Abstract
Recently it was found that dipotassium-trioxohydroxytetrafluorotriborate, K2(B3O3F4OH), is a potent and highly specific inhibitor of precancerous cell processes. We conducted gene expression profiling of human melanoma cells before and after treatment with two concentrations (0.1 and 1 mM) of this boron inorganic derivative in order to assess its effects on deregulation of genes associated with tumor pathways. Parallel trypan blue exclusion assay was performed to assess the cytotoxicity effects of this chemical. Treatment with K2(B3O3F4OH) induced a significant decrease of cell viability in melanoma cellline at both tested concentrations. Furthermore, these treatments caused deregulation of more than 30 genes known as common anti-tumor drug targets. IGF-1 and hTERT were found to be significantly downregulated and this result may imply potential use of K2(B3O3F4OH) as an inhibitor or human telomerase and insulin-like growth factor 1, both of which are associated with various tumor pathways.
Introduction
Cancer research continually identifies novel deregulated carcinogenesis-related genes elucidating new mechanisms of cancer progression or treatment evasion, and potentially leading to new avenues for drug development.
K2(B3O3F4OH), is a boron inorganic derivative recently explored as useful in treatment of benign and malignant skin changes, such as nevus or skin cancerCitation1,Citation2. First published effects of its bioactivity, revealed a potential for inhibition of lymphocytes proliferation and cell growth of basal cell carcinoma culture as well as certain clastogenic potentialCitation3. Recently it has been confirmed that selected flavonoids may inhibit damages of genetic material in human lymphocytes induced by K2(B3O3F4OH) in concentration of 0.1 mg/mlCitation4. K2(B3O3F4OH) has been recognized as a potent inhibitor of human carbonic anhydrases and catalase involved in the intracellular antioxidant mechanismCitation5. Preliminary investigations imply that acute toxicity of this substance is approximately 60 mg/kg suggesting reproducible biological effects on genetic material at low treatment concentrations (B. Galic, data not published). Previous findings of antitumor activity of the halogenated boroxine in vitro and in vivo on 4T1 mammary carcinoma, B16F10 melanoma and squamos cell carcinoma SCVII revealed inhibitory effects on cell proliferation in concentrations of 1 mM while concentrations of less than 0.1 mM do not significantly affect cell growthCitation6. In vivo, K2(B3O3F4OH) slows the growth of three tested tumors compared to control regardless of the route of administration (intraperitoneally, intratumor, per oral or as an ointment).
This experiment was designed to assess the effects on gene expression of selected antitumor targets under the treatment with previously established effective concentration limits of K2(B3O3F4OH) (0.1 mM and 1 mM) in comparison to untreated tumor cells (control). Differential gene expression between treated and untreated cell lines, as well as relative effects between two tested concentrations of tested substance have been evaluated. The qPCR assay that was used in this experiment precisely quantified expression of genes involved as human cancer drug targets in melanoma cell lines treated with K2(B3O3F4OH).
Material and methods
Chemistry
The boron inorganic heterocyclic compound dipotassium-trioxohydroxytetrafluorotriborate, K2(B3O3F4OH), was prepared as reported in the literatureCitation7. All other compounds used in this study were commercially available, highest purity reagents (Sigma-Aldrich, St. Louis, MO).
Melanoma cell line
The Human Caucasian melanoma cell line (GR-M) was obtained from Culture Collections, Public Health England, London, UK (Cat. No. 95032301). Cell line was cultured in RPMI 1640 (Gibco-Invitrogen, Grand Island, NY) with l-glutamine supplemented with 10% of fetal bovine serum (FBS) and 100 units/ml penicillin, 100 mg/ml streptomycin (Sigma-Aldrich, St. Louis, MO). T-25 flasks (NUNC, Rochester, NY) with vented caps were used as a culture dishes and cultures were maintained in CO2 incubator (EC 160, Nuve, Ankara, Turkey) at 37 °C in a 5%CO2 atmosphere with 95% humidity. Cell cultures were treated with K2(B3O3F4OH) in concentrations of 0.025 mg/ml and 0.25 mg/ml at 70% of confluence. Cells were exposed to K2(B3O3F4OH) for 48 h.
Trypan blue exclusion assay
For the cytotoxicity analysis trypan blue exclusion assay was performed after 48 h of incubation of melanoma cell cultures with K2(B3O3F4OH). Each treatment was carried out in triplicates. Cells were harvested by trypsinization and the cell viability (%) was determined as (number of viable cells/total number of viable + non-viable cells) × 100. ANOVA and Newman–Keuls multiple comparison test in WINKS 4.5 Professional software (TexaSoft, Cedar Hill, TX) were used for statistical analysis and pairwise comparisons. Statistical significance threshold was fixed at 0.05.
