Abstract
A series of new 7-arylpiperazinylalkyl-1,3-dimethyl-purine-2,6-dione derivatives with diversified 8-amino substituent in 8 position was synthesized and their 5-HT1A, 5-HT2A, 5-HT6, 5-HT7, and D2 receptor affinities were determined. The binding study allowed identifying some potent 5-HT1A/5-HT2A/5-HT7/D2 ligands. The most interesting because of their multireceptor profile were 8-piperidine (30–35) and 8-dipropylamine (45–47) analogs with four and five carbon aliphatic linkers. The selected compounds 24, 31, 34, 39, 41, 43, 45, and 46 in the functional in vitro evaluation for all targeted receptors showed significant partial D2 agonist, partial 5-HT1A agonist, and 5-HT2A antagonist properties. The advantageous in vitro affinity of compound 34 for 5-HT1A and D2 receptors has been explained by means of molecular modeling, taking into consideration its partial agonist activity towards the latter one. In behavioral studies, compounds 32 and 34 revealed antipsychotic-like properties, significantly decreasing d-amphetamine-induced hyperactivity in mice.
Introduction
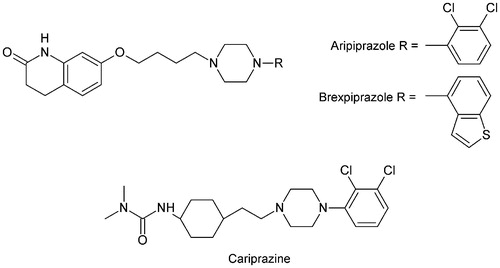
Development of the atypical antipsychotic drugs (AADs), such as olanzapine, quetiapine, and risperidone and more recently ziprazidone and paliperidone, was crucial innovation for the contemporary pharmacotherapy of schizophrenia. In comparison with the first-generation antipsychotics like, e.g. haloperidol, chlorpromazine, or fluphenazine, AADs interact with many serotoninergic receptors including 5-HT1A, 5-HT2A, 5-HT6, 5HT7, and dopaminergic D2 receptorsCitation1,Citation2. The multiple mechanism of their action significantly reduced the range of the observed adverse effects, such as extrapyramidal symptomsCitation3 but those drugs did not eliminate cognitive deficits in schizophrenia. Therefore, new therapeutic agents with dual effects, e.g. suppression of psychotic symptoms and elimination of cognition impairment are still neededCitation4. In the recent years, the new class of antipsychotics called “dopamine stabilizers”, exemplified by aripiprazole and compounds investigated in clinical trials, e.g. brexpiprazole and cariprazine have been identified (). Aripiprazole possessing broad spectrum of clinical benefits (antipsychotic, antidepressant, and anxiolytic effects) through unique receptor profile, partial agonist of D2 and 5-HT1 receptors and antagonist of 5-HT2A and 5-HT7 sites, was revealed to be effective against schizophrenia with anxiogenic and depressive symptoms. Moreover, aripiprazole received supplemental approval for the treatment of the acute manic episodes of bipolar disorder and as an adjunct therapy for major depressive disorderCitation5. The new class of dopamine stabilizers represents an important therapeutic innovation in psychopharmacology and is distinct from D2 antagonists in terms of side effects and improvement of negative and cognitive symptoms. Several series of aripiprazole analogs, e.g. quinoline- and isoquinoline – amide and sulfonamide – derivatives of long-chain arylpiperazines (LCAPs) have been explored as potential antipsychotic, antidepressant, and anxiolytic agents ()Citation6–8.
Among the currently numerous pharmacological approaches for schizophrenia treatments, considerable attention has been focused on 5-HT6 antagonists. Because of their distribution in cerebral cortex and limbic areas, 5-HT6 receptors were proposed to be involved in cognitive process. This modulatory activity including elevating extracellular levels of both glutamate and acetylcholine suggests potential utility for 5-HT6 receptor antagonist in the treatment of cognitive impairments associated with schizophreniaCitation9–11. Taking into account the above-mentioned findings, the multitarget strategy seems to be one of the most valuable approaches in the development of CNS active agents with potential antipsychotic, anxiolytic, and/or antidepressant-like activities.
For several years, our attention has been focused on the development of agents generally classified as long-chain arylpiperazines (LCAPs), containing 1,3-dimethylpurine-2,6-dione system as a terminal amide fragment, which were mainly evaluated toward 5-HT1A, 5-HT2A, and 5-HT7 receptorsCitation12–18 and recently also for 5-HT6 and D2 receptorsCitation19,Citation20. Among them, several compounds containing 8-alkoxy- or 8-morpholinyl-1,3-dimethylpurine-2,6-dione moiety showed features of a 5-HT1A/5-HT7 or 5-HT1A/5-HT2A receptor ligand and produced antidepressant- and anxiolytic-like properties in behavioral tests in miceCitation15,Citation18,Citation21. The multireceptor strategy was also explored among 8-unsubstituted 1,3-dimethylpurine-2,6-dione derivatives of LCAPsCitation19. The study allowed us to identify some potent 5-HT1A receptor ligands with additional moderate affinity for 5-HT2A, 5-HT7, and D2 receptors and a diversified 5-HT1A receptor functional profile which showed antidepressant- and anxiolytic-like activities even greater than those of the reference compounds (imipramine and diazepam, respectively)Citation19.
Continuing our study in a group of purine-2,6-dione derivatives of LCAPs and to extend the studies aimed at verification of the kind of substituent in a 8 position of the purine-2,6-dione system on the selected serotonin and dopamine receptor affinity, we designed a novel series of 7-arylpiperazinylalkyl-8-amino-1,3-dimethylpurine-2,6-dione derivatives (18–47). The influence of three structure features – the kind of amine moiety in the 8-position of purine-2,6-dione system, the linker length between the purine-2,6-dione core and phenylpiperazine (PhP) fragment, and the kind of substituent in the phenyl ring were explored.
Herein, we report on their synthesis and in vitro affinity evaluation for selected 5-HT1A, 5-HT2A, 5-HT6, 5-HT7, and D2 receptors, as well as determination of their intrinsic activity. Moreover, the potential antipsychotic-like properties of the selected compounds 32 and 34 in the behavioral test in mice will be presented. This is interesting in terms of validation of the multireceptor profile of the designed compounds which can lead to the formation of potential psychotropic (antipsychotic, antidepressant, and anxiolytic) agents.
Methods
Chemistry
Organic solvents (from Sigma-Aldrich, St. Louis, MO and Chempur, Karlsruhe, Germany) were of reagent grade and were used without purification. All other reagents were from Sigma-Aldrich (St. Louis, MO) and Alfa Aesar. Melting points (m.p.) were determined with a Büchi apparatus (BUCHI Labortechnik, Essen, Germany) and are uncorrected. All the compounds were routinely checked by TLC using Merck Kieselgel 60 F254 sheets (Merck, Darmstadt, Germany) with the following solvent: (A) dichloromethane/methanol (9.5:0.5). Spots were detected by UV absorption. Column chromatography separations were carried out on column with Merck Kieselgel 60 (Merck, Darmstadt, Germany) using the solvent: (A) dichloromethane/methanol (9.5:0.5). 1H-NMR spectra were taken with a Varian Mercury-VX (300 MHz) spectrometer (Merck, Darmstadt, Germany) in CDCl3 (6 and 10–17) or DMSO-d6 (18–47) solutions, using signal of solvent’s residual 1H atoms as an internal standard (δ = 7.26 ppm and 2.48 ppm, respectively). Chemical shifts were expressed in δ (ppm) and the coupling constants J in Hertz (Hz) and splitting patterns are designated as follows: s (singlet), d (doublet), t (triplet), q (quartet), and m (multiplet).
LC/MS analysis was performed on Waters Acquity TQD apparatus (Waters Corporation, Milford, MA) with eλ DAD detector. For mass spectrometry ESI + (electrospray positive), ionization mode was used. UV spectra were taken in the range of 200–700 nm. For establishing the purity of compounds, UV chromatograms were used. The UPLC/MS purity of all the investigated compounds was determined to be over 98%. Elemental analysis was taken with Elementar Vario EL III apparatus. All analyses were within ± 0.4% of the theoretical values.
The synthesis of the designed 8-amino-7-arylpiperazinylalkyl-1,3-dimethylpurine-2,6-diones hydrochlorides (18–47) is presented in the Scheme 1.
Scheme 1. The synthesis of 8-amino-purine-2,6-dione derivatives of LCAP 18–47. Reagents and conditions: (a) 1-chloro-3-bromopropane, 1-chloro-4-bromobutane, or 1-chloro-5-bromopentane, K2CO3, TEBA, acetone, boiling temp., 15–17 h; (b) 1-arylpiperazine, K2CO3, 1-propanol, 80°C, (c) conc. HCl, acetone.
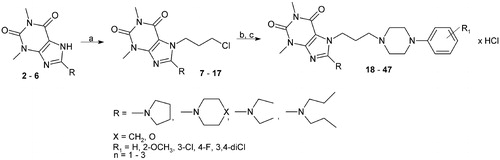
The starting 8-bromo-1,3-dimethylpurine-2,6-dione (1) was synthesized by a procedure published elsewhereCitation22. The 1,3-dimethyl-8-pyrrolidin-1-yl-7H-purine-2,6-dione (2)Citation23, 1,3-dimethyl-8-(1-piperidyl)-7H-purine-2,6-dione (3)Citation23, 1,3-dimethyl-8-morpholino-7H-purine-2,6-dione (4)Citation23, 8-(diethylamino)-1,3-dimethyl-7H-purine-2,6-dione (5)Citation24, and new 8-(dipropylamino)-1,3-dimethyl-7H-purine-2,6-dione (6) were obtained from 1 according to the previously described methodCitation23.
8-(Dipropylamino)-1,3-dimethyl-7H-purine-2,6-dione (6)
Yield: 75%, m.p. 188–190 °C, Rf = 0.51 (A), 1H-NMR (CDCl3) δ: 0.92 (t, J = 7.4 Hz, 6H, N(CH2CH2CH3)2), 1.56–1.74 (m, 4H, N(CH2CH2CH3)2), 3.40 (s, 3H, N1-CH3), 3.44–3.52 (m, 4H, N(CH2CH2CH3)2), 3.55 (s, 3H, N3-CH3), 10.55 (s, 1H, N7-H). LC/MS: m/z calc. 280.17, found 280.37. Anal. calcd. for C13H21N5O2: C, H, N.
The 7-(3-chloropropyl)-1,3-dimethyl-8-(1-piperidyl)purine-2,6-dione (7)Citation25, 7-(4-chlorobutyl)-1,3-dimethyl-8-morpholino-purine-2,6-dione (8)Citation18, 7-(3-chloropropyl)-8-(diethylamino)-1,3-dimethylpurine-2,6-dione (9)Citation25, and new 1,3-dimethyl-7-chloroalkyl-8-amino-purine-2,6-dione derivatives (10–17) were prepared in a reaction of 10 mmol of 2–6 with 20 mmol appropriate alkylating agent (1-bromo-3-chloropropane, 1-bromo-4-chlorobutane, and 1-bromo-5-chloropentane) according to the previously described methodCitation26. Compounds 7–17 were purified by crystallization from methanol (Scheme 1).
7-(3-Chloropropyl)-1,3-dimethyl-8-pyrrolidin-1-yl-purine-2,6-dione (10)
Yield 74%, m.p. 129–130 °C, Rf = 0.59 (A), 1H-NMR (CDCl3) δ: 1.98–2.03 (m, 4H, 3,4-pyrrolidine), 2.21–2.30 (m, 2H, N7-CH2CH2CH2Cl), 3.37 (s, 3H, N1-CH3), 3.55–3.59 (m, 2H, CH2Cl), 3.57 (s, 3H, N3-CH3), 3.65–3.68 (m, 4H, 2,5-pyrrolidine), 4.38–4.43 (m, 2H, N7-CH2CH2CH2Cl). LC/MS: m/z calc. 326.13, found 326.30. Anal. calcd. for C14H20ClN5O2: C, H, N.
