Abstract
In this study, the compounds having acrylophenone structure, 1-aryl-2-(N-methylpiperazinomethyl)-2-propen-1-one dihydrochlorides, were synthesized and their chemical structures were identified with 1H NMR, 13C NMR and HRMS spectra. The cytotoxicities of the compounds were tested towards Ca9-22 (human gingival carcinoma), HSC-2 (human oral squamous carcinoma), HSC-3 (human oral squamous carcinoma) and HSC-4 (human oral squamous carcinoma) cell lines as tumor cell lines and HGF (gingival fibroblasts), HPLF (periodontal ligament fibroblasts) and HPC (pulp cells) cell lines as non-tumor cell lines. PSE of the compound TA2, which has a methyl substituent on phenyl ring, pointed out the compound TA2 as a leader compound to be considered.
Introduction
One of the major problems in treating cancers with drugs is absence of drugs which has selective toxicity to malignant cells rather than normal cells. In addition, most of the drugs which are in clinical use have resistance or cross-resistance problems. Mannich bases are a group of compounds having several biological activities, such as antifungalCitation1,Citation2, anti-inflammatoryCitation3, cytotoxic and anticancerCitation4–12, and anticonvulsant activitiesCitation13,Citation14. One of the reported mechanisms of action of Mannich bases is thiol alkylation of α,β-unsaturated ketone moiety/ies which is available in the chemical structure or produced by deamination process of suitable Mannich bases in vivo or in vitro conditionsCitation15–18. The other mechanisms are inhibition of mitochondrial respirationCitation12,Citation19, inhibition of microtubular polimerizationCitation20,Citation21 or DNA topoisomerase enzyme inhibitionCitation9.
In the literature, conjugated stryrl ketones were designed as thiol alkylators having little or no affinity to interact with amino or hydroxy groups of cellular constituentsCitation22–24. Most of the drugs available in the market show genotoxicity. Being a thiol alkylator may provide to devoid of the genotoxic properties since thiol groups are not present in nucleic acidsCitation25. In addition to thiol specificity of α,β-unsaturated ketones, there is a study reporting that Mannich bases of stryryl ketones were free from cross-resistance to several drug resistant cell linesCitation26. The piperazine scaffold and its analogues are important pharmacophores, which show several bioactivities including anticancer activityCitation27–29. 4,4-1,2-(Ethanediyl)bis(1-isobutoxycarbonyloxy-methyl-2,6-piperazinedione (MST-16), which includes piperazine in its chemical structure, was recently approved as an oral anticancer drug for clinical use in JapanCitation30. The compound MST-16 has shown potent antiproliferative activity against colon, prostate, breast, lung and leukemia tumorsCitation30. In another study, piperazine-pyrimidine compound, (2Z,NE)-N-(3-cyclopropyl-1,2-dihydropyrazol-5-ylidene)-2-((3-isopropylisoxazol-5-yl)methylimino)-6-(4-methylpiperazin-1-yl)-2,3-dihydropyrimidin-4-amine (XL 228), a multitargeted protein kinase inhibitor, was found effective in patients with solid tumors or hematologic malignancies in phase I clinical trialsCitation31–33.
In the literature, 1-aryl-2-dimethylaminomethyl-2-propen-1-one hydrochlorides, which have acrylophenone structure, were reported as potent cytotoxins towards malignant cells whereby 77% of the CC50 values were less than 10 μMCitation34. The CC50 values of most of the compounds against HL60, HSC-2 and HSC-4 cells were also reported to be lower than melphalan in that studyCitation34. In this study, 1-aryl-2-(N-methylpiperazinomethyl)-2-propen-1-one dihydrochlorides, which have piperazine moiety in their structure, were synthesized and their cytotoxicity were tested against Ca9-22 (human gingival carcinoma), HSC-2 (human oral squamous carcinoma), HSC-3 (human oral squamous carcinoma) and HSC-4 (human oral squamous carcinoma) cell lines as tumor cell lines and HGF (gingival fibroblasts), HPLF (periodontal ligament fibroblasts) and HPC (pulp cells) cell lines as non-tumor cell lines.