RNA isolation and RT PCR
Upon the treatment of melanoma cells by K2(B3O3F4OH) in the above-mentioned manner, total RNA was extracted from harvested cells using RNA isolation kit (QIAGEN GmbH, Hilden, Germany). The quality and quantity of isolated RNA was estimated using RNA fluorometric assay on Qubit 2.0 (Life Technologies, London, United Kingdom). Additional purification step was introduced to eliminate any residual genomic DNA that might cause false positive signals. RT2 First strand kit (Qiagen GmbH, Hilden, Germany) was used for generation of cDNA for further genetic analysis according to manufacturer’s protocol (RT2 Profiler PCR Array Handbook, Qiagen N. V., Venlo, Limburg, Netherlands).
Gene expression analysis
Real Time PCR is a highly sensitive and reliable method for gene expression analysis at cell transcriptome level. It is a convenient method for simultaneous analysis of number of different genes in the same sample. Human Cancer Drug Targets RT2 Profiler™ PCR method is used according to manufacturer’s instructions (RT2 Profiler PCR Array Handbook, Qiagen N. V., Venlo, Limburg, Netherlands) for a multigene profiling of expression of 84 genes () on ABI 7300 real-time PCR apparatus (Applied Biosystems, Foster City, CA). The three cell populations used in the experiment (cell culture control sample and two cell cultures treated with K2(B3O3F4OH) in concentrations of 0.1 mM and 1 mM) were subsequently analyzed in three identical 96 wells array formats. The quality check up (false positive and false negative signals evaluation) was based on positive and negative controls included on arrays, as well as gDNA contamination probes.
Table 2. List of genes with changed expression profile after K2(B3O3F4OH) treatment with 1 mM and 0.1 mM: negative values show gene down-regulation of ≥2 fold compared to control – untreated melanoma cells; positive values show gene up-regulation of ≥2 fold compared to control – untreated melanoma cells).
Genetic data were collected during the real-time PCR procedure in a form of Ct value or the order number of PCR cycle when target (gene) signal amplification reached exponential phase. All samples that had ct value 35 and higher were considered as null gene expression. Fold change in gene expression between melanoma cell culture treated with K2(B3O3F4OH) (0.1 mM and 1 mM) and control (untreated cells) and between two treated samples was calculated using 2(−ΔΔCT) Method (www.SABiosciences.com).
Results and discussion
Cytotoxic effects
Exposure of GR-M cells to K2(B3O3F4OH) induced decrease in viable cell number in a concentration dependent manner. Data, presented in , are expressed as mean ± standard deviation (SD). Analysis of variance revealed that the decrease of cell viability was significant (p = 0.006) in both treatments as compared to controls, while there were no significant differences between treatments with two different concentrations of K2(B3O3F4OH).
Table 1. Results of trypan blue exclusion assay.
Gene expression effects
Prior to results readout, quality control assessment of the experiment was done. All gDNA controls were found to be above expected threshold level of 35 (below positive amplification), suggesting that any false positive signal in this experiment due to sample contamination with genomic DNA is unlikely. The cycle number deviation between triplicated signals from housekeeping genes (reference genes) was 20 ± 0.3 and within the acceptable range (20 ± 2.0 cycle), indicating that no PCR inhibitors were present in amplification runs.
Gene expression was evaluated for each well and Ct values were exported for further data analysis. The control sample (untreated melanoma cells), that was used as the gene expression reference, had a measurable expression of all 84 tested genes.
Pairwise comparison results in scatter diagram show clear difference in gene expression pattern between control and cells treated with K2(B3O3F4OH) in concentration of 1 mM () and 0.1 mM () and between cells treated with different K2(B3O3F4OH) concentrations (). If the threshold is set to two fold change in gene expression and higher, several genes’ expression is clearly grouped as those affected by the treatment ().
The treatment with 1 mM concentration of K2(B3O3F4OH) induced change in expression pattern of 36 genes from the tested panel of human cancer drug targets or 43% of all analyzed genes.
Six genes were up-regulated in 1 mM concentration: ERBB3, ESR1, ESR2, HSP90B, PARP2 and TERT, and 30 genes were down-regulated under equal treatment conditions (). Negative deregulation of TOP2A, PDGFRA, KDR, GRB2 and cathepsins was observed.
Treatment with 0.1 mM concentration of K2(B3O3F4OH) caused less effect on targeted genes expression profile (eight genes). Genes from the family of hormone receptors (ERBB3, ERBB4, ESR1, ESR2, PGR) and PRKCB (Protein kinase C-beta) were up-regulated in the range between 2.5 and 5-fold.
