Abstract
The inhibition and characterization of the α-class carbonic anhydrase (CA, EC 4.2.1.1) from the Halomonas sp. are reported for the first time. The enzyme was purified 91-fold with a yield of 39%, and a specific activity of 600 U/mg proteins was obtained. It has an optimum pH at 7.5, an optimum ionic strength at 20 mM and an optimum temperature at 20 °C. The following anions, SCN−, Br−, Cl−, I−, ,
,
,
,
and
showed inhibitory effects on the hydratase activity of the enzyme. Sulfate, sulfide, azide, nitrate, nitrite and iodide exhibited the strongest inhibitory activity, in the micromolar range (KI-s of 5.5–15.5 µM). SCN−, Br−, Cl−,
were moderate inhibitors, whereas other anions showed only weak activities. Our findings indicate that these anions inhibit the Halomonas sp. CA (HmCA) enzyme in a similar manner to other α-CAs from mammals investigated earlier, but the susceptibility to various anions differs significantly between the Halomonas sp. and other organism CAs.
Introduction
Carbon dioxide and bicarbonate are essential components in microorganisms as well as other living organism. The required CO2 by the cell is transported into the cell by hydration reaction depending upon the bicarbonate, carbon dioxide concentration inside and outside the cell. This transportation is performed depending upon the amount of carbon dioxide conversion into bicarbonate and occurs very frequently in the cell. The reaction of this conversion is very slow and should be speed up somehow. Carbonic anhydrase (CA, EC 4.2.1.1), one of the fastest known enzyme, catalyzes the reaction with typical catalytic rates of the different forms of this enzyme ranging between 104 and 106 reactions per secondCitation1–3. CAs are mainly zinc metalloenzymes that catalyze the reversible interconversion of carbon dioxide and water to bicarbonate and a protonCitation1–3. These isoenzymes are thus involved in many significant processes such as photosynthesis, respiration, pH regulation, CO2 fixation and CO2 transport in plants and bacteriaCitation4–7.
CAs have attracted attention in terms of drug design, clinical medicine, highlighting plant physiology and more recently as biocatalytic agents for industrial CO2 sequestrationCitation4–7. There are six known evolutionarily distinct classes of CA that are classified as α, β, γ, δ, ζ and ηCitation7. The α-CAs are found mostly in mammals and are the most catalytically efficient, β-CAs are found in several plants, prokaryotes and fungi and γ-CAs have been isolated in several strains of bacteria and archaea that are often found in extreme environmentsCitation8. The δ- and ζ-CAs are typically found in diatoms and types of archaea, and despite having significant sequence disparity between other classes of CAs, they have apparent structural homologyCitation8.
In mammals, 16 α-CA isozymes have been described in terms of their subcellular localization, catalytic activity and susceptibility to different classes of inhibitors. These isozymes are found in various locations as membrane bound (CA IV, CA IX, CA XII and CA XIV), cytosolic (CA I, CA II, CA III, CA VII and CA XIII), mitochondrial (CAVA and CA VB) and saliva (CA VI)Citation1–4. Some microorganisms (Neisseria gonorrhoeae, Helicobacter pylori, Vibrio cholerae, Sulfurihydrogenibium yellowstonense, Sulfurihydrogenibium azorense and Ralstonia eutropha) expressed CAs belonging to the α-classCitation9.
Halomonas is a member of halophilic proteobacteria. The member of this genus grows well in wide range of sodium chloride (5–25%). The member of this genus are Gram-negative rods and described as halotolerant or halophilic. The species of Halomonas have been isolated from different saline habitats, including salt lakes, saline soils and marine environmentsCitation10,Citation11. Recently, Halomonas species have been largely investigated for their potential use in biotechnologyCitation12,Citation13. The production of compatible solutes, exopolysaccharides and extracellular enzymes are among their potential use in biotechnologyCitation14,Citation15.
