Abstract
An α-carbonic anhydrase (CA, EC 4.2.1.1) was purified and characterized kinetically from gill of Acipenser gueldenstaedtii as an endangered sturgeon species. The carbonic anhydrase was purified 66-folds with yield 20.7% by Sepharose-4B-l-tyrosine-sulfanilamide affinity column and the specific activity was determined as 222.2 EU/mg protein. Km and Vmax kinetic values for gill carbonic anhydrase were calculated by a Lineweaver–Burk graph using p-nitrophenol acetate (p-NPA) as a substrate, and was defined as 2.5 mM and 5 × 106 μM/min, respectively. It was observed that CA from the sturgeon gill in the presence of the sulfanilamide and acetazolamide as an inhibitor had very low IC50 values such as 13.0 and 0.1 μM, respectively. In addition, it was determined that the enzyme was inhibited by Fe2+, Co2+, Ni2+, and Zn2+–Ba2+ with the IC50 values of 0.2, 1.7, 1.2, and 1.1 mM, respectively.
Introduction
Carbonic anhydrase (CA) (carbonate hydrolyase, EC 4.2.1.1) as a member of zinc metalloenzymes, an important class of enzymes used to regulate CO2 levels in the living organisms, catalyses the reversible hydration of CO2 to and H+Citation1–3. At least 16 different carbonic anhydrase isozymes playing key roles in diverse processes, such as physiological pH control and gas balance, calcification, and photosynthesis, have been described in higher vertebrates until nowCitation4,Citation5. The role of CA in fish gills is considered to be related to gas exchange, acid-base balance, osmoregulation, ion regulation, and clearance of the waste products from nitrogenous metabolismCitation6–9. The pattern of distribution of CA activity among gills pairs varies depending on habitat and osmoregulatory behavior of the animalCitation10. Gill epithelium is the first interface of the organism exposed to the aquatic environment, and for this reason, its a primary target for the action of environmental pollutants on fishCitation11,Citation12.
There are at least 26 species of sturgeons are known so far, and they belong to the Acipenseridae family, one of the oldest existent fish families. Sturgeons are spread in subtropical, temperate and sub-Arctic rivers, lakes and coastlines of Eurasia and North America, being harvested for caviar. Sturgeons are, however, slowly growing fish and mature late in life, being particularly sensitive to environmental pollution. Many of them are in decline or on the brink of extinction due to overfishing, pollution and other factors less well understood at this momentCitation5,Citation13.
Even though the carbonic anhydrase have been purified from erythrocyte of sturgeon and analyzed the effect of some inhibitorsCitation5, the enzyme has not been studied in its gill epithelium. This study includes the purification, characterization, and inhibition of a carbonic anhydrase from Acipenser gueldenstaedtii as the endangered sturgeon species. This enzyme was isolated from gill of this fish. It determined its catalytic activity for the physiological reaction such as esterase and inhibitory effect of some sulfonamides and metal ions using 4-nitrophenyl acetate (4-NPA) as a substrate. These findings may be useful for comparing CAs from various vertebrate species, their susceptibility to inhibition of various compound classes and also for understanding the toxic effect of some heavy metal ions in endangered fish species. Some physiological aspects of CA-mediated processes in this type of less investigated vertebrate are in fact poorly understood, since most of the CA pharmacological/environmental researches have been done with the human and rodent enzymesCitation4.
Material and methods
Materials
All chemicals used in this study were the highest grade purity available and were obtained from Sigma (Milan, Italy) and E. Merck (Darmstadt, Germany). Sepharose 4B activated by CNBr, protein assay reagents, and chemicals for electrophoresis were obtained from Sigma-Aldrich Chemie (Taufkirchen, Germany). 4-Aminobenzene sulfonamide, acetazolamide and l-tyrosine were from E. Merck (Darmstadt, Germany).
Preparation of homogenate
Acipenser gueldenstaedti gill were obtained from fish grown in the Aquaculture Department of Fisheries Faculty at Recep Tayyip Erdogan University in Rize, Turkey. Gill tissues (about 50 g) were cut off carefully with aid of scalpel for avoiding damage, and were washed three times with 0.9% NaCl to eliminate blood and other contaminants. After the tissues were lysed by liquid nitrogen, were smashed in 25 mM Tris HCl/0.1 M Na2SO4 buffer (pH 8.7) with a blender, and homogenized in an ice covered glass teflon homogenizer with up and down 10 strokes. The homogenate was centrifuged for 5 min (5000 rpm at +4 °C)Citation14,Citation15. The supernatant was centrifuged for 45 min (15 000 rpm at +4 °C) and stored at −20 °C until assayed. Protein concentration was measured by using bovine serum albumin as standard based on the method of LowryCitation16.