The rationals behind designing the chemical structure of 1-aryl-2-(N-methylpiperazinomethyl)-2-propen-1-one dihydrochlorides (), are as follows: (i) the chemical structure designed includes a conjugated enone group next to aryl part; (ii) there is a β-carbon atom which is sterically unhindered in the structures designed. This can make easy to take place thiol alkylation if the mechanism of action is thiol alkylation; (iii) there is an amino group in the protonated form in the chemical structure designed. Nitrogen in the structure is a quadrivalent nitrogen, which is more electrophilic than base form of amine to thiol alkylation. In addition, salt form of amine is more water soluble than base form, which increases the transportation of compound in body fluids. The other point to be considered is that, in the case of some tumor, extracellular pH around neoplasm may be lowCitation35,Citation36 and the pH of neoplasm is lower than normal tissue. It may be possible to lead preferential toxicity to neoplasm when the ions portion is higher in malignant cells.
Materials and methods
Melting points were determined using an Electrothermal 9100 (IA9100, Bibby Scientific Limited, Staffordshire, UK) instrument and are uncorrected. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were obtained using a Varian Mercury Plus Spectrometer (Varian Inc., Palo Alto, CA). Chemical shifts (δ) are reported as ppm.
Mass spectra were undertaken on an HPLC-TOF Waters Micromass LCT Premier XE mass spectrometer (Waters Corporation, Milford, MA) using an electrospray ion source (ESI).
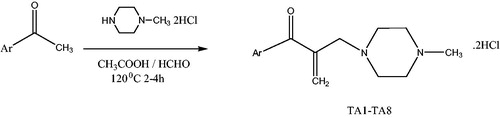
General synthesis of the 1-aryl-2-(N-methylpiperazinomethyl)-2-propen-1-one dihydrochlorides (TA1–TA8)
To a solution of suitable ketone in acetic acid (15 ml), paraformaldehyde and N-methylpiperazine dihydrochloride were added (). The mole ratios of the reagents used were in 1:2:1, respectively. The mixture was heated under reflux at 120 °C for 2–4 h. TA1 (2 h), TA2 (3.5 h), TA3 (3 h), TA4 (1 h), TA5 (4 h), TA6 (2.5 h), TA7 (3 h), TA8 (3 h) (). The reactions were monitored by TLC (chloroform:methanol (8:2)). After the solvent was removed under vacuum, the crude product was crystallized from methanol-diethylether. Ketone used and the amount of it (mmol) were as follows: TA1 (acetophenone, 17), TA2 (4-methylacetophenone, 14), TA3 (4-methoxyacetophenone, 13), TA4 (4-chloroacetophenone, 12), TA5 (4-bromoacetophenone, 10), TA6 (4-floroacetophenone, 14), TA7 (1-(thiophen-2-yl) ethanone, 16), TA8 (4-nitroacetophenone, 12).
2-(4-Methyl-piperazin-1-yl-methyl)-1-phenyl-2-propen-1-one dihydrochloride (TA1)
Yield: 17%; m.p. 218–220 °C, 231–233 °CCitation37; 1H NMR (CD3OD) δ 3.03 (3H, s, N-CH3), 3.69–3.80 (8H, m, CH2 piperazine ring), 4.33 (2H, s, CH2-N), 6.37 (1H, s, C = CH2), 6.77 (1H, s, C = CH2), 7.52–7.85 (5H, m, arom.); 13C NMR (CD3OD) δ 42.2, 48.8, 50.01, 56.6, 128.8, 129.7, 133.1, 136.2, 136.3, 138.2, 196.3; Mass spectrum: 245.16 (M++1); HRMS (ESI-MS); Calc.: 245.1654 for C15H21N2O [M+H+], Found: 245.1666.
2-(4-Methyl-piperazin-1-yl-methyl)-1-p-tolyl-2-propen-1-one dihydrochloride (TA2)
Yield: 15%; m.p. 208–212 °C. 1H NMR (CD3OD) δ 2.43 (3H, s, Ar-CH3), 3.03 (3H, s, N-CH3), 3.40–4.00 (8H, m, CH2 piperazine ring), 4.34 (2H, s, CH2-N), 6.35 (1H, s, C = CH2), 6.76 (1H, s, C = CH2), 7.35 (2H, d, arom., J = 8.2 Hz), 7.75 (2H, d, arom., J = 8.2 Hz); 13C NMR (CD3OD) δ 20.5, 42.2, 48.8, 49.9, 56.5, 129.2, 130.0, 133.4, 136.1, 138.0, 144.5, 195.9; Mass spectrum: 259.18 (M++1); HRMS (ESI-MS); Calc.: 259.1810 for C16H23N2O [M+H+], Found: 259.1828.