Although there are studies regarding purification of CA from various tissues, no reports have been found on purification and characterization of the enzyme from Halomonas sp. In the present study, we purified and characterized an α-CA from Halomonas sp. for the first time, and investigated its kinetic properties and inhibitory effects of anions including SCN−, Br−, Cl−, I−, ,
,
,
,
and
on enzyme activity.
Experiment section
Chemicals
NaSCN, KBr, KCl, KI, NaN3, NaNO2, NaNO3, Na2CO3, Na2SO3, Na2SO4, Sepharose-4B, protein assay reagents and 4-nitrophenyl acetate (NPA) were obtained from Sigma-Aldrich Co. (St. Louis, MO). All other chemicals were of analytical grade and obtained from Merck (Kenilworth, NJ).
Isolation and characterization data of strain owing CA activity
The microorganism was isolated from salt-affected soils in the south east Anatolian region, Turkey (Şanlıurfa). The bacterial isolate was incubated on moderate halophilic medium with salt consisting of (g/l): NaCl 81, MgSO4.7H2O 9.6, MgCl3.6H2O 7.0, CaCl2.2H2O 3.6, KCl 2.0, NaHCO3 0.06, NaHBr 0.026, agar 20Citation16. The isolate was screened for its Gram reaction and spore formation as described previouslyCitation17. This was followed by the determination of the optimal growth pH (5, 7, 9 and 11) in the 10% MH agar medium and salt concentrations (5, 10, 15 and 20) in nutrient agar. The motility of the isolates was determined with wet mounts. Other test that were used for the characterization of the isolate was the determination of catalase, oxidase, hydrolysis of casein, gelatin, esculin, Tween-80 and starch, nitrate and nitrite reduction and acid from carbohydrates (glucose, fructose, lactose, mannitol, maltose, mannose, inositol, sorbitol, sucrose and galactose)Citation18. Genomic DNA extraction of the isolate was performed with DNA extraction kit (Qiagen, Venlo, Netherlands). The 16S rRNA isolates were amplified by PCR using universal primers 27f (CATCTCAGTGCAACTAAA) and 1492r (CAGGAAACAGCTATGAC)Citation19. According to the conventional tests, the strain is Gram-negative, short rods, non-motile, catalase and oxidase-negative, nitrate reduction positive. The strain grew optimally in media containing 7.5–10% NaCl at pH 7.0–11.0. The strain was negative for hydrolysis of starch, casein, gelatin, and aesculin but positive for the hydrolysis Tween-80. The strain produces acid from D-fructose, α-D-glucose, myo-inositol, maltose, D-mannose, sucrose, lactose and D-galactose. The strain has an antibiotic susceptibility to kanamycin, tetracycline and neomycin. Based on the conventional and molecular techniques, the bacterial isolate was identified and characterized as Halomonas sp. (Accession number: KR706322).
Purification of CA from Halomonas sp. by affinity chromatography
Halomonas sp. CA (HmCA) was purified from bacteria grown in the liquid nutrient medium. Bacteria culture cells mixed lysis buffer and later samples were centrifuged at 10 000 rpm for 30 min, and plasma and precipitate were removed. The pH of the homogenate was adjusted to 7.5 with solid Tris. The homogenate was applied to the prepared sepharose-4B-L-tyrosine-sulfanilamide affinity column which had been equilibrated with 20 mM Tris-HCl/100 mM Na2SO4 (pH 7.5)Citation20. The affinity gel was washed with 20 mM Tris-HCl/25 mM Na2SO4 (pH 7.5), and the enzyme was eluted by 1 M NaCl/25 mM Na2HPO4 (pH 6.3). All procedures were performed at 4 °C.
Esterase activity assay
CA esterase activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25 °C using a spectrophotometer according to the method described by Verpoorte et al.Citation21 The enzymatic reaction, in a total volume of 3.0 ml, contained 1.4 ml 0.05 M Tris-SO4 buffer (pH 8.0), 1 ml 3 mM 4-NPA, 0.5 ml H2O and 0.1 ml enzyme solution.
Protein determination
Protein quantity was determined spectrophotometrically at 595 nm during the purification steps according to the Bradford method, using bovine serum albumin as the standardCitation22.