Purification of CA by affinity chromatography
The solution was applied to a Sepharose 4B-l-tyrosine sulfanilamide affinity column equilibrated with 25 mM Tris–HCl/0.1 M Na2SO4 (pH 8.7). CA was eluted with 0.1 M NaCH3COO/0.5 M NaClO4 (pH 5.6). The 280 nm absorbance of the protein in the column effluents was determined spectrophotometricallyCitation5. Because of inhibition of hydratase activity of the gill CA with the elution buffer, the fractions were combined and the buffer was changed by washing with 50 mM phosphate buffer pH 7.5 by Amicon Ultrafiltration Membrane (10 000 MWCO, 4000 × g). The enzyme solution was prepared by diluting to 5 mL with the same buffer.
Hydratase activity assay
CA activity was also assayed following the hydration of CO2 to bicarbonate and protons according to the Wilbur–Anderson methodCitation17 for measuring active enzyme samples during the purification procedures. CO2–hydratase activity as an enzyme unit (EU) was calculated by using the equation [(t0 − tc)/tc] where t0 and tc are the times for pH change of the non-enzymatic and the enzymatic reactions, respectively. Also, the method was used to determine inhibitory effects of acetazolamide, sulfanilamide, metal ions (Ni2+, Fe2+, Ba2+ Zn2+, Cu2+, and Co2+) and the forage waste solutions (the solutions were prepared by waiting 5 g forage used in the fish feed receptacle in 100 mL of destilled water at room temperature for 7 and 15 d, and then centrifuged for 15 min at 5000 rpm, +4 °C) on CA activity of sturgeon gill. Metal salts were dissolved in water.
Esterase activity assay
The CA activity was assayed by the esterase method by following the change in absorbance at 348 nm of 4-NPA hydrolyzed to 4-nitrophenolate ion in the presence of the enzyme, over a period of 3 min at 25 °C, using a UV–vis spectrophotometer according to the Verpoorte procedureCitation18. The total volume of the enzymatic assay mixture was of 3.0 mL and contained 1.4 mL 0.05 M Tris–SO4 buffer (pH 9.0), 1.0 mL 3 mM 4-NPA in acetone, 0.5 mL distilled–deionized water and 0.1 mL 0.1 μM enzyme solution. The absorbance of the non-catalyzed 4-NPA in this buffer was always subtracted from the enzymatic measurements reported in this article.
Polyacrilamide gel electrophoresis
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was carried out according to the method of LaemmliCitation19 for the determination of purity and molecular weight of the gill CA using a 5% (w/v) stacking gel and 12% (w/v) separating gel. The molecular weight of the enzyme was estimated using different protein markers from 10 kDa to 250 kDa. The molecular weight of the enzyme was estimated using different protein markers from 10 kDa to 250 kDa.
Results
The purification of the gill CA was carried out in one stage by chromatography column on l-tyrosine sulfonamide coupled to Sepharose 4BCitation5,Citation14,Citation15. The fish gill CA was purified with a specific activity of 222.2 EU/mg protein (66-folds) with a yield of 20.7% (). It was obtained a single protein band, evidence of purified enzyme having a molecular weight of the subunit was approximately 29 kDa, based on SDS-PAGE ().
Figure 1. SDS-polyacrylamide gel electrophoresis of the gill CA (line 1: purified sturgeon gill CA, line 2: the homogenate of sturgeon gill, line 3: standard proteins).
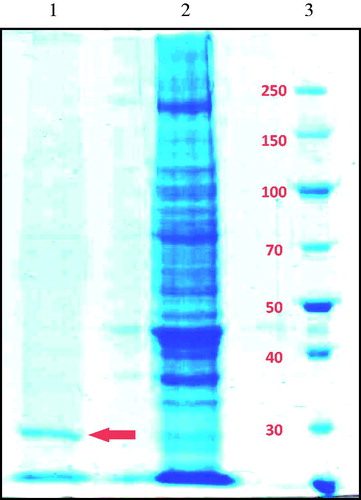
Table 1. Purification of sturgeon gill CA by affinity chromatography.
Kinetic parameters of the sturgeon CA for 4-NPA as a substrate were determined by the Lineweaver–Burk procedure, and are shown in . The Km, Vmax, and kcat values for 4-NPA hydrolysis were determined to be 2.5 mM, 5 × 106 μM/min and 134 408.6 s−1, respectively.
Table 2. Kinetic parameters of the sturgeon gill CA in the presence of 4-nitrophenyl acetate (pNPA) as a substrate.
shows the in vitro effect of sulfonamides, acetazolamide and some metal ions on the sturgeon gill CA activity. Very low IC50 values were obtained such as 13.0 and 0.1 μM in the presence of the sulfanilamide and acetazolamide as inhibitors. Additionally, IC50 values of Fe2+, Co2+, Ni2+, and Zn2+–Ba2+ cations were determined to be 200, 1700, 1200, and 1100 µM, respectively. Also, any inhibitory effect of waste feed solution was observed on the hydratase activity of the gill CA.