7-(4-Chlorobutyl)-1,3-dimethyl-8-pyrrolidin-1-yl-purine-2,6-dione (11)
Yield 62%, m.p. 97–99 °C, Rf = 0.58 (A), 1H-NMR (CDCl3) δ: 1.71–1.82 (m, 2H, N7-CH2CH2(CH2)2), 1.83–1.94 (m, 2H, N7-(CH2)2CH2CH2), 1.99–2.20 (m, 4 H, 3,4-pyrrolidine), 3.37 (s, 3H, N1-CH3), 3.51 (s, 3H, N3-CH3), 3.56 (m, 6H, 2,5-pyrrolidine & N7-(CH2)2CH2CH2Cl), 4.28 (t, J = 7.3 Hz, 2H, N7-CH2CH2(CH2)2). LC/MS: m/z calc. 340.15, found 340.33. Anal. calcd. for C15H22ClN5O2: C, H, N.
7-(4-Chlorobutyl)-1,3-dimethyl-8-(1-piperidyl)purine-2,6-dione (12)
Yield 70%, m.p. 101–103 °C, Rf = 0.58 (A), 1H-NMR (CDCl3) δ: 1.57–1.78 (m, 6H, (CH2)3 piperidine), 1.98–2.05 (m, 4H, N7-CH2CH2CH2CH2), 3.10–3.20 (m, 4H, N(CH2)2 piperidine), 3.38 (s, 3H, N1-CH3), 3.53 (s, 3H, N3-CH3), 3.56 (t, J = 6.0 Hz, 2H, CH2Cl), 4.10 (t, J = 7.3 Hz, 2H, N7-CH2). LC/MS: m/z calc. 354.16, found 354.06. Anal. calcd. for C16H24ClN5O2: C, H, N.
7-(5-Chloropentyl)-1,3-dimethyl-8-(1-piperidyl)purine-2,6-dione (13)
Yield 69%, m.p. 72–73 °C, Rf = 0.66 (A), 1H-NMR (DMSO) δ: 1.38–1.48 (m, 2H, N7-(CH2)2CH2(CH2)2), 1.63–1.78 (m, 6H, (CH2)3 piperidine), 1.82–1.88 (m, 4H, N7-CH2CH2CH2CH2) 3.10–3.20 (m, 4H, N(CH2)2 piperidine), 3.38 (s, 3H, N1-CH3), 3.53 (s, 3H, N3-CH3), 3.55 (t, J = 6.4 Hz, 2H, CH2Cl), 4.05 (t, J = 7.3 Hz, 2H, N7-CH2). LC/MS: m/z calc. 368.18, found 368.03. Anal. calcd. for C17H26ClN5O2: C, H, N.
7-(5-Chloropentyl)-1,3-dimethyl-8-morpholino-purine-2,6-dione (14)
Yield 75%, m.p. 87–88 °C, Rf = 0.58 (A), 1H-NMR (CDCl3) δ: 1.37–1.49 (m, 2H, N7-(CH2)2CH2(CH2)2), 1.84–1.93 (m, 4H, N7-CH2CH2CH2CH2), 3.19–3.25 (m, 4H, N(CH2CH2)2O), 3.39 (s, 3H, N1-CH3), 3.49–3.59 (m, 5H, N3-CH3, CH2Cl), 3.81–3.89 (m, 4H, N(CH2CH2)2O), 4.12 (t, J = 7.1 Hz, 2H, N7-CH2). LC/MS: m/z calc. 370.16, found 370.38. Anal. calcd. for C16H24ClN5O3: C, H, N.
7-(4-Chlorobutyl)-8-(diethylamino)-1,3-dimethyl-purine-2,6-dione (15)
Yield 65%, m.p. 84–86 °C, Rf = 0.71 (A), 1H-NMR (CDCl3) δ: 1.14 (t, J = 7.1 Hz, 6H, N(CH2CH3)2), 1.62–1.78 (m, 2H, N7-(CH2)2CH2CH2), 1.91–1.98 (m, 2H, N7-CH2CH2(CH2)2), 3.26 (q, J = 7.1 Hz, 4H, N(CH2CH3)2), 3.39 (s, 3H, N1-CH3), 3.47–3.56 (m, 5H, N3-CH3, CH2Cl), 4.25 (t, J = 7.1 Hz, 2H, N7-CH2). LC/MS: m/z calc. 342.16, found 342.39. Anal. calcd. for C15H24ClN5O2: C, H, N.
7-(3-Chloropropyl)-8-(dipropyloamino)-1,3-dimethyl-purine-2,6-dione (16)
Yield 73%, m.p. 88–90 °C, Rf = 0.45 (A), 1H-NMR (CDCl3) δ: 0.90 (t, J = 7.1 Hz, 6H, N(CH2CH2CH3)2), 1.52–1.65 (m, 4H, N(CH2CH2CH3)2), 2.25–2.34 (m, 2H, N7-CH2CH2CH2), 3.16–3.21 (m, 4H, N(CH2CH2CH3)2), 3.37 (s, 3H, N1-CH3), 3.52 (s, 3H, N3-CH3), 3.54–3.58 (t, J = 6.1 Hz, 2H, CH2Cl), 4.25 (t, J = 7,4 Hz, 2H, N7-CH2(CH2)2). LC/MS: m/z calc. 356.18, found 356.42. Anal. calcd. for C16H26ClN5O2: C, H, N.
7-(4-Chlorobutyl)-8-(dipropyloamino)-1,3-dimethyl-purine-2,6-dione (17)
Yield 71%, m.p. 38–40 °C, Rf = 0.58 (A), 1H-NMR (CDCl3) δ: 0.89 (t, J = 7.4 Hz, 6H, N(CH2CH2CH3)2), 1.52–1.62 (m, 4H, N(CH2CH2CH3)2), 1.70–1.79 (m, 2H, N7-(CH2)2CH2CH2), 1.90–1.98 (m, 2H, N7-CH2CH2(CH2)2), 3.14–3.19 (m, 4H, N(CH2CH2CH3)2), 3.37 (s, 3H, N1-CH3), 3.51 (s, 3H, N3-CH3), 3.54–3.56 (m, 2H, CH2Cl), 4.14 (t, J = 7.4 Hz, 2H, N7-CH2(CH2)3). LC/MS: m/z calc. 370.20, found 370.44. Anal. calcd. for C17H28ClN5O3: C, H, N.
General procedure for the preparation of 8-amino-7-arylpiperazinylalkyl-1,3-dimethylpurine-2,6-diones hydrochlorides (18–47)
A mixture of the appropriate 8-amino-7-chloroalkyl-1,3-dimethylpurine-2,6-dione derivatives 7–17 (10 mmol), respective 1-phenylpiperazine derivatives (1-phenylpiperazine, 1–(2-methoxyphenyl)piperazine, 1–(3-chlorophenyl)piperazine, 1–(2-fluorophenyl)piperazine, 1–(4-fluorophenyl)piperazine, 1–(3,4-dichlorophenyl)-piperazine (10 mmol), and anhydrous K2CO3 (20 mmol) were refluxed in 1-propanol (10 mL) for 40 h. Then the mixture was filtered off and the solvent was evaporated under reduced pressure. The residue was dissolved in a small amount of acetone, acidified with conc. HCl to pH = 3 and cooled. The precipitate hydrochloride salt was filtered off and purified by crystallization from ethanol.
1,3-Dimethyl-7-[3–(4-phenylpiperazin-1-yl)propyl]-8-pyrrolidin-1-yl-purine-2,6-dione hydrochloride (18)
Yield 62%, m.p. 157–159 °C, Rf = 0.49 (A), 1H-NMR (DMSO) δ: 1.21–1.26 (m, 2H, N7-(CH2)2CH2(CH2)2), 1.38 (t, J = 7.1 Hz, 3H, O-CH2CH3) 1.60–1.80 (m, 4H, N7-CH2CH2CH2CH2CH2), 2.82–3.18 (m, 6H, (CH2)2 N-Ph & N7-(CH2)3CH2CH2), 3.20 (s, 3H, N1-CH3), 3.37 (s, 3H, N3-CH3), 3.40–3.58 (m, 2H, N(CH2)2), 3.76 (s, 3H, Ph-OCH3), 4.00 (t, J = 6.5 Hz 2H, N7-CH2CH2), 4.50 (q, J = 7.0 Hz, 2H, O-CH2CH3), 6.82–7.02 (m, 4H, Ph), 10.77 (s, 1H, H+). LC/MS: m/z calc. 452.27, found 452.50. Anal. calcd. for C24H33N7O2···HCl: C, H, N.
7-{3-[4-(2-Methoxyphenyl)piperazin-1-yl]propyl}-1,3-dimethyl-8-pyrrolidin-1-yl-purine-2,6-dione hydrochloride (19)
Yield 46%, m.p. 241–243 °C, Rf = 0.20 (A), 1H-NMR (DMSO) δ: 1.86–1.98 (m, 4H, 3,4-pyrrolidine), 2.11–2.22 (m, 2H, N7-CH2CH2CH2), 2.98–3.14 (m, 6H, CH2NH+(CH2CH2)2N-Ph), 3.18 (s, 3H, N1-CH3), 3.34 (s, 3H, N3-CH3), 3.48–3.60 (m, 6H, 2,5-pyrrolidine, NH+(CH2CH2)2N), 3.63–3.76 (m, 2H, NH+(CH2CH2)2N), 3.77 (s, 3H, OCH3), 4.26 (t, J = 7.1 Hz, 2H, N7-CH2), 6.86–7.04 m, 4H, Ph), 10.84 (s, 1H, NH+). LC/MS: m/z calc. 482.28, found 482.34. Anal. calcd. for C25H35N7O2···HCl: C, H, N.
7-{3-[4-(3-Chlorophenyl)piperazin-1-yl]propyl}-1,3-dimethyl-8-pyrrolidin-1-yl-purine-2,6-dione hydrochloride (20)
Yield 53%, m.p. 237–240 °C, Rf = 0.26 (A), 1H-NMR (DMSO) δ: 1.85–1.97 (m, 4H, 3,4-pyrrolidine), 2.09–2.22 (m, 2H, N7-CH2CH2CH2), 2.96–3.15 (m, 6H, CH2NH+(CH2CH2)2N-Ph), 3.18 (s, 3H, N1-CH3), 3.36 (s, 3H, N3-CH3), 3.46–3.62 (m, 6H, 2,5-pyrrolidine, NH+(CH2CH2)2N), 3.65–3.78 (m, 2H, NH+(CH2CH2)2N), 4.25 (t, J = 7.1 Hz, 2H, N7-CH2), 6.85 (dd, 3J = 7.7 Hz, 4J = 1.8 Hz, 1H, Ph), 6.94 (dd, 3J = 8.4 Hz, 4J = 1.9 Hz, 1H, Ph), 7.03 (t, J = 2.2 Hz, 1H, Ph), 7.24 (t, J = 8.2 Hz, 1H, Ph), 10.86 (s, 1H, NH+). LC/MS: m/z calc. 486.23, found 486.40. Anal. calcd. for C24H32N7O2···HCl: C, H, N.
7-{3-[4-(4-Fluorophenyl)piperazin-1-yl]propyl}-1,3-dimethyl-8-pyrrolidin-1-yl-purine-2,6-dione hydrochloride (21)
Yield 48%, m.p. 248–250 °C, Rf = 0.34 (A), 1H-NMR (DMSO) δ: 1.84–1.97 (m, 4H, 3,4-pyrrolidine), 2.10–2.20 (m, 2H, N7-CH2CH2CH2), 2.96–3.15 (m, 6H, CH2NH+(CH2CH2)2N-Ph), 3.18 (s, 3H, N1-CH3), 3.34 (s, 3H, N3-CH3), 3.49–3.61 (m, 6H, 2,5-pyrrolidine, NH+(CH2CH2)2N), 3.65–3.75 (m, 2H, NH+(CH2CH2)2N), 4.27 (t, J = 7.0 Hz, 2H, N7-CH2), 6.94–7.03 (m, 2H, Ph), 7.03–7.13 (m, 2H, Ph), 10.90 (s, 1H, NH+). LC/MS: m/z calc. 470.26, found 470.49. Anal. calcd. for C24H32N7O2···HCl: C, H, N.
