Abstract
Drug resistance has become a major concern in the field of infection management, therefore searching for new antibacterial agents is getting more challenging. Our study presents an optimized and eco-friendly synthesis scheme for a panel of nitroalkenes bearing various functional groups in the aromatic moiety and bromine or cyano substituents in 1 position of nitrovinyl moiety. The presence of nitrolefine group outside the ring minimalizes genotoxic properties while conjugation of aryl group with nitrovinyl moiety increases stability of the compounds. Then our research focused on evaluation of biological properties of such obtained (E)-2-aryl-1-cyano-1-nitroethenes. As they exhibit strong bacteriostatic and bactericidal activities against reference bacteria and yeast species with no detectable cytotoxicity towards cultured human HepG2 and HaCaT cells, they could be promising candidates for the replacement of traditional nitrofurane-containing antibacterial drugs. Nevertheless, validation of the obtained data in an in vivo model and additional safety studies on mutagenicity are still required.
Introduction
Heterocyclic nitro compounds, including numerous 5-nitroimidazoles and 5-nitrofurans, are characterized by wide spectra of antimicrobial activityCitation1–3. Despite the extensive potency to fight bacteria, fungi, and parasites, some doubts arose over their use in human and veterinary medicine. The main concern was related to mutagenic and carcinogenic side-effects of the heterocyclic nitro compoundsCitation4,Citation5. In significance, it has been demonstrated that their nitroalkene derivatives, containing nitro group separated from the aromatic ring by alkene chain, are deprived of genotoxic propertiesCitation4,Citation6 or are much less genotoxic than the 5-nitrofuransCitation4,Citation7.
From the chemical perspective, nitroalkenes constitute an important group of reactants in organic chemistry. Their strong electrophilic character makes them extremely susceptible to react with diverse nucleophiles. As the nitro group can be easily reduced, they are good precursors for amines, hydroxylamines, esters and salts of nitronic acids and oximesCitation8,Citation9. However, the nitroalkenes are often unstable and may display some degree of cytotoxicity, especially these bearing short alkyl chainsCitation9,Citation10, while interactions of aryl moiety with nitrovinyl system increases their stability.
Considering these facts we decided to focus our attention on (E)-2-aryl-1-cyano-1-nitroethenes as potential antibacterial and antifungal agents, but without genotoxic properties. These nitroalkenes exist as stable compounds, due to conjugation of aryl with nitrovinyl moiety. On the other hand, their chemistry is very poorly understood. There are only a few reports available in the literature concerning their participation in [2 + 3]- and [2 + 4] cycloaddition reactionsCitation11–14. Moreover, nothing is known about their biological and pharmacological activity.
Here, we present an optimized and eco-friendly synthesis scheme for a panel of nitroalkenes bearing various functional groups in the aromatic moiety and bromine or cyano substituents in 1 position of nitrovinyl moiety. The obtained compounds were characterized on the basis of infrared (IR) and 1H-nuclear magnetic resonance (NMR) spectroscopy and by comparing the melting points with those reported in the literature. All synthesized compounds were screened for their antimicrobial activity against a wide spectrum of Gram-positive and Gram-negative bacteria strains as well as against fungi belonging to Candida spp. Cell selectivity constitutes formidable challenge in drug development process. Therefore, in order to determine the ability of these nitroalkenes to specifically target microorganisms over mammalian cells, we have employed two different cell lines, human hepatocellular carcinoma HepG2 cells and keratinocyte-like HaCaT cells, to evaluate the in vitro cytotoxicity of our compounds of interest.
Materials and methods
Analytical techniques
Knauer apparatus equipped with an UV-VIS detector and the Lichrospher 100-3 RP18 column (4 × 250) was applied for monitoring the reaction course. Methanol–water mixture (63:35, v/v) was used as an eluent at the flow rate of 0.6 mL/min. Melting points were determined on the Boetius apparatus and were not corrected. IR spectra were recorded on Bio-Rad 175C spectrophotometer in KBr pellets. 1H-NMR (500 MHz) spectra were taken on a Bruker AMX 500 spectrometer using CDCl3 as a solvent and TMS as an internal standard. Chemical shifts are given in a ppm scale.