1-(4-Methoxy-phenyl)-2-(4-methyl-piperazin-1-yl-methyl)-2-propen-1-on dihydrochloride (TA3)
Yield: 22%; m.p. 214–224 °C, 214–224 °CCitation37. 1H NMR (CD3OD) δ 3.03 (3H, s, N-CH3), 3.66–3.88 (8H, m, CH2 piperazine ring), 3.89 (3H, s, Ar-CH3O), 4.29 (2H, s, CH2-N), 6.30 (1H, s, C = CH2), 6.67 (1H, s, C = CH2), 7.05 (2H, d, arom., J = 8.8 Hz), 7.87 (2H, d, arom., J = 8.8 Hz); 13C NMR (CD3OD) δ 40.8, 42.2, 50.1, 55.0, 57.1, 128.4, 130.6, 132.3, 136.2, 136.4, 164.5, 194.8; Mass spectrum: 275.17 (M++1); HRMS (ESI-MS); Calc.: 275.1760 for C16H23N2O2 [M+H+], Found: 275.1769.
1-(4-Chloro-phenyl)-2-(4-methyl-piperazin-1-yl-methyl)-2-propen-1-one dihydrochloride (TA4)
Yield: 19%; m.p. 218–220 °C, 215–220 °CCitation37; 1H NMR (CD3OD) δ 3.03 (3H, s, N-CH3), 3.68–3.85 (8H, m, CH2 piperazine ring), 4.32 (2H, s, CH2-N), 6.38 (1H, s, C = CH2), 6.79 (1H, s, C = CH2), 7.55 (2H, d, arom., J = 8.4 Hz), 7.84 (2H, d, arom., J = 8.4 Hz); 13C NMR (CD3OD) δ 40.8, 42.2, 50.0, 56.4, 128.8, 131.4, 134.7, 136.1, 138.5, 139.4, 194.9; Mass spectrum: 279.12 (M++1); HRMS (ESI-MS); Calc.: 279.1264 for C15H20N2OCl [M+H+], Found: 279.1272.
1-(4-Bromo-phenyl)-2-(4-methyl-piperazin-1-yl-methyl)-2-propen-1-one dihydrochloride (TA5)
Yield: 23%; m.p. 220–222 °C. 1H NMR (CD3OD) δ 3.3 (3H, s, N-CH3), 3.69–3.74 (8H, m, CH2 piperazine ring), 4.22 (2H, s, CH2-N), 6.32 (1H, s, C = CH2), 6.71 (1H, s, C = CH2), 7.73 (2H, dd, arom., J = 8.4, 1.5 Hz), 7.96 (2H, dd, arom., J = 8.4, 1.5 Hz); Mass spectrum: 323.07 (M++1); HRMS (ESI-MS); Calc.: 323.0759 for C15H20N2OBr [M+H+], Found: 323.077.
1-(4-Fluoro-phenyl)-2-(4-methyl-piperazin-1-yl-methyl)-2-propen-1-one dihydrochloride (TA6)
Yield: 22%; m.p. 220–222 °C. 1H NMR (CD3OD) δ 3.04 (3H, s, N-CH3), 3.50–3.96 (8H, m, CH2 piperazine ring), 4.33 (2H, s, CH2-N), 6.36 (1H, s, C = CH2), 6.78 (1H, s, C = CH2), 7.26 (2H, dd, arom., JHF = 8.79 Hz, JHH = 8.43 Hz), 7.93 (2H, ddd, arom., JH3H2 = 7.7 Hz, JH3H4 = 2.2 Hz, JHF = 5.6 Hz); 13C NMR (CD3OD) δ 42.2, 48.8, 50.0, 56.4, 115.5 (d, 2 JC-F = 22 Hz), 132.7 (d, 3 JC-F = 9 Hz), 136.1, 138.3, 166 (d, JC-F = 253.3 Hz), 194.7; Mass spectrum: 263.15 (M++1); HRMS (ESI-MS); Calc.: 263.1560 for C15H20N2OF [M+H+], Found: 263.1578.