1,3-Dimethyl-7-[4-(4-phenylpiperazin-1-yl)butyl]-8-pyrrolidin-1-yl-purine-2,6-dione hydrochloride (22)
Yield 53%, m.p. 208–211 °C, Rf = 0.31 (A), 1H-NMR (DMSO) δ: 1.59–1.76 (m, 4H, N7-CH2CH2CH2CH2), 1.83–1.97 (m, 4H, 3,4-pyrrolidine), 2.98–3.24 (m, 6H, CH2NH+(CH2CH2)2N-Ph), 3.17 (s, 3H, N1-CH3), 3.34 (s, 3H, N3-CH3), 3.41–3.60 (m, 6H, 2,5-pyrrolidine, NH+(CH2CH2)2N), 3.71–3.82 (m, 2H, NH+(CH2CH2)2N), 4.28 (m, 2H, N7-CH2), 6.84 (t, J = 7.3 Hz, 1H, Ph), 6.93–7.03 (d, J = 8.0 Hz, 2H, Ph), 7.18–7.31 (m, 2H, Ph), 10.96 (s, 1H, NH+). LC/MS: m/z calc. 466.29, found 466.57. Anal. calcd. for C25H35N7O2···HCl: C, H, N.
7-{4-[4-(2-Methoxyphenyl)piperazin-1-yl]butyl}-1,3-dimethyl-8-pyrrolidin-1-yl-purine-2,6-dione hydrochloride (23)
Yield 49%, m.p. 177–179 °C, Rf = 0.14 (A), 1H-NMR (DMSO) δ: 1.58–1.75 (m, 4H, N7-CH2CH2CH2CH2), 1.85–1.95 (m, 4H, 3,4-pyrrolidine), 2.96–3.18 (m, 6H, CH2NH+(CH2CH2)2N-Ph), 3.18 (s, 3H, N1-CH3), 3.34 (s, 3H, N3-CH3), 3.48–3.60 (m, 6H, 2,5-pyrrolidine, NH+(CH2CH2)2N), 3.68–3.80 (m, 2H, NH+(CH2CH2)2N), 3.76 (s, 3H, OCH3), 4.28 (t, J = 6.9 Hz, 2H, N7-CH2), 6.86–7.02 (m, 4H, Ph), 10.94 (s, 1H, NH+). LC/MS: m/z calc. 496.30, found 496.20. Anal. calcd. for C26H37N7O3···HCl: C, H, N.
7-{4-[4-(3-Chlorophenyl)piperazin-1-yl]butyl}-1,3-dimethyl-8-pyrrolidin-1-yl-purine-2,6-dione hydrochloride (24)
Yield 51%, m.p. 226–228 °C, Rf = 0.23 (A), 1H-NMR (DMSO) δ: 1.60–1.77 (m, 4H, N7-CH2CH2CH2CH2), 1.90–1.93 (m, 4H, 3,4-pyrrolidine), 2.98–3.18 (m, 6H, CH2NH+(CH2CH2)2N-Ph), 3.18 (s, 3H, N1-CH3), 3.34 (s, 3H, N3-CH3), 3.45–3.58 (m, 6H, 2,5-pyrrolidine, NH+(CH2CH2)2N), 3.71–3.80 (m, 2H, NH+(CH2CH2)2N), 4.27 (t, J = 6.9 Hz, 2H, N7-CH2), 6.86 (d, J = 7.6 Hz, 1H, Ph), 6.95 (d, J = 8.4 Hz, 1H, Ph), 7.04 (s, 1H, Ph), 7.24 (t, J = 8.0 Hz, 1H, Ph), 10.96 (s, 1H, NH+). LC/MS: m/z calc. 500.25, found 500.43. Anal. calcd. for C25H34ClN7O2···HCl: C, H, N.
7-{4-[4-(4-Fluorophenyl)piperazin-1-yl]butyl}-1,3-dimethyl-8-pyrrolidin-1-yl-purine-2,6-dione hydrochloride (25)
Yield 58%, m.p. 211–213 °C, Rf = 0.31 (A), 1H-NMR (DMSO) δ: 1.70–1.80 (m, 4H, N7-CH2CH2CH2CH2), 1.90–1.92 (m, 4H, 3,4-pyrrolidine), 3.01–3.18 (m, 6H, CH2NH+(CH2CH2)2N-Ph), 3.18 (s, 3H, N1-CH3), 3.35 (s, 3H, N3-CH3), 3.46–3.56 (m, 6H, 2,5-pyrrolidine, NH+(CH2CH2)2N), 3.71–3.82 (m, 2H, NH+(CH2CH2)2N), 4.22 (t, J = 6.9 Hz, 2H, N7-CH2), 6.98–7.12 (m, 4H, Ph), 10.61 (s, 1H, NH+). LC/MS: m/z calc. 484.28, found 484.40. Anal. calcd. for C25H34FN7O2···HCl: C, H, N.
1,3-Dimethyl-7-[3–(4-phenylpiperazin-1-yl)propyl]-8-(1-piperidyl)-purine-2,6-dione hydrochloride (26)
Yield 58%, m.p. 243–245°C, Rf = 0.45 (A), 1H-NMR (DMSO-d6) δ: 1.51–1.72 (m, 6H, (CH2)3 piperidine), 2.16–2.27 (m, 2H, N7-CH2CH2CH2), 3.02–3.17 (m, 10H, CH2NH+(CH2CH2)2N-Ph, N(CH2)2 piperidine), 3.20 (s, 3H, N1-CH3), 3.37 (s, 3H, N3-CH3), 3.48–3.57 (m, 2H, NH+(CH2CH2)2N), 3.77–3.81 (m, 2H, NH+(CH2CH2)2N), 4.11 (t, 3J = 6.8 Hz, 2H, N7-CH2), 6.84 (t, J = 7.1 Hz, 1H, Ph), 6.97 (d, J = 8.0 Hz, 2H, Ph), 7.20–7.28 (m, 2H, Ph), 10.50 (s, 1H NH+). LC/MS: m/z calc. 466.29, found 466.02. Anal. calcd. for C25H35N7O2···HCl: C, H, N.
7-{3-[4-(2-Methoxyphenyl)piperazin-1-yl]propyl}-1,3-dimethyl-8-(1-piperidyl)-purine-2,6-dione hydrochloride (27)
Yield 67%, m.p. 241–242 °C, Rf = 0.35 (A), 1H-NMR (DMSO-d6) δ: 1.52–1.70 (m, 6H, (CH2)3 piperidine), 2.17–2.27 (m, 2H, N7-CH2CH2CH2), 2.92–3.18 (m, 10H, CH2NH+(CH2CH2)2N-Ph, N(CH2)2 piperidine), 3.20 (s, 3H, N1-CH3), 3.37 (s, 3H, N3-CH3), 3.48–3.52 (m, 2H, NH+(CH2CH2)2N), 3.76 (s, 3H, OCH3), 3.99–4.15 (m, 4H, NH+(CH2CH2)2N, N7-CH2), 6.86–7.03 (m, 4H, Ph), 10.57 (s, 1H NH+). LC/MS: m/z calc. 496.30, found 496.55. Anal. calcd. for C26H37N7O3···HCl: C, H, N.
7-{3-[4-(3,4-Dichlorophenyl)piperazin-1-yl]propyl}-1,3-dimethyl-8-(1-piperidyl)-purine-2,6-dione hydrochloride (28)
Yield 62%, m.p. 252–254 °C, Rf = 0.40 (A), 1H-NMR (DMSO-d6) δ: 1.51–1.69 (m, 6H, (CH2)3 piperidine), 2.12–2.23 (m, 2H, N7-CH2CH2CH2), 3.01–3.17 (m, 10H, CH2NH+(CH2CH2)2N-Ph, N(CH2)2 piperidine), 3.20 (s, 3H, N1-CH3), 3.37 (s, 3H, N3-CH3), 3.48–3.55 (m, 2H, NH+(CH2CH2)2N), 3.88–3.91 (m, 2H, NH+(CH2CH2)2N), 4.09 (t, J = 7.4 Hz, 2H, N7-CH2), 6.97–7.03 (m 1H, Ph), 7.20–7.25 (m, 1H, Ph), 7.42–7.47 (d, J = 8.7 Hz, 1H, Ph), 10.14 (s, 1H NH+). LC/MS: m/z calc. 534.21, found 533.90. Anal. calcd. for C25H33Cl2N7O2···HCl: C, H, N.
1,3-Dimethyl-7-[4-(4-phenylpiperazin-1-yl)butyl]-8-(1-piperidyl)-purine-2,6-dione hydrochloride (29)
Yield 57%, m.p. 200–201 °C, Rf = 0.34 (A), 1H-NMR (DMSO) δ: 1.58–1.65 (m, 8H, N7-(CH2)2CH2 (CH2)3, piperidine), 1.69–1.78 (m, 2H, N7-CH2CH2), 3.02–3.18 (m, 10H, CH2NH+(CH2CH2)2N-Ph, N(CH2)2 piperidine), 3.19 (s, 3H, N1-CH3), 3.37 (s, 3H, N3-CH3), 3.49–3.55 (m, 2H, NH+(CH2CH2)2N-Ph), 3.78–3.85 (m, 2H, NH+(CH2CH2)2N-Ph), 4.08 (t, J = 7.0 Hz, 2H, N7-CH2), 6.84 (t, J = 7.3 Hz, 1H, Ph), 6.98 (d, J = 8.0 Hz, 2H, Ph), 7.23 (t, J = 8.0 Hz, 2H, Ph), 10.22 (s, 1H, NH+). LC/MS: m/z calc. 480.30, found 480.57. Anal. calcd. for C26H37N7O2···HCl: C, H, N.
7-{4-[4-(2-Methoxyphenyl)piperazin-1-yl]butyl}-1,3-dimethyl-8-(1-piperidyl)-purine-2,6-dione hydrochloride (30)
Yield 71%, m.p. 198–199 °C, Rf = 0.28 (A), 1H-NMR (DMSO) δ: 1.57–1.78 (m, 10H, (CH2)3 piperidine, N7-CH2CH2CH2CH2), 3.05–3.17 (m, 10H, CH2NH+(CH2CH2)2N-Ph, N(CH2)2 piperidine), 3.19 (s, 3H, N1-CH3), 3.37 (s, 3H, N3-CH3), 3.44–3.56 (m, 4H, NH+(CH2CH2)2N-Ph), 3.75 (s, 3H, OCH3), 4.05 (t, J = 6.9 Hz, 2H, N7-CH2); 6,87–7,00 (m, 4H, Ph). 10.98 (s, 1H, NH+). LC/MS: m/z calc. 510.31, found 510.04. Anal. calcd. for C27H39N7O3···HCl: C, H, N.
7-{4-[4-(3-Chlorophenyl)piperazin-1-yl]butyl}-1,3-dimethyl-8-(1-piperidyl)-purine-2,6-dione hydrochloride (31)
Yield 58%, m.p. 223–224 °C, Rf = 0.38 (A), 1H-NMR (DMSO) δ: 1.57–1.65 (m, 8H, (CH2)3 piperidine, N7-(CH2)2CH2), 1.67–1.78 (m, 2H, N7CH2CH2), 3.05–3.18 (m, 10H, CH2NH+(CH2CH2)2N-Ph, N(CH2)2 piperidine), 3.19 (s, 3H, N1-CH3), 3.37 (s, 3H, N3-CH3), 3.40–3.45 (m, 2H, NH+(CH2CH2)2N-Ph), 3.80–3.89 (m, 2H, NH+(CH2CH2)2N-Ph), 4.06 (t, J = 6.9 Hz, 2H, N7-CH2), 6.85 (dd, 3J = 8.0 Hz, 4J = 1.7 Hz, 1H, Ph), 6.94 (dd, J = 8.0 Hz, 1H, Ph), 7.03 (s, 1H, Ph), 7.24 (t, J = 8.0 Hz, 1H, Ph), 10.25 (s, 1H, NH+). LC/MS: m/z calc. 514.26, found 514.00. Anal. calcd. for C26H36ClN7O2···HCl: C, H, N.