SDS polyacrylamide gel electrophoresis
SDS polyacrylamide gel electrophoresis was performed after purification of the enzyme. It was carried out in 10% and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to Laemmli procedureCitation23. The electrophoretic pattern was photographed (see ).
In vitro effects of anions
SCN−, Br−, Cl−, I−, ,
,
,
,
and
anions were tested. Five different volumes (0.1, 0.2, 0.3, 0.4 and 0.5 ml) of anion solutions at a constant concentration were added to HmCA activity determination medium (total volume: 4.2 ml). CA activities with anions were assayed by following the hydration of CO2Citation24. Activity % values of HmCA for five different concentrations of each anion were drawn by using regression analysis graphs on a computer. HmCA activity without an anion was accepted as 100% activity. For the anions having an inhibition effect, the inhibitor concentrations causing up to 50% inhibition (IC50 values) were determined from the graphsCitation25. The curve-fitting algorithm allowed us to obtain the IC50 values, working at the lowest concentration of substrate of 0.15 mM, from which KI values were calculated by using the Chenge–Prusoff equationCitation26.
Results and discussion
Purification and characterization of CA from Halomonas sp.
Bacteria encode enzymes belonging to the α-, β- and γ-CA classesCitation9. They contain zinc ion (Zn2+) in their active site, coordinated by three histidine residues and a water molecule/hydroxide ion (in the α- and γ-CAs) or by two Cys and one His residues (in the β class), with the fourth ligand being a water molecule/hydroxide ion. Some of the catalytically active α-CAs can also catalyze the hydrolysis of esters, for example, 4-NPA (and other hydrolytic reactions as well)Citation9,Citation27.
In this study, we have purified and characterized the HmCA enzyme. And then, anion inhibition of the HmCA enzyme investigated. The purification procedure was carried out by affinity chromatography on sepharose-4B-tyrosine-sulfanilamide, as this zinc enzyme has a high affinity for sulfonamides, which bind in deprotonated to the metal ion from the enzyme active siteCitation2.
The enzyme was purified up to 91-fold with a recovery ratio of 39% compared to homogenate (). After the sample had completely passed through, the column was washed with 20 mM Tris-HCl/100 mM Na2SO4 buffer whose pH was 7.5. During washing, absorbance of fractions were measured at 280 and 348 nm by means of the spectrometer.
Table 1. Summary of the carbonic anhydrase enzyme purification steps in Halomonas sp.
These values showed that some proteins, bound to the affinity material, have been removed from the column by the washing solutions. Then, the enzyme was eluted with 1 M NaCl/25 mM Na2HPO4 pH 7.0. At the end of the last step, a highly purified enzyme was obtained exhibiting a single band on SDS-PAGE ().
We used a single step chromatographic technique; employing sepharose-4B-tyrosine-sulfanilamide affinity chromatography which strongly binds α-CAsCitation28–31. The optimum pH for the purification of the enzyme was determined to be 7.5; the optimum temperature of 20 °C; optimum ionic strength 20 mM. The stable pH profile of the enzyme was determined at four different pHs in 50 mM Tris-HCl and five different pHs in 50 mM K-phosphate buffer. The enzyme maintained 83% of the maximum catalytic activity at the end of 14 d in 20 mM Tris-HCl buffer (pH 7.5), proving it to be stable, similar to many other α-CAsCitation28–31. To determine the native molecular weight of the enzyme, SDS-PAGE was carried out.
The molecular weight was determined to be 32 kDa. Similar results have been observed for the enzyme from different sources. For example, human erythrocyte CA is 29 kDa,Citation28–31 teleost fish Dicentrarchus labrax CA is 29 kDaCitation5 and rainbow trout gill CA is 29 kDaCitation28–31. The molecular weight was proved to be 32 kDa by SDS-PAGE ().
α-CAs have some catalytic versatility, acting also as esterases, phosphatasesCitation19,Citation32,Citation33. Thus, we have investigated the esterase activity of the HmCA enzyme with 4-NPA as a substrate. This enzyme has thus comparable esterase activity with the human isoforms hCA I and II, investigated earlierCitation19,Citation32,Citation33.