2-(4-Methyl-piperazin-1-yl-methyl)-1-thiophen-2-yl-2-propen-1-one dihydrochloride (TA7)
Yield: 23%; m.p. 217-219 °C. 1H NMR (CD3OD) δ 3.03 (3H, s, N-CH3), 3.59–3.69 (8H, m, CH2 piperazine ring), 4.31 (2H, s, CH2-N), 6.60 (1H, s, C = CH2), 6.68 (1H, s, C = CH2), 7.25 (1H, dd, J = 4.8, 3.7 Hz), 7.91 (1H, dd, J = 3.7, 1.1 Hz), 7.97 (1H, dd, J = 4.8, 1.1 Hz); Mass spectrum: 251.12 (M++1); HRMS (ESI-MS); Calc.: 251.1218 for C13H19N2OS [M+H+], Found: 251.1217.
2-(4-Methyl-piperazin-1-yl-methyl)-1-(4-nitro-phenyl)-2-propen-1-one dihydrochloride (TA8)
Yield: 22%; m.p. 228–230 °C. 1H NMR (DMSO) δ 3.02 (3H, s, N-CH3), 3.29–3.69 (8H, m, CH2 piperazine ring), 4.23 (2H, s, CH2-N), 6.36 (1H, s, C = CH2), 6.80 (1H, s, C = CH2), 8.02 (2H, d, arom., J = 7.0 Hz), 8.37 (2H, d, arom., J = 7.0 Hz); 13C NMR (DMSO) δ 42.5, 48.9, 50.6, 55.3, 124.2, 131.4, 138.1, 138.6, 142.5, 150.2, 195.2; Mass spectrum: 290.15 (M++1); HRMS (ESI-MS); Calc.: 290.1505 for C15H20N3O3 [M+H+], Found: 290.1507.
Biological activity
Cytotoxicity evaluation
The cytotoxicity of the compounds TA1-TA8 were assayed towards HSC-2 (human oral squamous carcinoma), HSC-3 (human oral squamous carcinoma), HSC-4 (human oral squamous carcinoma), Ca9-22 (human gingival carcinoma), HGF (gingival fibroblasts), HPC (pulp cells) and HPLF (periodontal ligament fibroblasts) cells as describedCitation38 with some minor modifications. In brief, cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) except the Ca9-22 cells were cultured in RPMI 1640 medium supplemented with 10% FBS. The following concentrations of the compounds in dimethylsulfoxide (DMSO) were added to the medium and incubated at 37 °C for 48 h: TA1∼TA8 and melphalan (3.12, 6.25, 12.5, 25. 50, 100, 200, 400 μM), 5-FU (7.8, 15.6, 31.3, 62.5, 125, 250, 500, 1000 μM). The media that contained the same concentration of DMSO (0.0078, 0.156, 0.03125, 0.0625, 0.125, 0.25, 0.5 or 1%) were used as controls, since DMSO above 0.25% is cytotoxic. The viable cell numbers were determined by the MTT method except the viability of Ca9-22 cells was obtained by cell counting with a hemocytometer after staining with 0.15% trypan blue. The CC50 values were determined from dose-response curves.
Results and Discussion
In this study, 1-aryl-2-(N-methylpiperazinomethyl)-2-propen-1-one dihydrochlorides type of compounds were designed and synthesized successfully. Except the compounds TA1, TA3 and TA4, all of the compounds reported here are new. The chemical structures of the compounds were confirmed by 1H NMR, 13C NMR and HRMS (see “Materials and methods” section) and reported here for the first time in detail. Ca9-22, HSC-2, HSC-3 and HSC-4 were used as tumor cell lines while HGF, HPLF and HPC cell lines were used as non-tumor cell lines. The cytotoxicity results are shown in .
Table 1. Cytotoxicities of the compounds.