7-{4-[4-(3,4-Dichlorophenyl)piperazin-1-yl]butyl}-1,3-dimethyl-8-(1-piperidyl)-purine-2,6-dione hydrochloride (32)
Yield 67%, m.p. 210–211 °C, Rf = 0.32 (A), 1H-NMR (DMSO) δ: 1.57–1.68 (m, 8H, (CH2)3 piperidine, N7-(CH2)2CH2), 1.70–1.77 (m, 2H, N7-CH2CH2), 3.06–3.17 (m, 10H, CH2NH+(CH2CH2)2N-Ph, N(CH2)2 piperidine), 3.19 (s, 3H, N1-CH3), 3.37 (s, 3H, N3-CH3), 3.41–3.50 (m, 2H, NH+(CH2CH2)2N-Ph), 3.80–3.88 (m, 2H, NH+(CH2CH2)2N-Ph), 4.06 (t, J = 6.9 Hz, 2H, N7-CH2), 6.96 (dd, 3J = 8.1 Hz, 4J = 2.8 Hz, 1H, Ph), 7.32 (d, J = 2.8 Hz, 1H, Ph), 7.45 (d, J = 9.0 Hz, 1H, Ph), 10.60 (s, 1H, NH+). LC/MS: m/z calc. 548.23, found 548.45. Anal. calcd. for C25H35Cl2N7O2···HCl: C, H, N.
1,3-Dimethyl-7-[5-(4-phenylpiperazin-1-yl)pentyl]-8-(1-piperidyl)-purine-2,6-dione hydrochloride (33)
Yield 58%, m.p. 229–230 °C, Rf = 0.45 (A), 1H-NMR (DMSO) δ: 1.18–1.25 (m, 2H, N7-(CH2)2CH2), 1.55–1.79 (m, 10H, N7-CH2CH2CH2CH2, N(CH2)3 piperidine), 3.06–3.18 (m, 10H, CH2NH+(CH2CH2)2N-Ph, N(CH2)2 piperidine), 3.19 (s, 3H, N1-CH3), 3.36 (s, 3H, N3-CH3), 3.43–3.52 (m, 2H, NH+(CH2CH2)2N), 3.78–3.87 (m, 2H, NH+(CH2CH2)2N), 4.06 (t, J = 6.9 Hz, 2H, N7-CH2,), 6.84 (t, J = 7.0 Hz, 1H, Ph), 6.98 (d, J = 6.0 Hz, 2H, Ph), 7.25 (t, J = 8.0 Hz, 2H, Ph), 10.61 (s, 1H, NH+). LC/MS: m/z calc. 494.32, found 494.54. Anal. calcd. for C27H39N7O2···HCl: C, H, N.
7-{5-[4-(2-Methoxyphenyl)piperazin-1-yl]pentyl}-1,3-dimethyl-8-(1-piperidyl)-purine-2,6-dione hydrochloride (34)
Yield 56%, m.p. 198–199 °C, Rf = 0.39 (A), 1H-NMR (DMSO) δ: 1.18–1.27 (m, 2H, N7-(CH2)2CH2), 1.55–1.79 (m, 10H, N7-CH2CH2CH2CH2, N(CH2)3 piperidine), 3.01–3.20 (m, 10H, CH2NH+(CH2CH2)2N-Ph, N(CH2)2-piperidine), 3.19 (s, 3H, N1-CH3), 3,36 (s, 3H, N3-CH3), 3.41–3.55 (m, 4H, NH+(CH2CH2)2N), 3.76 (s, 3H, OCH3), 4.06 (t, J = 6.9 Hz, 2H, N7-CH2), 6.86–7.00 (m, 4H, Ph), 10.15 (s, 1H, NH+). LC/MS: m/z calc. 524.33, found 524.46. Anal. calcd. for C28H41N7O3···HCl: C, H, N.
7-{5-[4-(3,4-Dichlorophenyl)piperazin-1-yl]pentyl}-1,3-dimethyl-8-(1-piperidyl)-purine-2,6-dione hydrochloride (35)
Yield 62%, m.p. 243–244 °C, Rf = 0.50 (A), 1H-NMR (DMSO) δ: 1.19–1.30 (m, 2H, N7-(CH2)2CH2(CH2)2), 1.55–1.79 (m, 10H, N7-CH2CH2CH2CH2, N(CH2)3 piperidine), 3.11–3.19 (m, 10H, CH2NH+(CH2CH2)2N-Ph, N(CH2)2 piperidine), 3.19 (s, 3H, N1-CH3), 3.36 (s, 3H, N3-CH3), 3.39–3.45 (m, 2H, NH+(CH2CH2)2N), 3.55–3.60 (m, 2H, NH+(CH2CH2)2N), 4.05 (t, J = 7.1 Hz, 2H, N7-CH2), 7.18–7.20 (m, 1H, Ph), 7.32–7.40 (m, 2H, Ph), 10.59 (s, 1H, NH+). LC/MS: m/z calc. 562.24, found 562.48. Anal. calcd. for C27H37Cl2N7O2···HCl: C, H, N.
7-{4-[4-(2-Fluorophenyl)piperazin-1-yl]butyl}-1,3-dimethyl-8-morpholino-purine-2,6-dione hydrochloride (36)
Yield 69%, m.p. 210–212 °C, Rf = 0.17 (A), 1H-NMR (DMSO-d6) δ: 1.67–1.81 (m, 4H, N7-CH2CH2CH2CH2), 3.04–3.19 (m, 10H, CH2NH+(CH2CH2)2N-Ph, N(CH2CH2)2O), 3.20 (s, 3H, N1-CH3), 3.38 (s, 3H, N3-CH3), 3.48–3.68 (m, 4H, N(CH2CH2)2O), 3.72–3.75 (m, 4H, CH2NH+(CH2CH2)2N-Ph), 4.09–4.14 (m, 2H, N7-CH2), 6.99–7.10 (m, 4H, Ph), 10.60 (s, 1H, NH+). LC/MS: m/z calc. 500.27, found 500.60. Anal. calcd. for C25H34ClN7O3···HCl: C, H, N.
7-{4-[4-(3,4-Dichlorophenyl)piperazin-1-yl]butyl}-1,3-dimethyl-8-morpholino-purine-2,6-dione hydrochloride (37)
Yield 66%, m.p. 209–211°C, Rf = 0.35 (A), 1H-NMR (DMSO-d6) δ: 1.67–1.78 (m, 4H, N7-CH2CH2CH2CH2), 3.09–3.17 (m, 10H, CH2NH+(CH2CH2)2N-Ph, N(CH2CH2)2O), 3.18 (s, 3H, N1-CH3), 3.36 (s, 3H, N3-CH3), 3.41–3.47 (m, 2H, NH+(CH2CH2)2N-Ph), 3.71–3.74 (m, 4H, N(CH2CH2)2O), 3.83–3.87 (m, 2H, NH+(CH2CH2)2N-Ph), 4.09 (t, J = 6.4 Hz, 2H, N7-CH2), 6.97 (dd, 3J = 8.9 Hz, 4J = 2.8 Hz, 1H, Ph), 7.20 (d, 4J = 2.8 Hz, 1H, Ph), 7.42 (d, 3J = 8.9 Hz, 1H, Ph), 10.85 (s, 1H, NH+). LC/MS: m/z calc. 550.20, found 550.40. Anal. calcd. for C25H33Cl2N7O3···HCl: C, H, N.
7-{5-[4-(2-Methoxyphenyl)piperazin-1-yl]pentyl}-1,3-dimethyl-8-morpholino-purine-2,6-dione hydrochloride (38)
Yield 62%, m.p. 207–209 °C, Rf = 0.16 (A), 1H-NMR (DMSO) δ: 1.19–1.26 (m, 2H, N7-(CH2)2CH2), 1.68–1.79 (m, 4H, N7-CH2CH2CH2CH2), 2.98–3.25 (m, 10H, CH2NH+(CH2CH2)2N-Ph, N(CH2CH2)2O), 3.20 (s, 3H, N1-CH3), 3.37 (s, 3H, N3-CH3), 3.44–3.48 (m, 4H, NH+(CH2CH2)2N-Ph), 3.72–3.75 m, 4H, N(CH2CH2)2O), 3.76 (s, 3H, OCH3), 4.06–4.14 (m, 2H, N7-CH2), 6.85–7.00 (m, 4H, Ph), 10.58 (s, 1H, NH+). LC/MS: m/z calc. 526.31, found 526.06. Anal. calcd. for C27H39N7O4···HCl: C, H, N.
8-(Diethylamino)-7-{3-[4-(2-methoxyphenyl)piperazin-1-yl]propyl}-1,3-dimethyl-purine-2,6-dione hydrochloride (39)
Yield 59%, m.p. 203–205 °C, Rf = 0.40 (A), 1H-NMR (DMSO-d6) δ: 1.09 (t, J = 7.1 Hz, 6H, N(CH2CH3)2), 2.16–2.27 (m, 2H, N7-CH2CH2CH2), 2.93–3.08 (m, 6H, CH2NH+(CH2CH2)2N-Ph), 3.20 (s, 3H, N1-CH3), 3.22–3.28 (m, 4H, N(CH2CH3)2), 3.39 (s, 3H, N3-CH3), 3.33–3.48 (m, 4H, NH+(CH2CH2)2N-Ph), 3.76 (s, 3H, OCH3), 4.12 (t, J = 6.9 Hz, 2H, N7-CH2), 6.87–7.04 (m, 4H, Ph), 10.20 (s, 1H, NH+). LC/MS: m/z calc. 484.30, found 484.52. Anal. calcd. for C25H37N7O3···HCl: C, H, N.
7-{3-[4-(3,4-Dichlorophenyl)piperazin-1-yl]propyl}-8-(diethylamino)-1,3-dimethyl-purine-2,6-dione hydrochloride (40)
Yield 61%, m.p. 264–266°C, Rf = 0.68 (A), 1H-NMR (DMSO-d6) δ: 1.08 (t, J = 7.1 Hz, 6H, N(CH2CH3)2), 2.06–2.20 (m, 2H, N7-CH2CH2CH2), 3.00–3.10 (m, 6H, CH2NH+(CH2CH2)2N-Ph), 3.12–3.16 (m, 4H, N(CH2CH3)2), 3.20 (s, 3H, N1-CH3), 3.36 (s, 3H, N3-CH3). 3.86–4.14 (m, 4H, NH+(CH2CH2)2N-Ph), 4.11 (t, J = 6.9 Hz, 2H, N7-CH2), 6.95–6.99 (dd, 3J = 8.9 Hz, 4J = 5.9 Hz, 1H, Ph), 7.21 (d, J = 2.8 Hz, 1H, Ph), 7.42–7.44 (d, J = 8.9 Hz, 1H, Ph), 10.20 (s, 1H, NH+). LC/MS: m/z calc. 522.21, found 522.48. Anal. calcd. for C24H33Cl2N7O2···HCl: C, H, N.
8-(Diethylamino)-7-{4-[4-(2-methoxyphenyl)piperazin-1-yl]butyl}-1,3-dimethyl-purine-2,6-dione hydrochloride (41)
Yield 72%, m.p. 224–226°C, Rf = 0.24 (A), 1H-NMR (DMSO-d6) δ: 1.08 (t, J = 6.9 Hz, 6H, N(CH2CH3)2), 1.60–1.80 (m, 4H, N7-CH2CH2CH2), 3.07–3.14 (m, 6H, CH2NH+(CH2CH2)2N-Ph), 3.19 (s, 3H, N1-CH3) 3.21–3.28 (m, 4H, N(CH2CH3)2), 3.36 (s, 3H, N3-CH3), 3.44–3.46 (m, 4H, NH+(CH2CH2)2N-Ph), 3.76 (s, 3H, OCH3), 4.08 (t, J = 6.9 Hz, 2H, N7-CH2), 6.85–7.03 (m, 4H, Ph), 11,06 (s, 1H, NH+). LC/MS: m/z calc. 498.31, found 498.43. Anal. calcd. for C26H39N7O3···HCl: C, H, N.