Preparation of nitroalkenes
(Z)-2-phenyl-1-bromo-1-nitroethene (A1)
Firstly, 2-phenyl-1,2-dibromo-1-nitroethane was obtained from commercially available (E)-2-phenyl-1-nitroethene in reaction with bromine in methylene chloride solution. Parham and Bleasdale methodology was appliedCitation15. Product with 90% yield was obtained. Next, (Z)-2-phenyl-1-bromo-1-nitroethene was prepared by dehydrobromination of 2-phenyl-1,2-dibromo-1-nitroethane in the presence of pyridine according to the previously described procedureCitation16. Product with 80% yield was obtained (m.p.: 65–66 °C; reported: 64–66 °CCitation15; 63–64 °CCitation17).
Nitroacetonitril
In 760 mL, four-neck glass equipped with a mechanic stirrer, a dropping funnel, a thermometer and a reflux condenser, 60 g of methazonic acid (prepared from nitromethane according to the procedure described previouslyCitation18) was dissolved in 300 mL of diethyl ether. Next, reaction mixture was heated to boiling, and dropped slowly 43 mL of tionyl chloride. During introduction of tionyl chloride to reaction system, temperature should be higher than 30 °C. Solution was stirred for 120 min, filtered and evaporated in argon atmosphere. Oil residue was treated by 240 mL of diethyl ether and 90 mL of water. Organic layer was separated, dried over anhydrous calcium chloride and evaporated in argon atmosphere. Oil residue was purified by column chromatography. Silica gel was used as stationary phase, and benzene as eluent. Fourteen grams (28%) of pure nitroacetonitrile was obtained as yellow liquid. Product was converted immediately in reaction with aldehydes without storage, as its long-term storage leads to the formation of explosive substances.
Selected spectral data: 1H NMR (CDCl3): 5.23 (s, 2H); IR (cm−1): 2963, 3015 (–CH2–), 2363 (–C ≡ N), 1573, 1363 (–NO2).
(E)-2-aryl-1-cyano-1-nitroethenes (A2–A7) – general procedure
Appropriate aldehyde (0.1 mol) was dissolved in 2 mL of 1-butyl-3-methylimidazolium chloride. Next, 0.2 g of A4 molecular sieves and 0.1 mol of nitroacetonitrile were added. Reaction mixture was stirred by 5 min. Product was separated by filtration and recrystallized from ethanol, thus the series of 6 (E)-2-aryl-1-cyano-1-nitroethenes was obtained. Yields and melting points for all compounds are collected in . After washing the ionic liquid with diethyl ether and dried in vacuo, it may be applied again for next synthesis.
Table 1. Yield of synthesis of nitroalkenes A1–A7 and their melting points.
Antibacterial and antifungal activity assessment
All the synthesized compounds were in vitro screened for their antibacterial and antifungal activities using a broth microdilution method according to European Committee on Antimicrobial Susceptibility Testing (EUCAST)Citation19 and Clinical and Laboratory Standards Institute guidelines (CLSI)Citation20 against a panel of 20 reference microorganisms strains. All microorganisms were obtained from American Type Culture Collection (ATCC, Rockville, MD). Bacteria strains were subcultured onto nutrient agar at 35 °C for 18–24 h. Fungal cultures were carried out on Sabouraud agar at 30 °C for 24 – 48 hCitation19–21.
Antibacterial properties of tested compounds were evaluated in both Gram-positive (Staphylococcus aureus ATCC 6538, Staphylococcus aureus ATCC 25923, Staphylococcus aureus ATCC 43300, Staphylococcus epidermidis ATCC 12228, Streptococcus pyogenes ATCC 19615, Streptococcus pneumoniae ATCC 49619, Streptococcus mutans ATCC 25175, Bacillus subtilis ATCC 6633, Bacillus cereus ATCC 10876, and Micrococcus luteus ATCC 10240) and Gram-negative bacteria (Escherichia coli ATCC 3521, Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 13883, Proteus mirabilis ATCC 12453, Bordetella bronchiseptica ATCC 4617, Salmonella typhimurium ATCC 14028, Pseudomonas aeruginosa ATCC 9027). Candida albicans ATCC 2091, Candida albicans ATCC 10231, and Candida parapsilosis ATCC 22019 were utilized in an antifungal activity assessment.