The first question to be answered is whether the antineoplastic properties of the compounds are available or not. shows that the CC50 values of the compounds (the concentration of the compounds which kills 50% of the cells as μM) were in low micromolar range towards Ca9-22, HSC-2, HSC-3 and HSC-4 cells. The potencies of TA1-TA8 towards the tumor cell lines were compared with an alkylating agent, melphalan. All compounds towards Ca9-22 cell line; TA1, TA2, TA3, TA6 and TA7 towards HSC-2 cell line; TA1, TA2, TA3, TA4, TA6 and TA7 towards HSC-3 cell line; all of the compounds except TA8 towards HSC-4 cell line were more potent than melphalan (). When the cytotoxicities of the compounds were compared with the other reference compound 5-Fluorouracil, it was observed that all compounds were more cytotoxic than 5-Fluorouracil towards Ca9-22; HSC-3, HSC-4 and HSC-2 cell lines, except TA8 towards HSC-2 cell line.
The second aspect of these compounds to be considered is whether they are tumor-specific cytotoxins since tumors are surrounded by different types of normal cells. Selectivity index (SI) figures, which are the quotients of the average CC50 values of the non-malignant cells and the CC50 figure of a compound towards a specific cell line, were generated. The compounds which have SI values of greater than 1 can be considered as tumor-specific antineoplastic agents (). Accordingly, it can be said that the compounds showed more remarkable tumor specificity towards HSC-3 cell line comparing with the other cell lines. However, the compounds synthesized showed slight specificity towards HSC-2 and HSC-4 cell lines while they are not tumour specific towards Ca9-22 cell line. SI values for tumor-specificity of the compounds are in the range of 1.29–2.88. SI values were 1.39 (TA1), 1.86 (TA2), 2.88 (TA3), 1.55 (TA4), 1.57 (TA5), 1.74 (TA6), 1.58 (TA7) and 1.29 (TA8) towards HSC-3 cell line. The most selective compounds towards HSC-3 is TA3, which has methoxy substituent on aryl ring. It is quite natural to observe different cytotoxicity for compounds toward different cell lines. Since the mechanism of action for any compound can be different towards a cell line. That is why cytotoxicity value and selectivity index are different for any compound.
Lead compound/s should possess both marked cytotoxic potencies and selective toxicity for tumors. In order to identify such molecule/s, a potency selectivity expression (PSE) was devised which is the product of the reciprocal of average CC50 values towards Ca9-22, HSC-2, HSC-3 and HSC-4 cells (a measure of potency, the column E, ) and the average SI values towards these cell lines (a determination of tumor-selectivity, the column J, ) expressed as a percentage. The PSE data are shown in . When the PSE values were considered, the compound TA2, had the highest PSE figure of 30.93. The differences in PSE values may result from the physical properties of the compounds itself and/or interaction of the compounds with a receptor/s at different ratio and/or the differences in the stabilities of the compounds in biological medium to provide active compound for alkylation and/or the life time of metabolite produced for optimum effect. Additionally, the type of cell line is important. When the cell lines change the results and PSE values change depending on the modification.
For further studies, the compounds can be modified chemically by changing amine part and/or aryl part to find out the more selective cytotoxic compounds and the compounds at issue can be tested toward different tumor cell lines to investigate a cell type to which these compounds may show more selectivity.
In conclusion, this study reports new cytotoxic compounds with their detailed spectral data and cytotoxicities. Compound TA2, which has the highest PSE value, seems to be a candidate compound for further studies.
Declaration of interest
The authors report no conflict of interest and are responsible for the contents and writing of the paper.
This research work was supported by Ataturk University Research Fund (Project No. BAP: 2010/166), Turkey. The authors are thankful to Dr Murat Sukuroglu (from Gazi University) for HRMS spectra.