7-{4-[4-(3,4-Dichlorophenyl)piperazin-1-yl]butyl}-8-(diethylamino)-1,3-dimethyl-purine-2,6-dione hydrochloride (42)
Yield 69%, m.p. 219–221°C, Rf = 0.24 (A), 1H-NMR (DMSO-d6) δ: 1.07 (t, J = 6.9 Hz, 6H, N(CH2CH3)2), 1.60–1.71 (m, 4H, N7-CH2CH2CH2CH2), 3.03–3.14 (m, 6H, CH2NH+(CH2CH2)2), 3.18 (s, 3H, N1-CH3), 3.20–3.27 (m, 4H, N(CH2CH3)2), 3.35 (s, 3H, N3-CH3), 3.42–4.04 (m, 4H, NH+(CH2CH2)2), 4.07 (t, J = 7.1 Hz, 2H, N7-CH2), 6.95–6.99 (d, J = 8.9, 1H, Ph), 7.20 (s, 1H, Ph), 7.40–7.43 (d, 1H, Ph), 11.30 (s, 1H, NH+). LC/MS: m/z calc. 536.23, found 536.38. Anal. calcd. for C25H35Cl2N7O2···HCl: C, H, N.
8-(Dipropylamino)-7-{3-[4-(2-methoxyphenyl)piperazin-1-yl]propyl}-1,3-dimethyl-purine-2,6-dione hydrochloride (43)
Yield 75%, m.p. 203–205 °C, Rf = 0.22 (A), 1H-NMR (DMSO-d6) δ: 0.85 (t, J = 7.4 Hz, 6H, N(CH2CH2CH3)2), 1.45–1.58 (m, 4H, N(CH2CH2CH3)2), 2.15–2.25 (m, 2H, N7-CH2CH2CH2); 3.05–3.60 (m, 10H, CH2NH+(CH2CH2)2N, C(8)N(CH2)2), 3.19 (s, 3H, N1-CH3), 3.36 (s, 3H, N3-CH3), 3.76 (s, 3H, OCH3), 3.86–4.04 (m, 4H, NH+(CH2CH2)2), 4.13 (t, J = 7.4 Hz, 2H, N7-CH2), 6.84–6.99 (m, 4H, Ph), 11.00 (s, 1H, NH+). LC/MS: m/z calc. 512.33, found 512.47. Anal. calcd. for C27H41N7O3···HCl: C, H, N.
7-{3-[4-(3,4-Dichlorophenyl)piperazin-1-yl]propyl}-8-(dipropylamino)-1,3-dimethyl-purine-2,6-dione hydrochloride (44)
Yield 67%, m.p. 246–249 °C, Rf = 0.34 (A), 1H-NMR (DMSO-d6) δ: 0.84 (t, J = 7,4 Hz, 6H, N(CH2CH2CH3)2), 1.45–1.57 (m, 4H, N(CH2CH2CH3)2), 2.17–2.22 (m, 2H, N7-CH2CH2CH2), 3.00–3.20 (m, 10H, NH+(CH2CH2)2N, C(8)N(CH2)2) 3.19 (s, 3H, N1-CH3), 3.36 (s, 3H, N3-CH3), 3.47–3.50 (m, 2H, NH+(CH2CH2)2N), 3.86–3.89 (m, 2H, NH+(CH2CH2)2N), 4.12 (t, J = 7.4 Hz, 2H, N7-CH2), 6.99–7.05 (d, 1H, Ph), 7.21 (s, 1H, Ph), 7.41–7.44 (d, 1H, Ph), 10.66 (s, 1H, NH+). LC/MS: m/z calc. 550.24, found 550.36. Anal. calcd. for C26H37Cl2N7O2···HCl: C, H, N.
8-(Dipropylamino)-7-{4-[4-(2-methoxyphenyl)piperazin-1-yl]butyl}-1,3-dimethyl-purine-2,6-dione hydrochloride (45)
Yield 67%, m.p. 185–186 °C, Rf = 0.22 (A), 1H-NMR (DMSO-d6) δ: 0.84 (t, J = 7.1 Hz, 6H, N(CH2CH2CH3)2), 1.44–1.54 (m, 4H, N(CH2CH2CH3)2), 1.60–1.71 (m, 4H, N7-CH2CH2CH2CH2), 2.97–3.11 (m, 10H, CH2NH+(CH2CH2)2N-Ph, C(8)N(CH2)2), 3.19 (s, 3H, N1-CH3), 3.36 (s, 3H, N3-CH3), 3.44–3.47 (m, 4H, NH+(CH2CH2)2N-Ph), 3.76 (s, 3H, OCH3), 4.07 (t, J = 6.9 Hz, 2H, N7-CH2), 6.87–6.99 (m, 4H, Ph), 10.68 (s, 1H, NH+). LC/MS: m/z calc. 526.35, found 526.51. Anal. calcd. for C28H43N7O3···HCl: C, H, N.
7-{4-[4-(3-Chlorophenyl)piperazin-1-yl]butyl}-8-(dipropylamino)-1,3-dimethyl-purine-2,6-dione hydrochloride (46)
Yield: 59%, m.p.: 138–140 °C, Rf = 0.46 (A), 1H-NMR (DMSO-d6) δ: 0.83 (t, J = 7.4 Hz, 6H, N(CH2CH2CH3)2), 1.43–1.55 (m, 4H, N(CH2CH2CH3)2), 1.60–1.71 (m, 4H, N7-CH2CH2CH2CH2), 2.97–3.18 (m, 10H, CH2NH+(CH2CH2)2N-Ph, C(8)N(CH2)2), 3.19 (s, 3H, N1-CH3), 3.36 (s, 3H, N3-CH3), 3.44–3.47 (m, 2H, NH+(CH2CH2)2N-Ph), 3.51–3.57 (m, 2H, (CH2CH2)2N-Ph), 4.07 (t, J = 6.9 Hz, 2H, N7-CH2), 6.85 (dd, 3J = 7.7 Hz, 4J = 1.5 Hz, 1H, Ph); 6.93 (dd, 3J = 8.2 Hz, 4J = 2.0 Hz, 1H, Ph), 7.02 (t, J = 2.3 Hz, 1H, Ph), 7.23 (t, J = 8.2 Hz, 1H, Ph), 10.42 (s, 1H, NH+). LC/MS: m/z calc. 530.30, found 530.47. Anal. calcd. for C27H40ClN7O2···HCl: C, H, N.
7-{4-[4-(3,4-Dichlorophenyl)piperazin-1-yl]butyl}-8-(dipropylamino)-1,3-dimethyl-purine-2,6-dione hydrochloride (47)
Yield: 68%, mp: 169–171 °C, Rf = 0.28 (A), 1H-NMR (DMSO-d6) δ: 0.84 (t, J = 6.9 Hz, 6H, N(CH2CH2CH3)2), 1.44–1.56 (m, 4H, N(CH2CH2CH3)2), 1.70–1.72 (m, 4H, N7-CH2CH2CH2CH2), 3.01–3.15 (m, 10H, CH2NH+(CH2CH2)2N-Ph, C(8)N(CH2)2), 3.18 (s, 3H, N1-CH3), 3.35 (s, 3H, N3-CH3), 3.43–3.47 (m, 2H, NH+(CH2CH2)2N), 3.84–3.88 (m, 2H, NH+(CH2CH2)2N), 4.07 (t, J = 7.1 Hz, 2H, N7-CH2), 7.06 (dd, 3J = 8.0 Hz, 4J = 2.8 Hz, 1H, Ph), 7.22 (d, J = 2.8 Hz, 1H, Ph), 7.45 (d, J = 9.0 Hz, 1H, Ph), 10.81 (s, 1H, NH+). LC/MS: m/z calc. 564.26, found 564.40. Anal. calcd. for C27H39Cl2N7O2···HCl: C, H, N.
X-ray structure determination and refinement
Good quality single crystals of the representative compounds 37 and 44 were obtained from ethanol solution by slow evaporation of the solvent at ambient condition. Few drops of water were added in order to increase solubility. X-ray diffraction data were collected at 120(2) K using SuperNova diffractometer (Agilent Technologies, Inc., Santa Clara, CA) with MoKα radiation (λ = 0.71073 Å) and processed with CRYSALISProCitation27. The phase problem was solved by direct methods with SHELXS-97 (Agilent Technologies, Inc., Santa Clara, CA)Citation28. Parameters of the obtained model were refined by full-matrix least-squares on F2 using SHELXL-97 (Agilent Technologies, Inc., Santa Clara, CA)Citation28. All non-hydrogen atoms were refined anisotropically. H atoms attached to nitrogen atom N15 and these in water molecule were found on the difference Fourier map.
Positions of hydrogen atoms attached to carbon atoms were calculated with C–H = 0.95 Å for aromatic, C–H = 0.99 Å for methylene, C–H = 0.98 Å for methyl groups and were refined using the riding model with the isotropic displacement parameter Uiso[H] = 1.2 Ueq[C] or Uiso[H] = 1.5 Ueq[C] (the last one constraint for the methyl groups only).
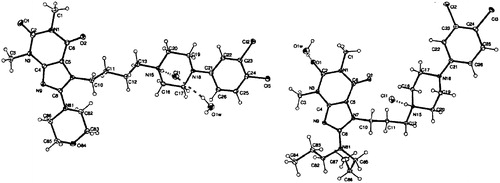
All crystallographic data for compounds 37 and 44 presented structures are shown in Supplemental Information\. WinGXCitation29 software (Gaussian Inc., Pittsburgh, PA) was used to prepare materials for publication. shows asymmetric units of presented structures which were obtained with ORTEP-3 for WindowsCitation30.
Figure 2. Asymmetric units of crystal structures of 37 (left panel) and 44 (right panel), showing the atom labeling scheme. Dashed lines represent a charge-assisted hydrogen bond N+-H … Cl- and hydrogen bonds formed by water molecule within the asymmetric unit. Displacement ellipsoids of non-hydrogen atoms are drawn at the 30% probability level. H atoms are presented as small spheres with an arbitrary radius.
Crystallographic data for the structures in this paper have been deposited with the Cambridge Crystallographic Data Center as supplementary publication nos. CCDC 1049600 (37), CCDC 1049601 (44). Copies of the data can be obtained, free of charge, by application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: +44 1223 336033 or E-mail: [email protected]).
In vitro radioligand binding assays
The compounds 18–47 were tested in competition binding experiments by displacement of respective radioligands from cloned human Rs, all stably expressed in HEK-293 cells: [3H]-8-OH-DPAT, [3H]-ketanserin), [3H]-LSD, [3H]-5-CT, and [3H]-raclopride for 5-HT1A, 5-HT2A, 5-HT6, 5-HT7, and dopamine D2 Rs, respectively according to the previously published proceduresCitation8,Citation31,Citation32. Each compound was tested in triplicate at 7–8 concentrations (10−11–10−4 M). The inhibition constants (Ki) were calculated based on the method described in the literatureCitation33. Results were expressed as means of at least two separate experiments.
Functional in vitro evaluation
The tested and reference compounds were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 1 mM. Serial dilutions were prepared in 96-well microplate in assay buffers and 8–10 concentrations were tested.
A cellular aequorin-based functional assay was performed with recombinant CHO-K1 cells expressing mitochondrially targeted aequorin, human GPCR, and the promiscuous G protein α16 for 5-HT6 and 5-HT2A receptors, Gαqi/5 for D2 receptor (PerkinElmer Health Sciences, Inc., Shelton, CT). Assay was executed according to previously described protocolCitation11. After thawing, cells were transferred to assay buffer (DMEM/HAM’s F12 with 0.1% proteasefree BSA) and centrifuged. The cell pellet was resuspended in assay buffer and coelenterazine h was added at final concentrations of 5 μM. The cell suspension was incubated at 16 °C, protected from light with constant agitation for 16 h and then diluted with assay buffer to a concentration of 12 500 cells/ml for 5HT6 and 5HT2A receptors and 3000 cells/ml for D2 receptor. After 1 h of incubation, 50 μl of the cell suspension was dispensed using automatic injectors built into the radiometric and luminescence plate counter MicroBeta2 LumiJET (PerkinElmer Health Sciences, Inc., Shelton, CT) into white opaque 96-well microplates preloaded with test compounds. Immediate light emission generated following calcium mobilization was recorded for 30−60 s. In antagonist mode, after 15−30 min of incubation the reference agonist was added to the above assay mix and light emission was recorded again. A final concentration of the reference agonist was equal to an EC80 value of 40 nM serotonin for the 5-HT6 receptor, 30 nM α-methylserotonin for the 5-HT2A receptor, and 30 nM apomorphine for the D2 receptor.