Interestingly, two genes, encoding for IGF1 and TERT were significantly down-regulated. Assessment of differential gene expression pattern between two different concentrations of K2(B3O3F4OH) revealed that: TERT gene was significantly up-regulated and IGF-1 was not deregulated at all under 0.25 mg/ml treatment.
Figure 1. Scatter plot diagram comparing expression profiles of the cells treated with 1 mM of K2(B3O3F4OH) and untreated melanoma cells. Empty dots represent genes whose expression did not change significantly in response to treatment. Filled dots represent genes that have been down-regulated and crossed dots genes with two or more fold up-regulation.
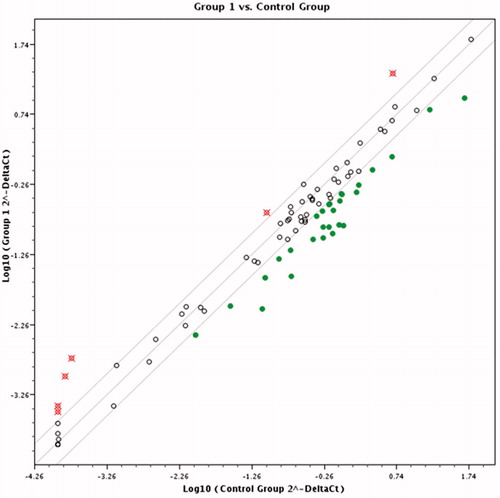
Figure 2. Scatter plot diagram comparing expression profiles of the cells treated with 0.1 mM of K2(B3O3F4OH) and untreated melanoma cells. Empty dots represent genes whose expression did not change significantly in respect to treatment. Filled dots represent genes that have been down-regulated and crossed dots genes with two or more fold up-regulation.
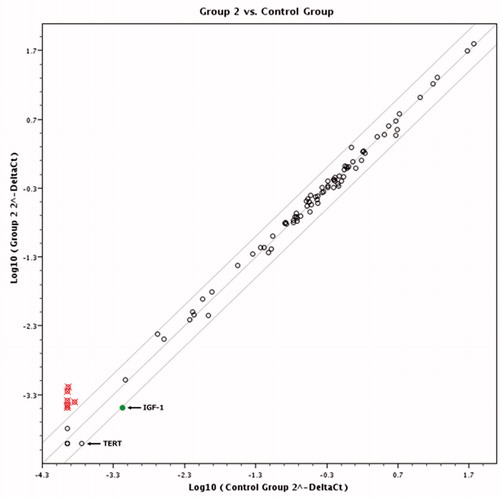
Figure 3. Scatter plot diagram comparing expression profiles of the cells treated with 1 mM and 0.1 mM of K2(B3O3F4OH). Empty dots represent genes whose expression did not change significantly in respect to treatment. Filled dots represent genes that have been down-regulated and crossed dots genes with two or more fold up-regulation.
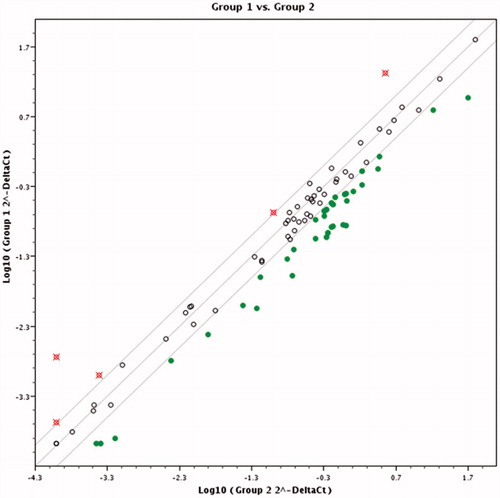
Dysregulation of the IGF system in tumor cells has been previously qualified as a key mechanism by which the balance of pro-survival and pro-apoptotic signaling shifts in favor of survival. This pro-survival predisposition of tumors may also have a major negative impact on the anti-tumor therapies that are used in clinical practice that rely on apoptosis: cytotoxic chemotherapy, biological therapies, hormonal therapies and radiation therapy. It has been observed that patient with melanoma exhibit significantly higher serum concentrations of IGF-1 which has also been associated with increased risk of lymph metastasisCitation8. Clinical trials have shown that patients with tumors expressing IGF-1 receptors are much less likely to obtain a clinical response to neoadjuvant therapy than non-expressers of IGF-1Citation9. From this perspective, blocking IGF system signaling has the potential for numerous clinically useful effects, including increasing the proportion, extent and duration of clinical responses from cytotoxic therapies when used in combination. Other studies showed the potential of IGF/IGF-IR pathway inhibition in antitumor and treatment of autoimmune disordersCitation10,Citation11.