The surface of Mueller–Hinton agar or Mueller–Hinton agar with 5% sheep blood (for bacteria) and RPMI 1640 with MOPS (for fungi) were inoculated with the suspensions of bacterial or fungal species. Microbial suspensions were prepared in sterile saline (0.85% NaCl) with an optical density of McFarland standard scale 0.5 [approximately 1.5 × 108 CFU (Colony Forming Units)/mL] for bacteria and 0.5 McFarland standard scale [approximately 5 × 105 CFU/mL)] for fungiCitation19–21.
Samples containing 5 mg, 1 mg or 0.5 mg of tested compounds A1–A7 were dissolved in 1 mL of DMSO. Subsequently, 50 µl of vehicle or the compounds of interest was applied into the wells (d = 6 mm) on the agar media mentioned above. The agar plates were preincubated at room temperature for 1 h and incubated at 37 °C for 24 h and at 30 °C for 48 h for bacteria and fungi, respectively. After the incubation period, the zones of growth inhibition were measured and average values were calculated. Vehicle-containing wells were used as controls. Furthermore, bacterial and fungal suspensions were applied onto Petri dishes with solid media containing 1 mg/mL of tested compounds A1–A7 and they were incubated. The inhibition of microorganisms growth was judged by comparing with a control culture prepared without any sample tested. Ciprofloxacin, vancomycin and fluconazole (Sigma-Aldrich, St. Louis, MO) were used as a reference antibacterial or antifungal compounds, respectivelyCitation19–21.
Subsequently, minimal inhibitory concentration (MIC) of the compounds was examined by the microdilution broth method, using their two-fold dilutions in Mueller–Hinton broth or Mueller–Hinton broth with 5% sheep blood (for bacteria) and RPMI 1640 broth with MOPS (for fungi) prepared in 96-well polystyrene plates. Final concentrations of the compounds ranged from 1000 to 0.488 µg/mL. Microbial suspensions were prepared in sterile saline (0.85% NaCl) with an optical density of 0.5 McFarland standard. Next, 2 µl of each bacterial or fungal suspension was added per each well containing 200 µl of broth and various concentrations of the examined compounds. After incubation (37 °C, 24 h), the MIC was assessed spectrofotometrically as the lowest concentration of the samples showing complete bacterial or fungal growth inhibition. Appropriate DMSO, growth and sterile controls were carried out. The medium without tested substances was used as a controlCitation19–21.
The MBC (Minimal Bactericidal Concentration) or MFC (Minimal Fungicidal Concentration) are defined as the lowest concentration of the compounds that is required to kill a particular bacterial or fungal species. MBC/MFC was determined by removing 20 μl of the culture using MIC determinations from each well and spotting onto appropriate agar medium. The plates were incubated at 37 °C for 24 h and at 30 °C for 48 h for bacteria and fungi, respectively. The lowest compound concentrations with no visible growth observed were assessed as a bactericidal/fungicidal concentration. All the experiments were repeated three times and representative data are presented in and Citation19–21.
Table 2. The antimicrobial activity data expressed as MIC (MBC or MFC) [µg/ml] against the reference strains of microorganisms for compounds A1–A7.
Table 3. The antimicrobial activity data expressed as MBC/MIC and MFC/MIC against the reference strains of microorganisms for compounds A1–A7.
In this study, no bioactivity was defined as a MIC >1000 µg/mL, mild bioactivity as a MIC in the range 501–1000 µg/mL, moderate bioactivity with MIC from 126 to 500 µg/mL, good bioactivity as a MIC in the range 26–125 µg/mL, strong bioactivity with MIC between 10 and 25 µg/mL and very strong bioactivity as a MIC <10 µg/mL. The MBC/MIC or MFC/MIC ratios were calculated in order to determine bactericidal/fungicidal (MBC/MIC ≤4, MFC/MIC ≤4) or bacteriostatic/fungistatic (MBC/MIC >4, MFC/MIC >4) effect of the tested compoundsCitation22.