For the 5-HT7 and 5-HT1A receptors, adenylyl cyclase activity was monitored using cryopreserved CHO-K1 cells, expressing the human serotonin 5-HT7 or 5-HT1A receptor. Thawed cells were resuspended in stimulation buffer (HBSS, 5 mM HEPES, 0.5 IBMX, and 0.1% BSA at pH 7.4) at 2000 cells/ml for 5-HT7 receptor and 4000 cells/ml for 5-HT1A receptor. Ten microliters of cell suspension was added to 10 μl of tested compounds for 5-HT7 receptor and 10 μl of tested compounds with 10 µM forskolin for 5-HT1A receptor loaded onto a white opaque half area 96-well microplate.
An antagonist response experiment was performed with 10 nM serotonin as the reference agonist for 5-HT7 receptor and 0.7 nM 5-carboxamidotryptamine as the reference agonist for 5-HT1A receptor. The agonist and the antagonist were added simultaneously. Cell stimulation was performed for 60 min at room temperature. After incubation, cAMP measurements were performed with homogeneous TR-FRET immunoassay using the LANCE Ultra cAMP kit (PerkinElmer Health Sciences, Inc., Shelton, CT). Ten microlites of EucAMP Tracer Working Solution and 10 μl of ULight-anti-cAMP Tracer Working Solution were added, mixed, and incubated for 1 h. The TR-FRET signal was read on an EnVision microplate reader (PerkinElmer Health Sciences, Inc., Shelton, CT). IC50 and EC50 values were determined by non-linear regression analysis using GraphPad Prism 5.0 software (GraphPad Software, Inc., San Diego, CA).
Molecular modeling
The homology models of human D2 dopamine and 5-HT1A serotonin receptors, which were engaged in docking studies, have been described in previously published papersCitation11,Citation34,Citation35. The detailed comparative modeling method and ligand-based optimization characteristics developed for this purpose have also been presented formerlyCitation36.
The D2 receptor models were built using dopamine D3 receptor crystal structure as a template (Protein Data Bank (PDB) database ID: 3PBL)Citation37 while the 5-HT1A receptor models were built on the basis of β2 adrenergic receptor crystal structure (PDB ID: 2RH1)Citation38. Sequence alignments between D2 and 5-HT1A receptors (UniProt database accession numbers P14416 and P08908, respectively)Citation39 and their templates were due to hhsearch tool via GeneSilico MetaserverCitation40. The crude receptor models were obtained using SwissModelCitation41, and were validated by processing in Protein Preparation WizardCitation42. For each receptor type, a set of bioactive compounds was selected for ligand-steered binding site optimization, which was completed using induced fit docking (IFD) workflowCitation43,Citation44. That procedure resulted in a variety of receptor models that served as molecular targets in docking studies, mimicking conformational freedom of the proteins.
Ligand structures were optimized using LigPrep tool. Glide XP flexible docking was carried out using default parameters, leaving ring conformations as defined during ligand preparation. H-bond constraints, as well as centroid of a grid box (22 × 22 × 22 Å) for docking studies were located on Asp3.32.
Glide, induced fit docking, LigPrep, and Protein Preparation Wizard were implemented in Small-Molecule Drug Discovery Suite (Schrödinger, Inc., New York, NY), which was licensed for Jagiellonian University Medical College.
Behavioral evaluation
The experiments were performed on male Albino Swiss or CD-1 mice (22–28 g). The animals were kept at a room temperature of 20 ± 1 °C, and had free access to food (standard laboratory pellets) and tap water before the experiment. All the experiments were conducted in the light phase between 9 a.m. and 2 p.m. All the experimental procedures were approved by the Local Ethics Commission for Animal Experiments of Jagiellonian University in Cracow. d-Amphetamine (Sigma-Aldrich, St. Louis, MO) and the investigated compounds (32 and 34) were dissolved in distilled water. With the exception of d-amphetamine that was administered subcutaneously (sc), the remaining compounds were injected intraperitoneally (ip). The investigated compounds were administered 60 min and d-amphetamine 30 min before the test. Each experimental group consisted of 6–10 animals, and all the animals were used only once.
d-Amphetamine-induced hyperactivity in CD-1 mice
Locomotor activity was recorded with an Opto M3 multi-channel activity monitor (MultiDevice Software v.1.3, Columbus Instruments, Columbus, OH). The mice were individually placed in plastic cages (22 × 12 × 13 cm) for 30 min habituation period, and then the crossings of each channel (ambulation) were counted during 1 h with data recording every 5 min. The cages were cleaned up with 70% ethanol after each mouse.
Spontaneous locomotor activity in CD-1 mice
Locomotor activity was recorded with an Opto M3 multi-channel activity monitor (MultiDevice Software v.1.3, Columbus Instruments, Columbus, OH). The CD-1 mice were individually placed in plastic cages (22 × 12 × 13 cm) for 30 min habituation period, and then the crossings of each channel (ambulation) were measured every 5 min for 60 min. The cages were cleaned up with 70% ethanol after each mouse.
Results and discussion
Comparative structural analysis
The X-ray crystal structure analysis was performed for 3,4-dichlorophenylpiperazine-purine-2,6-dione derivatives 37 and 44 in order to examine the molecular conformation and intermolecular interactions. The impact of the different amino substituent in the 8 position of purine-2,6-dione scaffold (morpholine and dipropylamine, respectively) and the length of the aliphatic linker (four and three methylene groups, respectively) were investigated.
Compounds 37 and 44 crystallise in the form of hydrochloride salt, with positive charge localized on N15 atom of the piperazine ring. Due to the presence of the cation, the charge-assisted hydrogen bond N15+–H15···Cl1− is formed in the crystal structure of 37 and 44. The co-crystallising water molecule additionally forms strong hydrogen bonds as a donor (O–H···Cl− and O−H···O) and as an acceptor of the weak C–H···O interactions ( and Supplemental Information).
There are two main differences in the molecular structures of both compounds with a great impact on the crystal packing. First is the length of the aliphatic linker, which consists of three and four methylene groups for 37 and 44, respectively. This linker adopts different conformation in both presented crystal structures. Conformation of linker and its influence on the biological activity was widely discussed in the literatureCitation18,Citation45–48. In most known LCAP crystal structures with tetramethylene linker, this aliphatic chain adopts extended conformation (anti–anti–anti)Citation18. Such conformation is also observed from 37, with torsion angles varying around 170°. However, in the shorter linker of 44, the conformation of the aliphatic trimethylene chain is anti-gauche, with torsion angles N7–C10–C11–C12 and C10–C11–C12–N15 being 178.3° and −70.8°, respectively.
In the molecule of the 37, the polar morpholine ring in the 8 position formed intermolecular interactions of C–H···O type, both as donor and acceptor of the hydrogen bond (Supplemental Information). The same type of interactions was observed in the previously reported 3-Cl and 2-OCH3 PhP analogsCitation18.
The bulky and hydrophobic dipropylamine substituent in the structure of compound 44 avoids polar intermolecular contacts, forming the hydrophobic planes normal to [001] direction. This hydrophobic association leads to such molecular arrangement which allow antiparallel stacking interaction. The 3,4-dichlorophenyl overlap alternately with the six-membered ring of the purine system (centroid–centroid distance in the range of 3.5–3.6 Å). This π–π interaction propagates continuously along [100] axis.
In vitro binding experiments
Numerous newly synthesized derivatives of 8-amine-purine-2,6-dione derivatives of LCAPs (18–47) displayed high-to-moderate affinity for 5-HT1A, 5-HT2A 5-HT7, and D2 Rs (Ki = 1–115 nM, 24–106 nM, 26–97 nM, and 2–78 nM, respectively). Moreover, several compounds possessed marked affinity (Ki < 100 nM) for 5-HT6 Rs ( and ). The 8-piperidine-purine-2,6-dione derivatives (26–35) were interesting because of their multireceptor (serotonin and dopamine) affinities. It seems that this cyclic amine system was preferable to obtain potent serotonin and D2 ligands. It was previously found in a series of 8-unsubstituted 7-phenylpiperazinylalkyl-purine-2,6-diones that the lack of substituent in a 8 position was rather preferential for the interaction with 5-HT1A RsCitation19. In this group of 8-piperidine analogs, the elongation of propylene spacer to butylene one increased the affinities for all evaluated receptors. This modification resulted in 273-fold higher affinities for 5-HT6, 148-fold for 5-HT7, and 34-fold for D2 Rs in the case of 2-OCH3 derivative 27 (yielding 30). Within a set of structural analogs, 3,4-diCl-PhP derivative (32) possessed high receptor affinities for the targeted receptors, similar to aripiprazole, except 5-HT1A sites to which was less active. This result may suggest that the 3,4-diCl substituent was preferable to obtain potent 5-HT2A, 5-HT6, 5-HT7, and D2 ligands (). Compound 31 containing 3-Cl substituent displayed higher affinity for 5-HT1A than 32 but was three-fold less active for 5-HT6 and over six-fold less active for D2 Rs. The elongation of the linker length between purine-2,6-dione core and PhP fragment from four- to five-carbon units increased threefold D2 affinity (29 and 30 versus 33 and 34) and 1.5–5 fold the 5-HT1A affinity (e.g. 29–31 versus 33–35). At the same time, it decreased 5-HT6 Rs affinity and either retained or slightly decreased the affinity for 5-HT2A Rs ().
Table 1. The binding data of the 8-pyrrolidino-, 8-piperidino-, and 8-morpholino-purine-2,6-dione derivatives for 5-HT and dopamine receptors.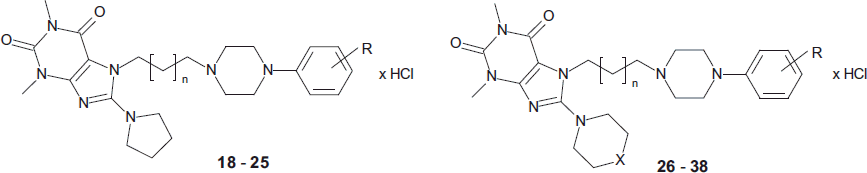
Table 2. The binding data of the 8-diethylamino- and 8-dipropylamino-purine-2,6-dione derivatives for 5-HT and dopamine receptors.
Hence, in the tested series, the four-methylene group spacer was preferable to obtain multifunctional receptor ligands. The replacement of the piperidine substituent with morpholine moiety with additional hydrogen bond acceptor generally decreased the affinity for the evaluated receptors as was previously evidenced in a series of 7-arylpiperazinylalkyl-8-morpholin-4-yl-purine-2,6-dione derivativesCitation18.
The 8-morpholine-3,4-diCl-PhP analog of 32, compound 37, explained similar 5-HT1A, 5-HT2, and 5-HT6 receptor affinity but was less active for 5-HT7 and D2 Rs. The elongation of the alkyl chain to five-carbon unit in a series of 8-morpholin-4-yl-purine-2,6-diones did not significantly change the affinity for the evaluated receptors in comparison with the previously reported analogs of the four aliphatic linkerCitation18.
Compound 38 with 2-OCH3 substituent and five methylene spacer was three-fold and 14-fold less active at 5-HT1A sites in comparison with the previously reported analogs with three-carbon and four-carbon linkers, respectivelyCitation18. At the same time, this modification retained its activity for evaluated 5-HT2A, 5-HT6, and 5-HT7 receptors.
The introduction of 3,4-diCl substituent into the PhP moiety significantly increased the affinity for 5-HT6 receptors. This effect was observed in the case of 32 and 37 among four-methylene analogs with piperidine (32) or morpholine (37) moiety in a 8-position of purine-2,6-dione core. Furthermore, this substituent in the PhP fragment is also preferable for D2 receptors. The reduction of cyclic amine system from six-membered piperidine to pyrrolidine ring generally decreased 5-HT1A, 5-HT2A, and D2 affinities but increased up to two-fold 5-HT7 affinity. Compound 24 with 3-Cl substituted phenylpiperazine displayed high affinity for 5-HT7 and D2 sites and moderate affinity to other evaluated receptors.
