROS inside cells, DCFH-DA was used as a probe. In cellular systems, non-fluorescent probe DCFH-DA readily crosses the cell membrane and undergoes hydrolysis by intracellular estrases to non-fluorescent 2′,7′-dichlorofluorescin (DCFH). DCFH is then rapidly oxidized in the presence of ROS to highly fluorescent 2′,7′- dichlorofluorescein (DCF)Citation29.
The protective effect of the newly synthesized MLT analogues against DCFH-DA oxidation was determined in CHO cells that were preloaded with the fluorescent probe. Oxidation of the probe located in the cytosol was screened at various time intervals up to 60 min. It is observed that all the newly synthesized hydrazone derivatives, except 3f, have potent antioxidant activity on DCFH oxidation, even higher than the parent compound MLT. Compound 3a, the only compound bearing no halogen on the aromatic ring, was found to be the most potent antioxidant (%24 decreased the control oxidation values) among all tested compounds. According to the structure activity relationship, mono-halogenated derivatives were found to have better antioxidant effect compared with the di-halogenated derivatives ().
Cytotoxic effect of the synthesized hydrazone derivatives
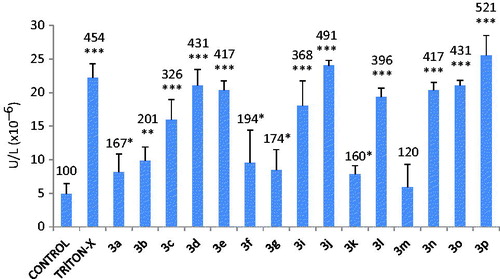
LDH is a cytoplasmic enzyme and its leakage from injured cells into the culture medium has been shown to be useful as an indicator of cellular membrane damage. In the present study, we determined LDH activity in the culture medium in order to assess effect of hydrazone derivatives on membrane integrity.
The MTT assay relies on the ability of live but not dead cells to reduce a water-soluble yellow dye, MTT, to water-insoluble purple formazan crystals. Since the substrate and the product absorb at very different wavelengths, no washing step is required which is a clear advantage of this assay and makes it a useful tool for drug screening studies.
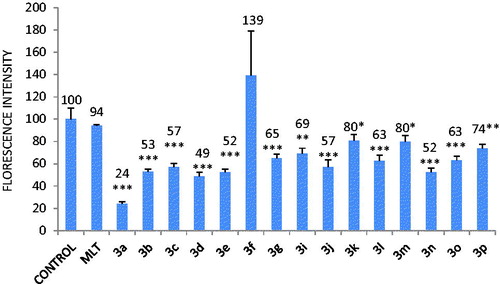
The cells were incubated with the compounds (10 µM) for 24 h in LDH assay. As can be seen in , most of hydrazone derivatives induced cell membrane damage. LDH leakage into the medium was statistically insignificant with 3 m and 3a. The most cytotoxic hydrazone derivative was found to be 3p which has p-bromine substitution on aromatic ring. In contrast, 3e, 3g and 3n were detected to have no cytotoxic effect in MTT assay. The possible reason for it is the diversity of parameters between assays. Results indicate that except 3a and 3m, other hydrazone derivatives have strong membrane damaging effect with longer exposure periods (24 h).
Figure 4. Lactate dehydrogenase (LDH) activity in the medium leaked from the cytosol of CHO-K1 cells incubated with 10 µM quinolone derivatives. Values are mean ± SD of three individual experiments. Values above the bars are % control values. *p < 0.05, **p < 0.005, ***p < 0.0005, compared with control values.
10 µM of 3b, 3c, 3d, 3f, 3i, 3j, 3k, 3l, and 3m decreased cell viability in MTT assay after incubation with CHO-K1 cells for 24 h ( and ). This effect was found to be related to the type of aromatic halogen substitution; mono-F and mono-Cl substances decreased cell viability while mono-Br did not show such an effect. Among dihalogenated compounds p-dihalogened ones were found to be more cytotoxic than m- and o-dihalogenated compounds. The only substance without any halogene substitution on aromatic ring, 3a, did not have any negative effect on cell viability.
Declaration of interest
The authors report no conflicts of interest. The chemical compounds of this work were supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK) Research and Development Grant 112S599. The cell culture facility was established by the support of TUBITAK 108S202 Grant.