Table 3. In vitro inhibition of some metal ions and sulfonamides against the hydratase activity of sturgeon gill CA.
Discussion
CAs are ubiquitous enzymes, catalyzing a physiologically crucial reaction, the reversible hydration of CO2 to and H+4,Citation20–24. These enzymes, as multiple isoforms in most organisms studied so far, are involved in a wide range of physiological processes, being present in high amounts in most tissues, for example, in erythrocytesCitation4. In mammals, these enzymes are involved in processes connected with respiration and transport of CO2/
, pH, and CO2 homeostasis, electrolyte secretion in a variety of tissues/organs, biosynthetic reactions (such as gluconeogenesis, lipogenesis and ureagenesis), bone resorption, calcification, tumorigenicity, and many other physiological or pathological processes (thoroughly studied in many vertebrates), whereas in algae, plants, and some bacteria, they play an important role in photosynthesis and other biosynthetic reactionsCitation4,Citation20–24.
CA is also an important enzyme in several other key physiological processes. For example, the gills of aquatic organisms function in respiratory gas exchange, salt transport, and acid–base balance and CA can function in all three processes. The multiple functions of CA are a result of multiple isoforms that are localized to specific subcellular compartments/fractions within the gillCitation25,Citation26. As gill carbonic anhydrases contact primary with habitat of fish, investigation of these functions' performance is necessary how affected by environmental factors.
In this study, the carbonic anhydrase was purified the first time from sturgeon gill, in a single step, by using affinity chromatography. The purified enzyme showed very good catalytic activity for the physiological reaction, possessing kcat and Km values rather similar to those of its human counterpart, hCA II, which is one of the best catalysts known in natureCitation4. Furthermore, this enzyme has been inhibited by the classical sulfonamide inhibitors acetazolamide and sulfonamide, with the IC50 values of 0.1 μM and 13.0 μM, respectively.
CA was purified from sturgeon fish erythrocytes, the same technique and esterase activity of the enzyme showed optimal activity at pH 9.0 and 30 °C. Km, Vmax, and kcat values of sturgeon erythrocytes CA were determined using 4-nitrophenyl acetate as a substrate to be 4 mM, 20 000 μM/min and 20.8 s−1, respectivelyCitation5. Hisar et al.Citation27 reported that the CA was purified 104.8-folds with a specific activity of 55.6 EUxmg−1 from the rainbow trout gills by using Sepharose 4B-l-tyrosine-sulfanilamide affinity column and Km and the Vmax values were obtained as 8.13 mM and 2.10 mmol/min mg for 4-NPA substrate. Soyut and BeydemirCitation28 purified a fish enzyme from the rainbow trout liver and achieved a purification of 2260-folds with a specific activity of 4318 EUxmg−1 and recovery of 38% using the identical technique.
The subunit molecular weight of the enzyme was estimated to be about 29 kDa by SDS-PAGE. It is indicated that the carbonic anhydrases derived from different organisms have the mono, trimer, tetramer, and octamer structures with the subunit molecular mass of from 18.9 to 29.3 kDaCitation29. Kolayli et al.Citation5 reported that the molecular weight of the erythrocyte CA from Russian Sturgeon Fish is about 29 kDa while Hisar et al.Citation27 reported that it to be 27 kDa from the rainbow trout gills and 25 kDa from gilthead sea bream liver CACitation18.
Data of show that the gill CA was weakly inhibited by divalent heavy metal ions, with IC50 values in the range of 200–1700 µM. The most inhibitory cation was Fe (II) and the least inhibitory was Co (II). Thus, it is probable that heavy metal ions do not constitute a great danger for this fish, or at least they do not inhibit significantly the CA found in the gill of this sturgeon. In addition, inhibitory effect of sulfonamides as acetazolamide and sulfonamide, inhibitors of CA, was investigated on the carbonic anhydrase. Data of show that acetazolamide is an effective CA inhibitor (IC50 value of 0.10 μM) also for the hydratase activity of this enzyme, whereas sulfanilamide is a weaker CA inhibitor having an IC50 value of 13 μM. The heavy metals were found to be a very weak inhibitor for gill CA. Contrarily, the aromatic and heterocyclic sulfonamides strongly inhibit this enzyme, as they do with most α-CAs investigated so far in all types of organisms, from bacteria to protozoa, fish, and mammalsCitation5.