CA inhibition
It is well-known that metal-complexing anions bind to CAs, representing a rather well investigated class of inhibitorsCitation34. Most anions coordinate directly to the metal ion from the enzyme active site, replacing the coordinated water molecule/hydroxide ion, or add to the coordination sphere, leading to trigonal bipyramidal geometries of the Zn (II) ionCitation1–3,Citation34. In our study, we examined the inhibitory activities of anions on the alpha class CA from Halomonas sp., by assaying the inhibition of the CO2 hydratase activity mentioned above.
Inhibition data of the current paper and previous studies were discussed in . As seen from data of and , all investigated anions show inhibitory activity, but sulfate was the strongest one, with KI value of 5.5 µM, which is similar to that of the (coral Stylophora pistillata CA) STPCACitation35, but quite different from α-isozyme hCA I, II, β-isozyme (Methanobacterium Thermoautotrophicum) Cab CA and γ-isozyme (Methanosarcina thermophila) Zn-Cam CA reported earlierCitation36. Iodide and sulfite ions were also an excellent HmCA inhibitor, with a potency intermediate between that of sulfate on one part, and azide and nitrate on the other one. Azide and nitrate exhibited quite effective inhibition that is similar for STPCA, but rather different than hCA II, Cab CA and Cam CACitation35–37. Interestingly, nitrate showed much more effective inhibition on HmCA compared to human isoforms CA I, II, IV, Cab CA and Cam CACitation19,Citation29–31,Citation32,Citation33. Carbonate was found to be rather weak inhibitor of the HmCA with inhibition value of 121 µM, which are very close to that of hCA IV, whereas they are much lower than those of hCA I, II, Cab CA and Cam CACitation35–37.
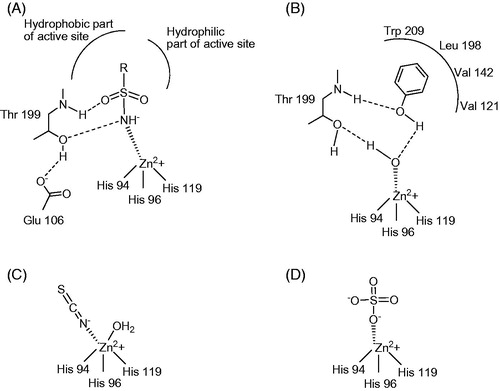
Figure 2. CA inhibition with: (A) zinc binders such as sulfonamide compounds (B) anchoring to the zinc-bound water/hydroxide ion, such as phenol, (C) inorganic anion SCN− and (D) inorganic anion . Figures represent distances (in Å), as determined by X-ray crystallographic techniquesCitation35–37. Hydrogen bonds are represented as dashed lines. All these binding modes have been proven by means of X-ray crystallography on enzyme-inhibitor adductsCitation36.
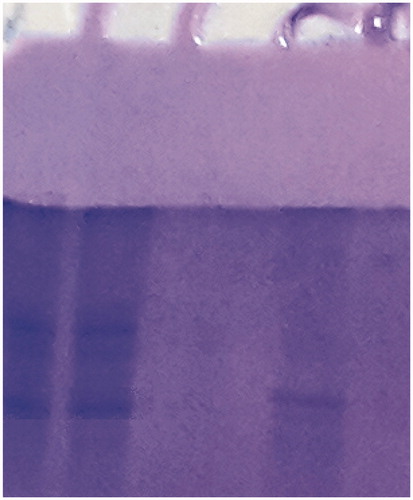
Table 2. Inhibition constants of anionic inhibitors against HmCA, for the CO2 hydration reaction, at 20 °C.
Thiocyanate, bromide and chloride were found to be moderate inhibitors of the HmCA with inhibition values of 27.5–39.0 µM, which were close to that of Pseudomonas gingivalis (PgiCA), whereas they are much lower than those of hCA I, II, VI, STPCA, Zn-Cam and Cab CACitation35–37.