Cell culture
Human hepatocellular carcinoma HepG2 cells were obtained from ATCC (#HB-8065) and spontaneously transformed human keratinocytes HaCaT were from CLS (#300493, Eppelheim, Germany). Culture of HepG2 cells was maintained in Eagle’s minimal essential medium (EMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin and 0.1 mg/mL streptomycin. HaCaT cells were cultured in high-glucose (4.5 g/L) Dulbecco’s Modified Eagle’s Medium (DMEM) modified with 10% FBS and 2 mM l-glutamine, 100 U/mL penicillin and 0.1 mg/mL streptomycin. All cell culture media components were from Life Technologies (Carlsbad, CA). The cells were cultured in humidified atmosphere of 95% air and 5% CO2 at 37 °C and were routinely passaged twice a week.
MTS assay
Cytotoxic effects of the synthetized compounds were assessed using CellTiter MTS reagent (Promega, Mannheim, Germany) according to manufacturer’s protocol. In brief, the cells were seeded out at 1 × 104 cells per well onto a 96-well flat-bottom plate and cultured overnight to enable proper cell attachment. Then, the cells were treated with increasing concentrations of the compounds of interest (up to 100 µM) or vehicle (DMSO, 0.1%) in serum-free media (100 µl of total volume) for 24 h. The assay was terminated by adding MTS reagent (20 µl/well). Three hours later, the absorbance was measured at 490 nm with reference wavelength of 620 nm using ELx800 plate reader (BioTek Instruments, Winooski, VT). All experiments were performed in sextuplicates and were performed three times.
Statistical analysis
Data on cellular viability were curve-fitted to four-parameter logistic equation and plotted as mean ± standard deviation (SD) using GraphPad Prism v6 (GraphPad Software, Inc., San Diego, CA).
Results and discussion
Preparation of conjugated nitroalkenes
(Z)-2-phenyl-1-bromo-1-nitroethene (compound A1) was obtained by dehydrobromination of 2-phenyl-1,2-dibromo-1-nitroethane, which was obtained from commercially available (E)-2-phenyl-1-nitroethene using previously described procedure (see “Materials and methods” section for details). Yield and melting point of A1 are available in .
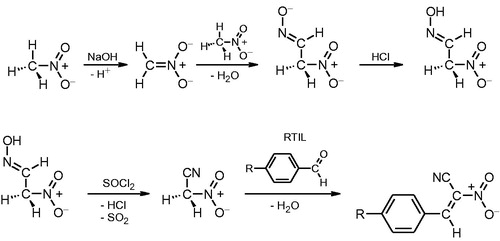
Preparation of (E)-2-aryl-1-cyano-1-nitroethenes (compounds A2–A7) required more complex approach. Initially, we employed a previously reported three-step reaction scheme to obtain methazonic acid from commercially available nitromethaneCitation23. Next, we have prepared nitroacetonitril via interaction between methazonic acid and thionyl chloride. Subsequently, the nitroacetonitril was purified by column chromatography and applied for synthesis of a series of (E)-2-aryl-1-cyano-1-nitroethenes in reaction with carbonyl compounds (Scheme 1).
Conventional procedures for the preparation of this type of nitroalkenes (via Knovanagel reaction) require primary amines as catalystCitation18. Under these conditions reaction proceeds for several hours. It is well established that dialkylimidazolium cations linked by carbonyl group are capable of activating many addition and condensation reactionsCitation24–27. Thus, we applied relatively inexpensive and widely available 1-butyl-3-methylimidazolium chloride as a bifunctional component: catalyst and reaction medium. Hereby, the reactions proceeded only for several minutes and gave high-purity product and significant yield. Ionic liquid was applied for the next synthesis round after washing with diethyl ether. Crude products were recrystallized from ethanol-yielding compounds of purity higher than 99%. Measured melting points correlated with literature data. A series of 6 nitroalkenes was prepared employing this approach (see “Materials and methods” section for details). Yields and melting points of obtained products are summarized in .
Microbiology
Compounds A1–A7 displayed inhibitory effects on the growth of reference strains of both Gram-positive and Gram-negative bacteria as well as on the growth of reference strains of Candida spp. yeasts.