References
- Mete E, Ozelgul C, Kazaz C, et al. Synthesis and antifungal activity of 1-Aryl-3-phenethylamino-1-propanone hydrochlorides and 3-Aroyl-4-aryl-1-phenethyl-4-piperidinols. Arch Pharm 2010;343:291–300
- Mete E, Gul HI, Bilginer S, et al. Synthesis and antifungal evaluation of 1-Aryl-2-dimethylaminomethyl-2-propen-1-one hydrochlorides. Molecules 2011;16:4660–71
- Gul HI, Suleyman H, Gul M. Evaluation of the anti-inflammatory activity of N,N-bis(3-dimethylamino-1-phenyl-propylidene)hydrazine dihydrochloride. Pharm Biol 2009;47:968–72
- Gul HI, Gul M, Hanninen O. Cytotoxic activities of some mono and bis Mannich bases derived from acetophenone in brine shrimp bioassay. Arzneimittelforschung 2002;52:840–3
- Gul HI, Yerdelen KO, Gul M, et al. Synthesis of 4-hydroxy-3-piperidinomethylchalcone derivatives and their cytotoxicity against PC-3 cell lines. Arch Pharm 2007;340:195–201
- Gul HI, Yerdelen KO, Das U, et al. Synthesis and cytotoxicity of novel 3-aryl-1-(3-dibenzylaminomethyl-4-hydroxyphenyl)-propenones and related compounds. Chem Pharm Bull 2008;56:1675–81
- Gul HI, Das U, Pandit B, Li PK. Evaluation of the cytotoxicity of some mono-mannich bases and their corresponding azine derivatives against androgen-independent prostate cancer cells. Arzneimittelforschung 2006;56:850–4
- Gul M, Mete E, Atalay M, et al. Cytotoxicity of 1-aryl-3-buthylamino-1-propanone hydrochlorides against Jurkat and L6 cells. Arzneimittelforschung 2009;59:364–9
- Mete E, Gul HI, Canturk P, et al. Biological activity of 1-aryl-3-phenethylamino-1-propanone hydrochlorides and 3-aroyl-4-aryl-1-phenethyl-4-piperidinols on PC-3 cells and DNA topoisomerase I enzyme. Z Naturforsch C 2010;65:647–52
- Mete E, Gul HI, Cetin-Atalay R, et al. The design and cytotoxic evaluation of some 1-aryl-3-isopropylamino-1-propanone hydrochlorides towards human Huh-7 hepatoma cells. Arch Pharm 2011;344:333–9
- Bilginer S, Gul HI, Mete E, et al. 1-(3-aminomethyl-4-hydroxyphenyl)-3-pyridinyl-2-propen-1-ones: a novel group of tumour-selective cytotoxins. J Enzyme Inhib Med Chem 2013;28:974–80
- Kucukoglu K, Gul HI, Cetin-Atalay R, et al. Synthesis of new N,N′-bis[1-aryl-3-(piperidine-1-yl)propylidene]hydrazine dihydrochlorides and evaluation of their cytotoxicity against human hepatoma and breast cancer cells. J Enzyme Inhib Med Chem 2014;29:420–6
- Gul HI, Calis U, Vepsalainen J. Synthesis of some mono-Mannich bases and corresponding azine derivatives and evaluation of their anticonvulsant activity. Arzneimittelforschung 2004;54:359–64
- Gul HI, Calls U, Ozturk Z, et al. Evaluation of anticonvulsant activities of bis(3-aryl-3-oxo-propyl) ethylamine hydrochlorides and 4-aryl-3-arylcarbonyl-1-ethyl-4-piperidinol hydrochlorides. Arzneimittelforschung 2007;57:133–6
- Gul M, Gul HI, Das U, Hanninen O. Biological evaluation and structure-activity relationships of bis-(3-aryl-3-oxo-propyl)-methylamine hydrochlorides and 4-aryl-3-arylcarbonyl-1-methyl-4-piperidinol hydrochlorides as potential cytotoxic agents and their alkylating ability towards cellular glutathione in human leukemic T cells. Arzneimittelforschung 2005;55:332–7
- Gul HI, Gul M, Vepsalainen J, et al. Cytotoxicity of some azines of acetophenone derived mono-Mannich bases against Jurkat cells. Biol Pharm Bull 2003;26:631–7
- Gul M, Gul HI, Hanninen O. Effects of Mannich bases on cellular glutathione and related enzymes of Jurkat cells in culture conditions. Toxicol In Vitro 2002;16:107–12