It should be noted the important difference of affinity of these anions for HmCA and human CA enzymes. All tested anions here exhibited competitive inhibition which might indicate that these anions are in competition with the CO2 binding site.
Conclusion
We purified CA from the Halomonas sp. which has not been reported earlier, and analyzed the features of this enzyme. As a strong esterase and hydratase, enzyme activity was determined from raw homogenate obtained from a liquid medium, the colonization to a microorganism (e.g. Escherichia coli) were not tested. The kinetic data for the hydratase activity of this enzyme with CO2 as substrate are in good agreement with others reported in the literature. The inhibitory effects of several anions on the enzyme activity were reported. Our findings indicate that these anions inhibit the HmCA enzyme in a similar manner to other α-CAs from mammals investigated earlier, but the susceptibility to various anions differs between the Halomonas sp. and mammalian CAs. All tested anions exhibited competitive inhibition with the CO2 substrate, which indicates that these anions are in competition with CO2 for binding to the active site.
Soil salinization severely affects the health of the soil (socio-economic wellbeing) and thus the production of foodCitation38. Throughout the world, salinity is an increasing problem in arid and semi-arid lands. Saline soils are estimated to increase at a rate of 7% in the worldCitation39. The increasing salinity threatens the sustainable agriculture and food production of the world. In this respect, the sustainable use of natural sources (arable lands) for the human beings is of utmost importance. As Halomonas is a genus inhabit in saline soil, the investigation of the presence of CA and anions interfering CA physiological function are of vital importance in the maintenance of the food security.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Duda DM, McKenna R. Handbook of metalloproteins. New York: John Wiley & Sons; 2006
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Mahon BP, Díaz-Torres NA, Pinard MA, et al. Activity and anion inhibition studies of the α-carbonic anhydrase from Thiomicrospira crunogena XCL-2 Gammaproteobacterium. Bioorg Med Chem Lett 2015. Available from: http://dx.doi.org/10.1016/j.bmcl.2015.05.001
- Frost SC, McKenna R. Carbonic anhydrase mechanism, regulation, links to disease, and industrial applications. Dordrecht: Springer; 2014
- Ekinci D, Ceyhun SB, Senturk M, et al. Characterization and anions inhibition studies of an α-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–8
- Guney M, Cavdar H, Senturk M, Ekinci D. Synthesis and carbonic anhydrase inhibitory properties of novel uracil derivatives. Bioorg Med Chem Lett 2015;25:3261–3
- Tripp BC, Smith K, Ferry JG. Carbonic anhydrase: new insights for an ancient enzyme. J Biol Chem 2001;276:48615–8
- Sawaya MR, Cannon GC, Heinhorst S, et al. The structure of beta-carbonic anhydrase from the carboxysomal shell reveals a distinct subclass with one active site for the price of two. J Biol Chem 2006;281:7546–55
- Capasso C, Supuran CT. An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32
- Kaye JZ, Marquez MC, Ventosa A, Baross JA. Halomonas neptunia sp. nov., Halomonas sulfidaeris sp. nov., Halomonas axialensis sp. nov. and Halomonas hydrothermalis sp. nov.: halophilic bacteria isolated from deep-sea hydrothermal-vent environments. Int J Syst Evol Microbiol 2004;54:499–511
- Azeredo J, Oliveira R. A new method for precipitating bacterial exopolysaccharides. Biotechnol Tech 1996;10:341–4
- Ventosa A, Nieto JJ. Biotechnological applications and potentialities of halophilic microorganisms. World J Microbiol Biotechnol 1995;11:85–94
- Margesin R, Schinner F. Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 2001;5:73–83