Minimum concentrations of compounds A1–A7, obtained by the broth microdilution method, which inhibited the growth of Gram-positive bacteria belonging to staphylococci (Staphylococcus aureus ATTC strains and Staphylococcus epidermidis ATCC 12228), micrococci and bacteria from Bacillus spp. ranged from 3.91 to 62.5 µg/ml and MBC ranged from 15.62 to 250 µg/ml. The compounds A1, A3, A5 and A6 showed very strong or strong bacteriostatic and bactericidal activity (MBC/MIC = 2 – 64) against these microorganisms. In turn, the compounds A2, A4 and A7 exhibited good bacteriostatic and bactericidal activity to the same bacteria (MIC = 15.62–62.5 µg/mL, MBC = 31.25–500 µg/mL and MBC/MIC = 2–8). Moreover, all tested compounds showed much lower activity against remaining isolates of Gram-positive bacteria belonging to streptococci (Streptococcus spp. ATCC). The bioactivity toward these microorganisms was good or moderate (MIC = 15.62–500 µg/mL, MBC = 31.25–1000 µg/mL and MBC/MIC = 1–8) ( and ).
The tested compounds were found to be active against reference Gram-negative bacteria (Bordetella spp. ATTC, Pseudomonas spp. ATTC and rod-shaped bacteria of the Enterobacteriaceae family) with MIC = 7.81–500 µg/mL, MBC = 15.62–1000 µg/mL and MBC/MIC = 1–8. Among them, Pseudomonas aeruginosa ATCC 9027 and Bordetella bronchiseptica ATCC 4617 were especially sensitive to compound A1 with MIC = 7.81 µg/mL ( and ).
The growth of the reference yeast strains belonging to Candida spp. was inhibited by compounds A1–A7. On the basis of MIC values, it was shown that compounds A1, A2 and A6 had very strong fungicidal activity against reference strains of C. albicans ATCC 2091 and C. parapsilosis ATCC 22019 (MIC = 3.91–7.81 µg/mL) and slightly lower fungicidal activity against C. albicans ATCC 10231 (MIC = 15.62 µg/mL) ( and ). Compounds A1, A2 and A6 were characterized by MFC of 15.62–31.25 µg/mL and MFC/MIC of 1–4. Moreover, compounds A3, A4 and A5 displayed good fungicidal activity with MIC = 31.25–125 µg/mL, while compound A7 demonstrated only moderate activity with MIC = 62.5–500 µg/mL.
Cytotoxicity
Effects of the synthetized compounds on the viability of hepatocellular carcinoma HepG2 cells () and normal keratinocytic HaCaT cells () were evaluated in MTS assay. Compounds A2–A5 and A7 displayed no cytotoxic properties up to the concentration of 100 µM in both tested cell lines. Addition of A1 to the culture media of HepG2 and HaCaT cells caused viability inhibition with IC50 of 113.76 and 69.18 µM, respectively (). Compound A6 was the most toxic and caused half-maximal cytotoxicity at the concentration of 17.02 µM in HepG2 cells and at 36.56 µM in HaCaT cells ().
Figure 1. Cytotoxicity of compounds A1–A7 (a–g) against cultured HepG2 cells. HepG2 cells were treated with increasing concentrations of the compounds of interest (A1–A7, a–g) or vehicle (DMSO, 0.1%) for 24 h. Cell viability was assessed using MTS assay. Mean absorbance value for DMSO-treated cells was set to 1. The data are expressed as mean ± SD from three independent experiments carried out in sextuplicates. Obtained IC50 values are listed in .
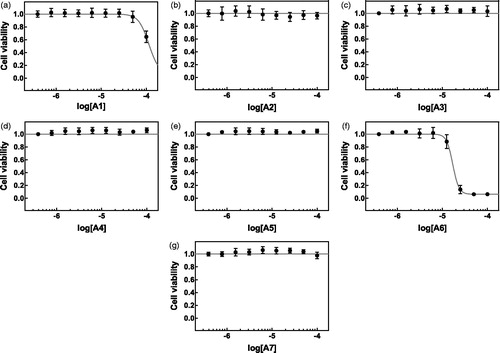
Figure 2. Cytotoxicity of compounds A1–A7 (a–g) against cultured HaCaT cells. HaCaT cells were treated with increasing concentrations of the compounds of interest (A1–A7, a–g) or vehicle (DMSO, 0.1%) for 24 h. Cell viability was assessed using MTS assay. Mean absorbance value for DMSO-treated cells was set to 1. The data are expressed as mean ± SD from three independent experiments carried out in sextuplicates. Obtained IC50 values are listed in .