Generally, compounds with a dipropylamine substituent in an 8-position (43–47) may be classified as multimodal serotonin and dopamine receptor ligands with high-to-moderate affinity for 5-HT1A, 5-HT2A, 5-HT6, 5-HT7, and D2 receptors (). This modification was significantly beneficial for 5-HT6 affinity. Compound 45 was 68-fold more active than its 8-piperidinyl counterpart 30. This may suggest that the hydrophobic substituent in a 8-position of purine-2,6-dione core may account for higher affinity for 5-HT6 sites. The replacement of the dipropylamine group by diethylamine moiety significantly reduced 5-HT6 affinity. The impact of this substituent for the affinities for other targeted receptors was not so decisive. Summing up, the binding study allowed us to identify some multimodal 5-HT/D receptor ligands. Among them, the most interesting compounds were selected for the determination of their functional profile.
Functional in vitro evaluation
On the basis of the multireceptor binding affinity results, a series of 11 compounds was selected for the functional profile characterization in vitro at all five targets, according to the previously published methodsCitation11. Potent D2R antagonists were identified among the selected 8-aminopurine-2,6-dione derivatives, achieving single-digit nanomolar IC50 values (34, 41, and 45) and, in this way, were comparable with the chlorpromazine (). Other evaluated compounds (24, 31, 32, 39, and 46) displayed antagonistic properties at this receptor with double-digit nanomolar IC50 values. In addition, 43 and 47 showed antagonistic activity with IC50 values > 100 nM. On the contrary, the majority of the evaluated compounds showed agonistic activity at D2R, achieving double-digit nanomolar value in the case of 34 and 45 (). Regarding the functional profile the tested compounds are similar to aripiprazole (partial agonist of D2R) that is in line with the recent trends in the development of modern atypical antipsychotics. Moreover, five from the evaluated compounds (31, 34, 39, 41, and 43) behaved as partial agonists of 5-HT1A receptors (). It is well known that 5-HT1A receptor agonists or partial agonists may attenuate the catalepsy induced by the D2R blockade, as well as reduce the cataleptogenic potential of novel antipsychotic agentsCitation50. In addition, compounds 31, 32, 43, 45, and 46 showed antagonistic properties at 5-HT2A receptors in a range of IC50 value = 34.6–616 nM, and, at the same time, displayed 5-HT2A agonistic activity ( and ). The use of 5-HT2A antagonists in dopamine-deficient areas of the brain, including the frontal and nigrostriatal pathways, may improve the negative symptoms of schizophrenia and decrease the incidence of extrapyramidal side effectsCitation51,Citation52. Among the evaluated compounds, weak 5-HT7R antagonistic properties were observed in the case of 34 (). Taking into account the functional profile, the evaluated compounds corresponded to aripiprazole which exerts not only partial dopaminergic activity but also a 5-HT1A and 5-HT2C receptor partial agonist and 5-HT2A and 5-HT7 antagonist and this mechanism of action may contribute to its broad efficacy – antipsychotic, antidepressant, and anxiolyticCitation53–55.
Table 3. Functional data (agonist and antagonist mode) for the selected compounds for dopamine D2 receptor.
Table 4. Functional data (agonist mode) for the selected compounds for serotonin receptors.
Table 5. Functional data (antagonist mode) for the selected compounds for serotonin receptors.
Moreover, among the evaluated compounds, 43 and 46 showed 5-HT6 antagonistic properties (IC50 values = 676 and 847 nM, respectively) (). It seems important in terms of the recent reports that proposed 5-HT6 receptor antagonists as potential drugs to treat cognitive impairments in schizophreniaCitation56,Citation57.
Molecular modeling
Molecular modeling studies have been performed in order to characterize putative binding mode of the bioactive representative of the chemical class of arylpiperazinylalkyl derivatives of 8-amino-1,3-dimethylpurine-2,6-dione – compound 34. Docking studies extensively facilitated interpretation of SAR data and defined possible structural modifications.
Compound 34 displayed potent affinity for 5-HT1A receptor, which is of major interest in the presented research. The molecule adopts consistent conformation in the homology models of 5-HT1A receptor (). The protonated nitrogen atom of the arylpiperazine moiety binds to Asp3.32 (charge-reinforced H-bond), while the aryl ring interacts with Phe6.52 (π–π stacking) and Lys191 (π-cation). Binding mode analysis confirmed that substitution of the methoxy group in position 2 of the aryl ring is the most beneficial in the series, since it enables H bonding with Lys191 and does not interfere with other aminoacid residues constituting the binding pocketCitation59. The 1,3-dimethylpurine-2,6-dione moiety stabilizes the ligand–receptor complex by π–π stacking with Tyr2.64, while its substituent in position 8 fills the non-specific cavity below the second extracellular loop (ECL 2). The latter moiety does not determine affinity for this receptor subtype, but may introduce activity towards other subtypes of receptors, e.g. 5-HT6, which is evident in the presented series, although further investigation is required to explain it.
Figure 3. Binding modes of compound 34 in the site of 5-HT1A receptor (A) and D2 receptor (C). Proposed bioactive conformations and important interactions of compound 34 (green) and aripiprazole (cyan) in the binding site of D2 receptor (B). Amino acid residues engaged in ligand binding (within 4 A from the ligand atoms) are displayed as sticks, whereas those forming important H-bonds (dotted yellow lines) or π–π stacking/π-cation interactions (dotted green lines) are represented as thick sticks. For the sake of clarity, ECL2 residues were hidden. TMH, transmembrane helix; ECL, extracellular loop.
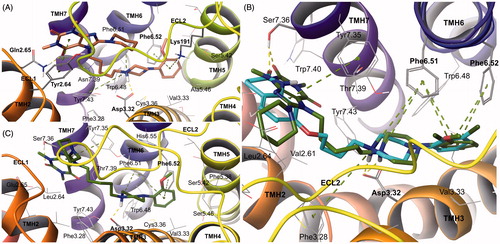
The present series of compounds display pronounced affinity for dopamine D2 receptor, which has been secured by introducing privileged arylpiperazine scaffoldCitation60. Functional studies revealed that several compounds of the series were characterized as D2 receptor partial agonists. Analysis of SAR data in this class of ligands suggests that the second terminal part of molecules may be responsible for elicitation of intrinsic activityCitation61,Citation62. Arylpiperazine part of the analyzed compound 34 interacts with D2 receptor’s Asp3.32 and Phe6.51/6.52, as described for 5-HT1A receptor, while 8-piperidine-1,3-dimethylpurine-2,6-dione moiety occupies less conserved cavity, having aromatic contacts with Tyr7.35 and H-bond with Ser7.36 ().
The proposed binding mode resembles the one of aripiprazole, antipsychotic drug, exhibiting well-balanced partial agonism for D2 receptorCitation63. The docking poses of compound 34 and aripiprazole shown to be very consistent. Besides the arylpiperazine moieties, which took the same position in the orthosteric pocket, the 8-piperidine-1,3-dimethylpurine-2,6-dione and 3,4-dihydroquinolin-2(1H)-one fragments appeared to interact in the same manner, both accepting H-bond from the hydroxy group of Ser7.36 and filling fairly hydrophobic cavity between THMs 2 and 7 (). Such binding site interactions are supposed to cause conformational changes promoting receptor activation (compare in vitro data in ).
The results of the described X-ray studies confirmed the outcomes of structure generation by means of molecular modeling, e.g. chair conformation of piperazine ring which is substituted equatorially. The aliphatic linker adopts extended conformation and the heterocyclic substituent in a 8 position of purine-2,6-dione core preserved the same equatorially substituted chair conformation.
Behavioral evaluation
Both the receptor profile and the functional activity of compounds 32 and 34 prompted us to evaluate potential antipsychotic activity using d-amphetamine-induced hyperactivity in CD-1 mice. d-Amphetamine produces locomotor hyperactivity in animals as a result of increased dopaminergic activity in the mesolimbic systemCitation64,Citation65. There are many evidences that its effect is blocked by antipsychotics having antagonist properties toward dopamine receptorsCitation66. The obtained, in our experiment, results indicate that both investigated compounds 32, at dose of 20 mg/kg, and 34, administered at doses of 10 and 20 mg/kg, revealed antipsychotic-like properties, significantly decreasing d-amphetamine-induced hyperactivity in mice by 77.5, 80, and 97%, respectively ().
Table 6. The effect of compounds 32 and 34 on the d-amphetamine-induced locomotor activity in mice.
The effect seems to be specific, since 32 administered at effective dose had no influence on the spontaneous locomotor activity in mice. In contrary, the reduction of d-amphetamine induced hyperactivity caused by compound 34 is probably due to its sedative potential (Supplemental information). The present preclinical results demonstrate that compound 32 has pharmacological characteristics of an antipsychotic agent and encourage to pursue further more advanced studies of this structure.
Conclusion
Extending the studies in a group of 7-arylpipiperazinylalkyl-8-amino-1,3-dimethylpurine-2,6-diones, some potent multireceptor 5-HT1A/5-HT2A/5-HT7/D2 ligands were identified. The 8-piperidine (26–35) and 8-dipropyloamino (45–47) derivatives were interesting because of their multireceptor (serotonin and dopamine) affinities which may indicate that this cyclic amine systems were preferable to obtain potent ligands for evaluated receptors. In these series, the four- and the five-methylene group alkyl spacer was beneficial for binding affinity for targeted receptors. A strong positive influence of 3-chloro- and 3,4-dichloro-phenylpiperazinyl moieties for 5-HT6 receptor affinity should be pointed out. Compounds 32, 37, and 44–46 were active at these sites similar to aripiprazole. The selected compounds 24, 31, 34, 39, 41, 43, 45, and 46 in the functional in vitro evaluation for all targeted receptors showed significant partial D2 agonist, partial 5-HT1A agonist, and 5-HT2A antagonist properties. In the behavioral studies, compounds 32 and 34 revealed antipsychotic-like properties, which in the case of 32 seems to be specific. The obtained results imply that the series of the novel long-chain arylpiperazine derivatives of 8-aminopurine-2,6-dione is worthy of future research for their potential antipsychotic- and/or antidepressant-like activity.
Supplementary material available online
IENZ_1088844_Supp.pdf
Download PDF (64.1 KB)Acknowledgements
This study was supported by the National Science Centre (NCN) - Poland funded Grant No. UMO-2011/01/B/NZ4/00695 and No. 2012/07B/NZ7/01173. The crystallographic research was carried out with the equipment purchased using financial support of the European Regional Development Fund in the framework of the Polish Innovative Economy Operational Program (contract no. POIG.02.01.00-12-023/08).
Declaration of interest
The authors report that they have no conflict of interest.