In conclusion, a sturgeon gill CA that has not been reported earlier and analyzed characteristic features was purified in a one-step affinity chromatography procedure. Its kinetic properties show that the CA activity is similar to hCA II. In light of these results, the sturgeon gill CA is inhibited by classical sulfonamide inhibitors (sulfonamide and acetazolamide) and some environmental pollutants as heavy metal ions by using the hydratase activity of this enzyme.
Declaration of interest
This work was financially supported by the Recep Tayyip Erdoğan University.
References
- Beydemir S, Ciftci M, Ozmen I, et al. Effects of some medical drugs on enzyme activities of carbonic anhydrase from human erythrocytes in vitro and from rat erythrocytes in vivo. Pharmacol Res 2000;42:187–91
- Esposito EX, Baran K, Kelly K, Madura JD. Docking of sulfonamides to carbonic anhydrase II and IV. J Mol Graph Mod 2000;18:283–9
- Boztaş M, Çetinkaya Y, Topal M, et al. Synthesis and carbonic anhydrase isoenzymes I, II, IX, and XII inhibitory effects of dimethoxy-bromophenol derivatives incorporating cyclopropane moieties. J Enzyme Inhib Med Chem 2015;58:640–50
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Kolayli S, Karahalil F, Sahin H, et al. Characterization and inhibition studies of an α-carbonicanhydrase from the endangered sturgeon species Acipenser gueldenstaedti. J Enzyme Inhib Med Chem 2011;26:895–900
- Wilkie MP. Mechanisms of ammonia excretion across fish gills. Comp Biochem Physiol 1997;118A:39–50
- Henry RP, Boutilier RG, Tufts BL. Effects of carbonic anhydrase inhibition on the acid-base status in lamprey and trout. Respir Phys 1995;99:241–8
- Evans D, Cameron JN. Gill ammonia transport. J Exp Zool 1986;239:17–23
- Randall DJ, Daxbaeck C. Oxygen and carbon dioxide transfer acroos fish gills. In: Hoar WS, Randall DJ, eds. Fish histology. Vol. 10A. New York: Academic Press; 1984
- Lopez Mananes AA, Magnoni LJ, Goldemberg AL. Brancial carbonic anhydrase (CA) of gills of Chasmagnathus granulata (Crustacea decapoda). Comp Biochem Physiol 2000;127B:85–95
- Lionetto MG, Giordano ME, Vilella S, Schettino T. Inhibition of eel enzymatic activities by cadmium. Aquat Toxicol 2000;48:561–71
- Hisar Aras S, Hisar O, Yanık T, Aras SM. Inhibitory effects of ammonia and urea on gill carbonic anhydrase enzyme activity of rainbow trout (Oncorhynchus mykiss). Environ Toxicol Pharm 2004;17:125–8
- Bemis WE, Findeis EK, Grande L. An overview of Acipenseriformes. Environ Biol Fish 1997;48:25–71
- Kaya ED, Söyüt H, Beydemir S. Carbonic anhydrase activity from the gilthead sea bream (Sparus aurata) liver: the toxicological effects of heavy metals. Environ Toxicol Pharmacol 2013;36:514–21
- Böttcher K, Siebers D, Becker W. Carbonic anhydrase in branchial tissues of osmoregulating shore crabs, Carcinus maenas. J Exp Zool 1990;255:251–61
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265–75
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948;176:147–54
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–9
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5
- Supuran CT. Carbonic anhydrase inhibition/activation: trip of a scientist around the world in the search of novel chemotypes and drug targets. Curr Pharm Des 2010;16:3233–45
- Alterio V, Hilvo M, Di Fiore A, et al. Crystal structure of the catalytic domain of the tumorassociated human carbonic anhydrase IX. Proc Natl Acad Sci USA 2009;106:16233–8
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–89
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–50
- Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors and activators and their use in therapy. Exp Opin Ther Pat 2006;16:1627–64
- Cudennec B, Rousseau M, Lopez E, Fouchereau-Peron M. CGRP stimulates gill carbonic anhydrase activity in molluscs via a common CT/CGRP receptor. Peptides 2006;27:2678–82
- Sender S, Böttcher K, Cetin Y, Gros G. Carbonic anhydrase in the gills of seawaterand freshwater-acclimated flounders Platichthys flesus: purification, characterization, and immunohistochemical localization. J Histochem Cytochem 1999;47:43–50
- Hisar O, Beydemir Ş, Bülbül M, Yanık T. Kinetic properties of carbonic anhydrase purified from gills of rainbow trout (Oncorhynchus mykiss). J Appl Anim Res 2006;30:185–8
- Soyut H, Beydemir S. Purification and some kinetic properties of carbonic anhydrase from rainbow trout (Oncorhynchus mykiss) liver and metal inhibition. Protein Pept Lett 2008;15:528–35
- Smith KS, Ferry JG. Prokaryotic carbonic anhydrases, reviews. FEMS Microbiol 2000;24:335–66