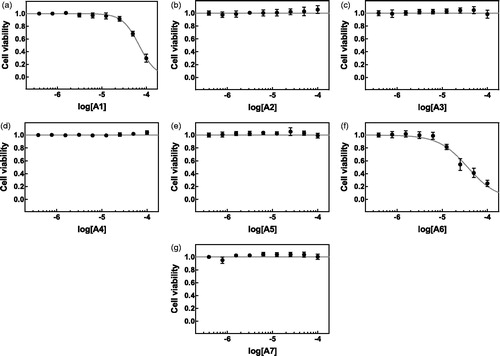
Table 4. Summary of cytotoxic activities of A1–A7 compounds in HepG2 and HaCaT cell lines.
Conclusions
In conclusion, first time a rapid, efficient, and eco-friendly method for the synthesis of (E)-2-aryl-1-cyano-1-nitroethenes has been developed. Application of the reusable ionic liquid environment not only decreased the synthesis cost, but also minimized chemical waste production. Our results indicated that compounds A2–A7 possess a wide spectrum of antimicrobial activity against reference bacteria and yeast species. Among them, compounds A3 and A5 showed the highest activity with bacteriostatic or bactericidal effect against Gram-positive bacteria, especially staphylococci, micrococci and Bacillus spp. with no detectable cytotoxicity towards cultured human HepG2 nor HaCaT cells. In addition, some of the tested compounds, especially A1, A2 and A6, exhibited very high fungicidal activity against yeasts belonging to Candida spp.
Several studies indicated that the metabolism-dependent detoxification can occur with respect to genotoxic furyl nitroethenes, compound of structure similar to A1–A7 derivatives in respect to the presence of nitrolefine group outside the heterocyclic ring. Thus, it is tempting to speculate that the compounds described in this study are also deprived of genotoxic properties in vivo. Consequently, they may find application as novel antimicrobial pharmaceuticals of increased safety, especially when compared with conventional 5-nitrofuranes. As drug resistance has become a major concern in the field of infection management, searching for new antibacterial agents is getting more challenging. Taking into account strong bacteriostatic and bactericidal activity of the studied compounds as well as low genotoxity of structurally similar compounds, the A2–A7 seem to be promising candidates for the replacement of traditional nitrofurane-containing antibacterial drugs. Nevertheless, validation of the obtained data in an in vivo model and additional safety studies on mutagenicity are still required.
Declaration of interest
The authors state no conflict of interest.
This work was partially supported by Polpharma Scientific Foundation scholarship (to A.W.).
References
- Guay DR. An update on the role of nitrofurans in the management of urinary tract infections. Drugs 2001;61:353–64
- Raether W, Hanel H. Nitroheterocyclic drugs with broad spectrum activity. Parasitol Res 2003;90:S19–39
- Kim P, Zhang L, Manjunatha UH, et al. Structure-activity relationships of antitubercular nitroimidazoles. 1. Structural features associated with aerobic and anaerobic activities of 4- and 5-nitroimidazoles. J Med Chem 2009;52:1317–28
- Gonzalez Borroto JI, Perez Machado G, Creus A, Marcos R. Comparative genotoxic evaluation of 2-furylethylenes and 5-nitrofurans by using the comet assay in TK6 cells. Mutagenesis 2005;20:193–7
- Moreno SN, Docampo R. Mechanism of toxicity of nitro compounds used in the chemotherapy of trichomoniasis. Environ Health Perspect 1985;64:199–208
- Estrada E. Structure-mutagenicity relationships in 2-furylethylene derivatives: a molecular orbital study of the role of nitro groups. Mutat Res 1998;420:67–75
- Gonzalez Borroto JI, Creus A, Marcos R. Genotoxic evaluation of the furylethylene derivative 2-furyl-1-nitroethene in cultured human lymphocytes. Mutat Res 2001;497:177–84
- Jasiński R, Ziółkowska M, Demchuk O, Maziarka A. Regio- and stereoselectivity of polar [2 + 3] cycloaddition reactions between (Z)-C-(3,4,5-trimethoxyphenyl)-N-methylnitrone and selected (E)-2-substituted nitroethenes. Cent Eur J Chem 2014;12:586–93