References
- Stahl S. Describing an atypical antipsychotic: receptor binding and its role in pathophysiology. Prim Care Companion J Clin Psychiatry 2003;5:9–13
- Roth BL, Sheffler D, Potkin SG. Atypical antipsychotic drug actions: unitary or multiple mechanism for ‘atypicality’? Clin Neurol Res 2003;3:108–17
- Divac N, Prostran M, Jakovcevski I, Cerovac N. Second-generation antipsychotics and extrapyramidal adverse effects. Biomed Res Int 2014. http://dx.doi.org/10.1155/2014/656370
- Lameh J, McFarland K, Ohlsson J, et al. Discovery of potential antipsychotic agents possessing pro-cognitive properties. Naunyn Schmiedebergs Arch Pharmacol 2012;385:313–23
- Hirose T, Kikuchi T. Aripiprazole, a novel antipsychotic agent: dopamine D2 receptor partial antagonist. J Med Invest 2005;52:284–90
- Butini S, Gemme S, Campiani G, et al. Discovery of a new class of potential multifunctional atypical antipsychotic agents targeting dopamine D3 and serotonin 5-HT1A and 5-HT2A receptors: design, synthesis, and effects on behavior. J Med Chem 2009;52:151–69
- Zajdel P, Marciniec K, Maślankiewicz A, et al. Quinoline-and isoquinoline-sulfonamide derivatives of LCAP as potent CNS multi-receptor-5-HT1A/5-HT2A/5-HT7 and D2/D3/D4-agents: the synthesis and pharmacological evaluation. Bioorg Med Chem 2012;20:1545–56
- Zajdel P, Marciniec K, Maślankiewicz A, et al. Antidepressant and antipsychotic activity of new quinoline- and isoquinoline-sulfonamide analogs of aripiprazole targeting serotonin 5-HT1A/5-HT2A/5-HT7 and dopamine D2/D3 receptors. Eur J Med Chem 2013;60:42–50
- Marazziti D, Baroni S, Bosini F, et al. Serotonin receptors of type 6 (5-HT6): from neuroscience to clinical pharmacology. Curr Med Chem 2013;20:371–7
- Glennon RA, Siripurapu U, Roth BL, et al. The medicinal chemistry of 5-HT6 receptor ligands with a focus on arylsulfonyltryptamine analogs. Curr Top Med Chem 2010;10:579–95
- Kołaczkowski M, Marcinkowska M, Bucki A, et al. Nowel arylsulfonamide derivatives with 5-HT6/5-HT7 receptor antagonism targeting behavioral and psychological symptoms of dementia. J Med Chem 2014;57:4543–57
- Pawłowski M, Chłoń G, Obniska J, et al. Synthesis, 5-HT1A and 5-HT2A receptor affinity of new 1-phenylpiperazinylpropyl derivatives of purine-2,6-and pyrrolidine-2,5-diones. Il Farmaco 2000;55:461–8
- Chłoń G, Pawłowski M, Duszyńska B, et al. Synthesis, 5-HT1A and 5-HT2A receptor activity of new 1-phenylpiperazinylpropyl derivatives with arylalkyl substituents in position 7 of purine-2,6-dione. Pol J Pharmacol 2001;53:359–68
- Zajdel P, Bojarski AJ, Byrtus H, et al. Preliminary study on application of impregnated synthetic peptide TLC stationary phases for the pre-screening of 5-HT1A ligands. Biomed Chromatogr 2003;17:312–7
- Chłoń-Rzepa G, Żmudzki P, Zajdel P, et al. 7Arylpiperazinylalkyl and 7-tetrahydroisoquinolinylalkyl derivatives of 8-alkoxy-purine-2,6-dione and some of their purine-2,6,8-trione analogs as 5-HT1A, 5-HT2A, and 5-HT7 serotonin receptor ligands. Bioorg Med Chem 2007;15:5239–50
- Jastrzębska-Więsek M, Partyka A, Chłoń-Rzepa G, et al. Potential anxiolytic, but not antidepressant activity of new 7-arylpiperazinylbutyl-8-morpholinyl-purine-2,6-dione analogs in mice. Acta Biol Cracov Ser Zool 2011;53:31–7
- Chłoń-Rzepa G, Żmudzki P, Satała G, et al. New 8-aminoalkyl derivatives of purine-2,6-dione with arylalkyl, allyl or propynyl substituents in position 7, their 5-HT1A, 5-HT2A, and 5-HT7 receptor affinity and pharmacological evaluation. Pharmacol Rep 2013;65:15–29
- Chłoń-Rzepa G, Żmudzki P, Pawłowski M, et al. New 7-arylpiperazinylalkyl-8-morpholin-4-yl-purine-2,6-dione derivatives with anxiolytic activity – synthesis, crystal structure and structure–activity study. J Mol Struct 2014;1067:243–51
- Partyka A, Chłoń-Rzepa G, Wasik A, et al. Antidepressant-and anxiolytic-like activity of 7-phenylpiperazinylalkyl-1,3-dimethyl-purine-2,6-dione derivatives with diversified 5-HT1A receptor functional profile. Bioorg Med Chem 2015;23:212–21
- Chłoń-Rzepa G, Zagórska A, Bucki A, et al. New arylpiperazinylalkyl derivatives of 8-alkoxy-purine-2,6-dione and dihydro[1,3]oxazolo[2,3-f]purinedione targeting the serotonin 5-HT1A/5-HT2A/5-HT7 and dopamine D2 receptors. Arch Pharm (Weinheim) 2015;348:242–53
- Zygmunt M, Sapa J, Chłoń-Rzepa G, et al. 7-3-Chlorophenypiperazinylalkyl derivatives of 8-alkoxy-purine-2,6-dione as a serotonin receptor ligands with potential antidepressant activity. Pharmacol Rep 2014;66:505–10
- Eckstein M, Gorczyca M, Zejc A. About the oxidative bromination of methylxanthines. Acta Pharm Yugoslav 1972;22:133–6
- Gorczyca M, Mogilnicka E, Wantuch C. Synthesis of 1-and 7-β-hydroxypropyl-8-cycloalkylamino-dimethylxanthines. Dissert Pharm Pharmacol 1970;22:403–7
- Seela F, Ramzaeva N, Rosemeyer H. Purines. Sci Synth 2003;16:945–1108
- Klingler KH. 8-Substituted xanthine derivatives. Ger. Offen, DE 2253075 A1 19730524, 1973
- Klinger KH. Synthesis of bronchospasmolytically effective beta-phenylethylaminoalkyl xanthines. Arzneimittelforschung 1977;27:4–14
- Agilent. CrysAlis PRO. Yarnton, England: Agilent Technologies UK Ltd; 2011
- Sheldrick GM. A short history of SHELX. Acta Crystallogr., A, Found. Crystallogr 2008;64:112–22
- Farrugia LJ. WinGX suite for small-molecule single-crystal crystallography. J Appl Cryst 1999;32:837–8
- Farrugia LJ. WinGX and ORTEP for Windows: an update. J Appl Cryst 2012;45:849–54
- Bojarski AJ, Cegła MT, Charakchieva-Minol S, et al. Structure-activity relationship studies of CNS agents. Part 9: 5-HT1A and 5-HT2 receptor affinity of some 2-and 3-substituted 1,2,3,4-tetrahydro-beta-carbolines. Pharmazie 1993;48:289–94
- Paluchowska MH, Bugno R, Duszyńska B, et al. The influence of modifications in imide fragment structure on 5-HT1Aand 5-HT7 receptor affinity and in vivo pharmacological properties of some new 1-(m-trifluoromethylphenyl)piperazines. Bioorg Med Chem 2007;15:7116–25
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 1973;22:3099–108
- Czopek A, Kołaczkowski M, Bucki A, et al. Novel spirohydantoin derivative as a potent multireceptor-active antipsychotic and antidepressant agent. Bioorg Med Chem 2015;23:3436–47
- Kowalski P, Jaśkowska J, Bojarski AJ, et al. Evaluation of 1-arylpiperazine derivative of hydroxybenzamides as 5-HT 1A and 5-HT7 serotonin receptor ligands: an experimental and molecular modeling approach. J Heterocyclic Chem 2011;48:192–8
- Kołaczkowski M, Bucki A, Feder M, Pawłowski M. Ligand-optimized homology models of D1 and D2 dopamine receptors: application for virtual screening. J Chem Inf Model 2013;53:638–48
- Chien EYT, Liu W, Zhao Q, et al. Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 2010;330:1091–5
- Cherezov V, Rosenbaum DM, Hanson MA, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 2007;318:1258–65
- Jain E, Bairoch A, Duvaud S, et al. Infrastructure for the life sciences: design and implementation of the UniProt Website. BMC Bioinf 2009;10:136
- Kurowski MA, Bujnicki JM. GeneSilico protein structure prediction meta-server. Nucleic Acids Res 2003;31:3305–7
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinforma Oxf Engl 2006;22:195–201
- Sastry GM, Adzhigirey M, Day T, et al. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des 2013;27:221–34
- Sherman W, Beard HS, Farid R. Use of an induced fit receptor structure in virtual screening. Chem Biol Drug Des 2006;67:83–4
- Sherman W, Day T, Jacobson MP, et al. Novel procedure for modeling ligand/receptor induced fit effects. J Med Chem 2006;49:534–53
- Paluchowska MH, Bojarski AJ, Charakchieva-Minol S, Wesołowska A. Active conformation of some arylpiperazine postsynaptic 5-HT(1A) receptor antagonists. Eur J Med Chem 2002;37:273–83
- Lopez-Rodriguez ML, Morcillo MJ, Rovat TK, et al. Synthesis and structure-activity relationships of a new model of arylpiperazines. 4. 1-[omega-(4-Arylpiperazin-1-yl)alkyl]-3-(diphenylmethylene)-2, 5-pyrrolidinediones and-3-(9H-fluoren-9-ylidene)-2, 5-pyrrolidinediones: study of the steric requirements of the terminal amide fragment on 5-HT1A affinity/selectivity. J Med Chem 1999;42:36–49
- Bojarski AJ, Duszyńska B, Kołaczkowski M, et al. The impact of spacer structure on 5-HT7 and 5-HT1A receptor affinity in the group of long-chain arylpiperazine ligands. Bioorg Med Chem Lett 2004;14:5863–6
- Lewgowd W, Bojarski AJ, Szczesio M, et al. Synthesis and structural investigation of some pyrimido[5,4-c]quinolin-4(3H)-one derivatives with a long-chain arylpiperazine moiety as potent 5-HT(1A/2A) and 5-HT(7) receptor ligands. Eur J Med Chem 2011;46:3348–61
- Shapiro DA, Renock S, Arrington E, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 2003;28:1400–11
- Kleven MS, Barret-Glévoz C, Bruins Slot L, Newman-Tancredi A. Novel antipsychotic agents with 5-HT(1A) agonist properties: role of 5-HT(1A) receptor activation in attenuation of catalepsy induction in rats. Neuropharmacology 2005;49:135–43
- Schmidt CJ, Sorensen SM, Kenne JH, et al. The role of 5-HT2A receptors in antipsychotic activity. Life Sci 1995;56:2209–22
- Herbert Y, Meltzer MD. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 1999;21:106S–15
- Burda K, Czubak A, Kus K, et al. Influence of aripiprazole on the antidepressant, anxiolytic and cognitive functions of rats. Pharmacol Rep 2011;63:898–907
- Blier P, Blondeau C. Neurobiological bases and clinical aspects of the use of aripiprazole in treatment-resistant major depressive disorder. J Affect Disord 2011;128:S3–10
- Norquist RE, Risterucci C, Moreau JL, et al. Effects of aripiprazole/OPC-14597 on motor activity, pharmacological models of psychosis, and brain activity in rats. Neuropharmacology 2008;54:405–16
- Morozowa MA, Lepilkina TA, Rupchev GE, et al. Add-on clinical effects of selective antagonist of 5HT6 receptors AVN-211 (CD-008-0173) in patients with schizophrenia stabilized on antipsychotic treatment: pilot study. CNS Spectr 2014;19:316–23
- Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav 2011;99:245–53
- Tadori Y, Miwa T, Tottori K, et al. Aripiprazole's low intrinsic activities at human dopamine D2L and D2S receptors render it a unique antipsychotic. Eur J Pharmacol 2005;515:10–19
- Zagórska A, Kołaczkowski M, Bucki A, et al. Structure-activity relationships and molecular studies of novel arylpiperazinylalkyl purine-2,4-diones and purine-2,4,8-triones with antidepressant and anxiolytic-like activity. Eur J Med Chem 2015;97:142–54
- Wang Q, Mach RH, Luedtke RR, Reichert DE. Subtype selectivity of dopamine receptor ligands: insights from structure and ligand-based methods. J Chem Inf Model 2010;50:1970–85
- Shonberg J, Herenbrink CK, López L, et al. A structure–activity analysis of biased agonism at the dopamine D2 receptor. J Med Chem 2013;56:9199–221
- Kołaczkowski M, Marcinkowska M, Bucki A, et al. Novel 5-HT6 receptor antagonists/D2 receptor partial agonists targeting behavioral and psychological symptoms of dementia. Eur J Med Chem 2015;92:221–35
- Urban JD, Vargas GA, von Zastrow M, Mailman RB. Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signalling pathways. Neuropsychopharmacology 2007;32:67–77
- Costall B, Domeney AM, Naylor RJ. Locomotor hyperactivity caused by dopamine infusion into the nucleus accumbens of rat brain: specificity of action. Psychopharmacology 1984;82:174–80
- Cools AR. Mesolimbic dopamine and its control of locomotor activity in rats: differences in pharmacology and light/dark periodicity between the olfactory tubercle and the nucleus accumbens. Psychopharmacology 1986;88:451–9
- Ellenbroek BA. Treatment of schizophrenia: a clinical and preclinical evaluation of neuroleptic drugs. Pharmacol Ther 1993;57:1–78