References
- Suzen S. Recent developments of melatonin related antioxidant compounds. Com Chem High T Synt 2006;9:409–19
- Suzen S. Antioxidant activities of synthetic indole derivatives and possible activity mechanisms. In: Khan MTH, ed. Heterocyclic chemistry, bioactive heterocycles, Vol. 11. Berlin, Heidelberg: Spinger-Verlag; 2007:145–78 (Chapter V)
- Suzen S. Evaluation synthetic melatonin analogue antioxidant compounds. In: Srinivasan V, Gobbi G, Shillcutt DS, Suzen S, eds. Melatonin: therapeutic value and neuroprotection. Oxford: CRC Press, Taylor and Francis; 2014:250–68
- Karaaslan C, Kadri H, Coban T, et al. Synthesis and antioxidant properties of substituted 2-phenyl-1H-indoles. Bioorg Med Chem Lett 2013;23:2671–4
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002;82:47–95
- Suzen S, Tekiner-Gulbas B, Shirinzadeh H, et al. Antioxidant activity of indole-based melatonin analogues in erythrocytes and their voltammetric characterization. J Enz Inhib Med Chem 2013;28:1143–55
- Yilmaz Ad, Coban T, Suzen S. Synthesis and antioxidant activity evaluations of melatonine based analogue indole-hydrazide/hydrazone derivatives. J Enz Inhib Med Chem 2012;27:428–36
- Gurkok G, Coban T, Suzen S. Melatonin analogue new indole hydrazide/hydrazone derivatives with antioxidant behavior: synthesis and discussion on structure activity relationships. J Enzym Inhib Med Chem 2009;24:506–15
- Shirinzadeh H, Eren B, Gurer-Orhan H, et al. Novel indole-based analogs of melatonin: synthesis and in vitro antioxidant activity studies. Molecules 2010;15:2187–202
- Mathé-Allainmat M, Andrieux J, Langlois M. Recent developments in melatonin receptor ligands. Expert Opin Therapeut Patents 1997;7:1447–58
- Iakovou K, Varvaresou A, Kourounakis AP, et al. Design, synthesis and biological evaluation of novel conformationally restrained melatoninergic analogs. J Pharm Pharmacol 2002;54:147–56
- Suzen S. Melatonin and synthetic analogs as antioxidants. Curr Drug Deliv 2013;10:71–5
- Ancizu S, Castrillo N, Pérez-Silanes S, et al. New quinoxaline derivatives as potential MT1 and MT2 receptor ligands. Molecules 2012;17:7737–57
- Puskullu MO, Tekiner B, Suzen S. Recent studies of antioxidant quinoline derivatives. Mini Rev Med Chem 2013;13:365–72
- Larsen RD, Corley EG, King AO, et al. Practical route to a new class of LTD4 receptor antagonists. J Org Chem 1996;61:3398–405
- Roma G, Braccio MD, Grossi G, et al. 1,8-Naphthyridines IV, 9-substituted N,N-dialkyl-5-(alkylamino or cycloalkyl amino) [1,2,4] triazolo [4,3-a] [1,8] naphthyridine-6-carboxamides, new compounds with antiaggressive and potent anti- inflammatory activities. Eur J Med Chem 2000;35:1021–35
- Chen YL. Fang KC, Sheu JY, et al. Synthesis and antibacterial evaluation of certain quinolone derivatives. J Med Chem 2001;44:2374–7
- Kauffman GS, Harris GD, Dorow RL, et al. An efficient chiral moderator prepared from inexpensive (+)-3-carene: synthesis of the HIV-1 non-nucleoside reverse transcriptase inhibitor DPC 963. Org Lett 2000;2:3119–21
- Kumar S, Bawa S, Gupta H. Biological activities of quinoline derivatives. Mini Rev Med Chem 2009;9:1648–54
- Subashini R, Roopan SM, Khan FN. Synthesis and free radical scavenging property of some quinoline derivatives. J Chil Chem Soc 2010;55:317–19
- Panteleon V, Kostakis IK, Marakos P, et al. Synthesis of some new spiropyranoquinolines and evaluation of their free radical scavenging activity. Chem Pharm Bull (Tokyo) 2009;57:446–52
- Korrichi L, Dalila B, Dalila S. Quinolines antioxydant activity structure activity relation-ship. Eur J Biol Sci 2009;1:32–6
- Sankaran M, Kumarasamy C, Chokkalingam U, Mohan PS. Synthesis, antioxidant and toxicological study of novel pyrimido quinoline derivatives from 4-hydroxy-3-acyl quinolin-2-one. Bioorg Med Chem Lett 2010;20:7147–51
- Nayyar A, Malde A, Coutinho E, Jain R. Synthesis, anti-tuberculosis activity, and 3D-QSAR study of ring-substituted-2/4-quinolinecarbaldehyde derivatives. Bioorg Med Chem 2006;14:7302–10
- Kidwai M, Negi N, Gupta SD. Synthesis and antifertility activity of 1,5-diaryl-3(3′-indolyl)formazans. Chem Pharm Bull (Tokyo) 1994;42:2363–4
- Puntarulo S, Cederbaum AI. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic Biol Med 1998;24:1324–30
- Hassoun EA, Roche VF, Stohs JS. Release of enzymes by ricin from macrophages and Chinese hamster ovary cells in culture. Toxicol Methods 1993;3:119–29
- Moss DW, Henderson AR, Kochmar JR. Enzymes. In: Tietz NW, ed. Textbook of clinical chemistry. Philadelphia, USA: WB Saunders; 1986:619–63
- Lautraite S, Bigot-Lasserre D, Bars R, Carmichael N. Optimisation of cell-based assays for medium throughput screening of oxidative stress. Toxicol In Vitro 2003;17:207–20