- Gul HI, Gul M, Erciyas E. Syntheses and stability studies of some Mannich bases of acetophenones and evaluation of their cytotoxicity against Jurkat cells. Arzneimittelforschung 2002;52:628–35
- Hamon NW, Bassendowski DL, Wright DE, et al. Effect of anti-neoplastic and cytotoxic Mannich-bases derived from conjugated styryl ketones on mitochondrial respiration in rat-liver cells. J Pharm Sci 1978;67:1539–42
- Delacourte A, Boutteau F, Biserte G, et al. Mannich bases: β-(4-methyl-piperazinyl)propiophenone hydrochloride: a new antimicrotubular drug. Acta Ther 1980;6:121–31
- Mallevais ML, Delacourte A, Lesieur I, et al. 2-(4-Methyl-1-piperazinylmethyl)acrylophenone dihydrochloride: a new antimicrotubular drug. Biochimie 1984;66:477–82
- Pati HN, Das U, Sharma RK, Dimmock JR. Cytotoxic thiol alkylators. Mini Rev Med Chem 2007;7:131–9
- Mutus B, Wagner JD, Talpas CJ, et al. 1-Para-chlorophenyl-4,4-dimethyl-5-diethylamino-1-penten-3-one hydrobromide, a sulfhydryl-specific compound which reacts ırreversibly with protein thiols but reversibly with small molecular-weight thiols. Anal Biochem 1989;177:237–43
- Dimmock JR, Raghavan SK, Logan BM, Bigam GE. Anti-leukemic evaluation of some Mannich-bases derived from 2-arylidene-1,3-diketones. Eur J Med Chem 1983;18:248–54
- Dimmock JR, Kumar P. Anticancer and cytotoxic properties of Mannich bases. Curr Med Chem 1997;4:1–22
- Dimmock JR, Kumar P, Quail JW, et al. Synthesis and cytotoxic evaluation of some styryl ketones and related-compounds. Eur J Med Chem 1995;30:209–17
- Gillet R, Jeannesson P, Sefraoui H, et al. Piperazine derivatives of butyric acid as differentiating agents in human leukemic cells. Cancer Chemother Pharmacol 1998;41:252–5
- Eilon GF, Gu J, Slater LM, et al. Tumor apoptosis induced by epoxide-containing piperazines, a new class of anti-cancer agents. Cancer Chemother Pharmacol 2000;45:183–91
- Hulme C, Ma L, Romano J, Morisette M. Novel applications of ethyl glyoxalate with the ugi MCR. Tetrahedron Lett 1999;40:5295–9
- Yoshida M, Maehara Y, Sugimachi K. MST-16, a novel bis-dioxopiperazine anticancer agent, ameliorates doxorubicin-induced acute toxicity while maintaining antitumor efficacy. Clin Cancer Res 1999;5:4295–300
- Font M, Gonzalez A, Palop JA, Sanmartin C. New insights into the structural requirements for pro-apoptotic agents based on 2,4-diaminoquinazoline, 2,4-diaminopyrido[2,3-d]pyrimidine and 2,4-diaminopyrimidine derivatives. Eur J Med Chem 2011;46:3887–99
- Scagliotti GV, Novello S. The role of the insulin-like growth factor signaling pathway in non-small cell lung cancer and other solid tumors. Cancer Treat Rev 2012;38:292–302
- Schenone S, Brullo C, Musumeci F, Botta M. Novel dual Src/Abl inhibitors for hematologic and solid malignancies. Expert Opin Invest Drugs 2010;19:931–45
- Pati HN, Das U, Kawase M, et al. 1-Aryl-2-dimethylaminomethyl-2-propen-1-one hydrochlorides and related adducts: a quest for selective cytotoxicity for malignant cells. Bioorg Med Chem 2008;16:5747–53
- Ojugo ASE, McSheehy PMJ, McIntyre DJO, et al. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous F-19 and P-31 probes. NMR Biomed 1999;12:495–504
- Stubbs M, McSheehy PMJ, Griffiths JR, Bashford CL. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today 2000;6:15–19
- Cazin M, Lesieur I, Lesieur D, et al. 2-(Aminoalkyl)acrylophenones and their use. From European Patent Applications, EP0158785A1, 1985
- Motohashi N, Wakabayashi H, Kurihara T, et al. Biological activity of barbados cherry (acerola fruits, fruit of Malpighia emarginata DC) extracts and fractions. Phytother Res 2004;18:212–23