- Sanchez-Porro C, Martín S, Mellado E, Ventosa A. Diversity of moderately halophilic bacteria producing extracellular hydrolytic enzymes. J Appl Microbiol 2003;94:295–300
- Oren A. Industrial and environmental applications of halophilic microorganisms. Environ Technol 2010;31:825–34
- Ventosa A, Queseda E, Rodríguez-Valera F, et al. Numerical taxonomy of moderately halophilic Gram-negative rods. J Gen Microbiol 1982;128:1959–68
- Gerhardt P, Murray RGE, Wood WA, Krieg NR. Methods for general and molecular bacteriology. Washington, DC: American Society for Microbiology; 1994
- Barrow GH, Feltham RKA. Cowan and steel’s manual for identification of medical bacteria. 3rd ed. Cambridge: Cambridge University Press; 1993
- Orhan F, Gulluce M. Isolation and characterization of salt-tolerant bacterial strains in salt-affected soils of Erzurum, Turkey. Geomicrobiol J 2015;32:521–9
- Durdagi S, Senturk M, Ekinci D, et al. Kinetic and docking studies of phenol-based inhibitors of carbonic anhydrase isoforms I, II, IX and XII evidence a new binding mode within the enzyme active site. Bioorg Med Chem 2011;19:1381–9
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–9
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–51
- Laemmli DK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–3
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948;176:147–54
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–66
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 1973;22:3099–108
- Nishimori I, Onishi S, Takeuchi H, Supuran CT. The alpha and beta classes’ carbonic anhydrases from Helicobacter pylori as novel drug targets. Curr Pharm Des 2008;14:622–30
- Ekinci D, Al-Rashida M, Abbas G, et al. Chromone containing sulfonamides as potent carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:744–7
- Ekinci D, Cavdar H, Durdagi S, et al. Structure-activity relationships for the interaction of 5,10-dihydroindeno[1,2-b]indole derivatives with human and bovine carbonic anhydrase isoforms I, II, III, IV and VI. Eur J Med Chem 2012;49:68–73
- Ekinci D, Kurbanoglu NI, Salamci E, et al. Carbonic anhydrase inhibitors: inhibition of human and bovine isoenzymes by benzenesulphonamides, cyclitols and phenolic compounds. J Enzyme Inhib Med Chem 2012;27:845–8
- Ceyhun SB, Sentürk M, Erdogan O, Kufrevioglu OI. In vitro and in vivo effects of some pesticides on carbonic anhydrase enzyme from rainbow trout (Oncorhynchus mykiss) gills. Pestic Biochem Phys 2010;97:177–81
- Kazancioğlu EA, Güney M, Sentürk M, Supuran CT. Simple methanesulfonates are hydrolyzed by the sulfatase carbonic anhydrase activity. J Enzyme Inhib Med Chem 2012;27:880–5
- Cavdar H, Ekinci D, Talaz O, et al. α-Carbonic anhydrases are sulfatases with cyclic diol monosulfate esters. J Enzyme Inhib Med Chem 2012;27:148–54
- Alterio V, Di Fiore A, D’Ambrosio K, et al. X-ray crystallography of carbonic anhydrase inhibitors and its importance in drug design. In: Supuran CT, Winum JY, eds. Drug design of zinc-enzyme inhibitors: functional, structural, and disease applications. Hoboken, NJ: Wiley; 2009:73–138
- Bertucci A, Innocenti A, Zoccola D, et al. Carbonic anhydrase inhibitors: inhibition studies of a coral secretory isoform with inorganic anions. Bioorg Med Chem Lett 2009;19:650–3
- Innocenti A, Zimmerman S, Ferry JG, et al. Carbonic anhydrase inhibitors. Inhibition of the beta-class enzyme from the methanoarchaeon Methanobacterium thermoautotrophicum (Cab) with anions. Bioorg Med Chem Lett 2004;14:4563–7
- De Luca V, Vullo D, Del Prete S, et al. Cloning, characterization and anion inhibition studies of a new γ-carbonic anhydrase from the Antarctic bacterium Pseudoalteromonas haloplanktis. Bioorg Med Chem 2015;23:4405–9
- Rengasamy P. Soil salinity and sodicity. In: Stevens D, Kelly J, McLaughlin M, Unkovich M, ed. Growing crops with reclaimed wastewater. Collingwood: CSIRO Publishing; 2006:125–38
- Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann Bot 2003;91:503–27