Another notable effect of K2(B3O3F4OH) treatment at concentration of 0.1 mM is its downregulating effect on TERT gene that is involved in telomerase activity. Telomerase is active in the majority of cancer cell types and is virtually absent in somatic cells with rare exceptions. This difference allows us to consider inhibition of telomerase activity as a possible approach to antitumor therapyCitation12,Citation13.
Our results are in favor of further assessment of antitumor targets deregulated by K2(B3O3F4OH) at gene expression level and proof of its inhibitory effect on various cancers via defined molecular targets and implicated pathway analysis – i.e. Ras/Raf/MEK/ERK/MAPK (Ras pathway) and PI3K/AKT (AKT pathway) that are mean of oncogenic mediation of IGF-1 signaling or telomerase components: the reverse transcriptase (hTERT), which is a catalytic subunit, and telomerase RNA (hTR) availability and interactionsCitation14.
Conclusion
This work represents first evaluation of antitumor effects of K2(B3O3F4OH) on expression of 84 genes that encode for proteins qualified as common anti-cancer drug targets. Tested substance shows high potency, polyvalence and observable effects on gene expression of a number of tumor associated proteins and their implicated pathways. Both tested concentrations (1 and 0.1 mM) significantly inhibit cell proliferation of melanoma cells and specifically deregulate genes associated with protumor activity revealing potential antitumor molecular targets of K2(B3O3F4OH). Targeted pathway assays upstream from deregulated genes (suggestively TERT and IGF-1) should be challenged in order to explain molecular mechanism action of K2(B3O3F4OH) in detail.
Declaration of interest
This work has been supported in part by Croatian Science Foundation under the project number (IP-2014-09-6897). The authors report no conflict of interest.
References
- Galic B. Removal of skin changes, European patent, EP1996514; 2013
- Galic B. Boroxine composition for removal of skin changes. Inventor: Galic B. Int. Cl. A61K3169, USA patent US8278289; 2012
- Haveric S, Haveric A, Bajrovic K, et al. Effects of dipotassium trioxohydroxytetrafluorotriborate (K2[B3O3F4OH]) on genetic material and inhibition of cell division in human cell cultures. Drug Chem Toxicol 2011;34:250–4
- Hadzic M, Haveric S, Haveric A, Galic B. Inhibitory effects of delphinidin and luteolin on genotoxicity induced by K2(B3O3F4OH) in human lymphocytes in vitro. Biologia 2015;70:553–8
- Vullo D, Milos M, Galic B, et al. Dipotassium-trioxohydroxytetrafluorotriborate, K2(B3O3F4OH), is a potent inhibitor of human carbonic anhydrases. J Enzyme Inhib Med Chem 2015;30:341–4
- Ivankovic S, Stojkovic R, Galic Z, et al. In vitro and in vivo antitumor activity of the halogenated boroxine dipotassium trioxohydroxytetrafluorotriborate (K2[B3O3F4OH]). J Enzyme Inhib Med Chem 2014;18:1–6
- Ryss IG, Slutskaya MM. Report on the platinum sector. Akad Nauk SSSR 1951;26:216–18
- Kucera R, Treskova I, Vrzalova J, et al. Evaluation of IGF1 serum levels in malignant melanoma and healthy subjects. Anticancer Res 2014;34:5217–20
- Weroha SJ, Haluska P. IGF-1 receptor inhibitors in clinical trials – early lessons. J Mammary Gland Biol Neoplasia 2008;13:471–83
- Sharon C, Baranwal S, Patel NJ, et al. Inhibition of insulin-like growth factor receptor/AKT/mammalian target of rapamycin axis targets colorectal cancer stem cells by attenuating mevalonate-isoprenoid pathway in vitro and in vivo. Oncotarget 2015;6:15332–47
- Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol Rev 2010;62:199–236
- Zvereva MI, Zatsepin TS, Azhibek DM, et al. Oligonucleotide inhibitors of telomerase: prospects for anticancer therapy and diagnostics. Biochemistry (Mosc) 2015;80:251–9
- Ferguson LR, Chen H, Collins AR, et al. Genomic instability in human cancer: molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin Cancer Biol 2015. [Epub ahead of print]. doi: 10.1016/j.semcancer.2015.03.005
- Pal D, Sharma U, Khajuria R, et al. Augmented telomerase activity, reduced telomere length and the presence of alternative lengthening of telomere in renal cell carcinoma: plausible predictive and diagnostic markers. Gene 2015;562:145–51