- Lancianesi S, Palmieri A, Petrini M. Synthetic approaches to 3-(2-nitroalkyl) indoles and their use to access tryptamines and related bioactive compounds. Chem Rev 2014;114:7108–49
- Perekalin VV. Nitroalkenes: conjugated nitro compounds. Chichester (NY): Wiley; 1994
- Fringuelli F, Lanari D, Pizzo F, Vaccaro L. Amberlite IRA900F as a solid fluoride source for a variety of organic transformations under solvent-free conditions. Eur J Org Chem 2008;23:3928–32
- Amantini D, Fringuelli F, Piermatti O, et al. Synthesis of 4-aryl-1H-1,2,3-triazoles through TBAF-catalyzed [3 + 2] cycloaddition of 2-aryl-1-nitroethenes with TMSN3 under solvent-free conditions. J Org Chem 2005;70:6526–9
- Jasiński R, Kubik M, Łapczuk-Krygier A, et al. An experimental and theoretical study of the hetero Diels–Alder reactions between (E)-2-aryl-1-cyano-1-nitroethenes and ethyl vinyl ether: one-step or zwitterionic, two-step mechanism? Reac Kinet Mech Cat 2014;113:333–45
- Łapczuk-Krygier A, Ponikiewski Ł, Jasiński R. The crystal structure of (1RS,4RS,5RS,6SR)-5-cyano-5-nitro-6-phenyl-bicyclo[2.2.1]hept-2-ene. Crystallogr Rep 2014;59(7):961–3
- Parham WE, Bleasdale JL. The condensation of diazo compounds with nitroölefins. II. 3-Bromo- and 3-nitropyrazoles. J Am Chem Soc 1951;73:4664–6
- Łapczuk-Krygier A, Ponikiewski Ł. Single crystal X-ray structure of (Z)-1-bromo-1-nitro-2-phenylethene. Curr Chem Lett 2015;4:21–6
- Ganesh M, Namboothiri INN. Stereospecific approach to α,β-disubstituted nitroalkenes via coupling of α-bromonitroalkenes with boronic acids and terminal acetylenes. Tetrahedron 2007;63:11973–83
- Ried W, Köhler E, Königstein FJ. Reaktionen mit nitro-acetonitril. Justus Liebigs Annalen der Chemie 1956;598:145–58
- European Committee for Antimicrobial Susceptibility Testing of the European Society of Clinical M, Infectious D. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin Microbiol Infect 2003;9:9–15
- Institute CaLS. Reference method for broth dilution antifungal susceptibility testing of yeasts. M27-S4. Wayne (PA): Clinical and Laboratory Standards Institute; 2012
- Popiołek Ł, Biernasiuk A, Malm A. Synthesis and antimicrobial activity of new 1,3-thiazolidin-4-one derivatives obtained from carboxylic acid hydrazides. Phosphorus Sulfur Silicon Relat Elem 2015;190:251–60
- O’Donnell F, Smyth TJ, Ramachandran VN, Smyth WF. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int J Antimicrob Agents 2010;35:30–8
- Jasiński R. Preparatyka alifatycznych nitrozwiązków. Radom (Poland): Radomskie Towarzystwo Naukowe; 2013
- Narayanaperumal S, da Silva RC, Feu KS, et al. Basic-functionalized recyclable ionic liquid catalyst: a solvent-free approach for Michael addition of 1,3-dicarbonyl compounds to nitroalkenes under ultrasound irradiation. Ultrason Sonochem 2013;20:793–8
- Zhao G, Jiang T, Gao H, et al. Mannich reaction using acidic ionic liquids as catalysts and solvents. Green Chem 2004;6:75–7
- Angueira EJ, White MG. Arene carbonylation in acidic, chloroaluminate ionic liquids. J Mol Catal A 2005;227:51–8
- Gholap AR, Venkatesan K, Daniel T, et al. Ionic liquid promoted novel and efficient one pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones at ambient temperature under ultrasound irradiation. Green Chem 2004;6:147–50
- Brillon D, Sauvg G. Silica gel-catalyzed knoevenagel condensation of peptidyl cyanomethyl functionality for C-modified peptides: the benzylidene and alkylidene cyanomethyl ketone function ketones with aromatic aldehydes and ketones. A novel Michael acceptor. J Org Chem 1992;57:1838–42
- Jasiński R, Kwiatkowska M, Barański A. Synthesis of (E)-2-aryl-1-cyano-1-nitroethenes. Czasopismo Techn PK (Chemia) 2006;103:41–6