Abstract
A novel library based on quinolin-4-ylimidazoline core was designed to incorporate a general quinoline antimicrobial pharmacophore. A synthesis of the well-characterized library of 36 compounds was achieved using the Pd-catalyzed Buchwald–Hartwig-type imidazoline arylation chemistry developed earlier. Compounds were tested for biological activity and were found to possess no antimalarial activity. However, the library delivered two promising antitubercular leads, which are non-cytotoxic and can be further optimized with respect to antimycobacterial potency.
Introduction
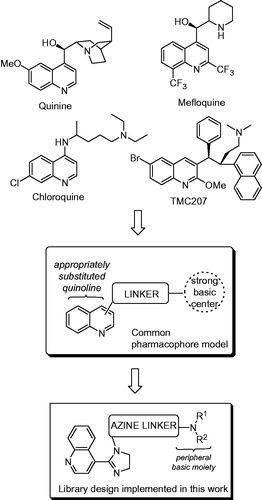
Recently, we developed a convenient method for Pd-catalyzed N-arylation of 2-imidazolinesCitation1. 2-Imidazolines are important, privileged motifs in drug discovery, as demonstrated by numerous biologically active compounds designed around 2-imidazoline coreCitation2. Following the initial success of applying imidazoline arylation chemistry toward the synthesis of known selective cyclooxygenase-2 inhibitorsCitation3, we promptly developed novel imidazoline-based chemical series endowed with anti-inflammatoryCitation4 and kinase-inhibitoryCitation5 activities. Enthused with this initial success positioning N-(hetero)aryl imidazoline moiety as a basis for focused library design, we sought to design a chemical series that would incorporate moieties that would allow developing new leads for anti-infective (in particular, antimalarialCitation6 and antitubercularCitation7) therapies.
One such moiety that found particular prominence in anti-infective drug design is quinoline. While generally considered a privileged motifCitation8, quinolin-4-yl group is omnipresent in quinoline antimalarials. Quinine itself, its synthetic congeners (mefloquine and chloroquine) and even the quinoline-pyrimidine hybrids recently disclosed by Kumar et al.Citation9 – all appear to align with the same pharmacophoreCitation10. Moreover, recent developments in quinoline-based antitubercular agents, such as TMC207 or mefloquine (which is just one example of the significant cross-talk between antitubercular and antimalarial pharmacology)Citation11, emphasize the importance of this class of compounds as a new-generation therapy for multidrug-resistant tuberculosis (TB)Citation12. Considering the special position of quinoline in anti-infective drug design, we set off to design and synthesize a library of N-heteroaryl 2-(quinolin-4-yl)imidazolines that would position these compounds well within the above-mentioned pharmacophore model ()Citation13. Herein, we present the details of the chemical synthesis and the results obtained while evaluating the antimalarial and antitubercular potential of this library.
Materials and methods
Chemical syntheses – general
All reagents were used as received from the suppliers. Column chromatography was performed using silica gel 60 (particle size 0.040–0.063 mm, 230–400 mesh ASTM). Analytical thin-layer chromatography was performed with silica gel 60 F254 aluminum sheets. 1H NMR was acquired at 500 MHz, and 13C NMR at 125 MHz at 30 °C. 1H and 13C NMR were acquired in CDCl3, chemical shifts (δ) are reported in ppm relative to the solvent residual peak: proton (δ 7.26 ppm) and carbon (δ 77.0 ppm) signals, respectively. Melting points are uncorrected. High- and low-resolution electrospray ionization mass spectra were acquired using electrospray as the ionization technique in positive ion and/or negative ion modes as stated. All MS analysis samples were prepared as solutions in MeOH.
4-(4,5-Dihydro-1H-imidazol-2-yl)quinoline (1)
Compound was obtained according to the literature methodCitation14. Yield 78%, m.p.: 98–100 °C. ES-MS m/z 198 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.83 (d, J = 4.35 Hz, 1H), 8.59 (d, J = 8.4 Hz, 1H), 8.07 (d, J = 8.45 Hz, 1H), 7.70 (t, J = 8.0 Hz, 1H), 7.54 (t, J = 8.0 Hz, 1H), 7.48 (d, J = 4.35 Hz, 1H), 3.81 (s, 4H); 13C NMR (125 MHz, CDCl3): δC 163.1, 149.8, 148.8, 137.1, 129.8, 129.7, 127.4, 126.1, 125.5, 120.1, 50.6, 50.5.
General procedure for the preparation of compounds 2a–e
A thick-glass screw-capped pressure tube (50 mL) was charged with a suspension of 4-(4,5-dihydro-1H-imidazol-2-yl)quinoline (1, 5.0 mmol), appropriate dihalo azine (5.0 mmol), Cs2CO3 (5.0 mmol), and toluene (25 mL). Pd(OAc)2 (0.20 mmol) and BINAP (0.40 mmol) were weighed as solids into a 15 mL screw-capped vial and toluene (10 mL) was added. The resulting suspension was heated at 110 °C until a clear purple solution was obtained. The latter was rapidly transferred, while still hot, to the screw-capped pressure tube using a Pasteur pipette. The reaction vessel was purged with argon and the reaction mixture was vigorously stirred at 100 °C for 16–20 h. It was cooled to room temperature and filtered through a Celite pad. The latter was washed with ethyl acetate, and the combined filtrate and washings were evaporated to dryness. The crude material was purified on silica (CH2Cl2:MeOH, 95:5) to provide the title N-heteroarylation product.
4-[1-(6-Bromopyridin-2-yl)-4,5-dihydro-1H-imidazol-2-yl]quinoline (2a)
Yield 73%, m.p.: 162–164 °C. ES-MS m/z 354 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.95 (d, J = 4.3 Hz, 1H), 8.13 (d, J = 8.45 Hz, 1H), 7.85 (d, J = 8.3 Hz, 1H), 7.70 (t, J = 7.2 Hz, 1H), 7.47 (t, J = 7.15 Hz, 1H), 7.42 (d, J = 4.3 Hz, 1H), 6.99 (t, J = 7.95 Hz, 1H), 6.79 (d, J = 7.6 Hz, 1H), 6.01 (d, J = 8.25 Hz, 1H), 4.30–4.26 (m, 2H), 4.24–4.20 (m, 2H); 13C NMR (125 MHz, CDCl3): δC 157.3, 152.0, 150.0, 148.5, 139.7, 139.2, 139.1, 130.1, 129.9, 127.6, 125.7, 125.0, 120.7, 120.6, 109.3, 53.8, 49.6.
4-[1-(2-Chloropyrimidin-4-yl)-4,5-dihydro-1H-imidazol-2-yl]quinoline (2b)
Yield 68%, m.p.: 146–148 °C. ES-MS m/z 311 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 9.01 (d, J = 4.3 Hz, 1H), 8.18 (d, J = 8.4 Hz, 1H), 7.81 (d, J = 6.0 Hz, 1H), 7.77 (d, J = 7.85 Hz, 2H), 7.53 (t, J = 7.75 Hz, 1H), 7.45 (d, J = 4.3 Hz, 1H), 5.75 (d, J = 5.95 Hz, 1H), 4.36–4.32 (m, 2H), 4.29–4.25 (m, 2H); 13C NMR (125 MHz, CDCl3): δC 160.5, 158.2, 158.1, 155.7, 150.1, 148.5, 138.0, 130.4 (2 carbons), 128.2, 125.3, 124.4, 120.7, 105.1, 54.0, 48.8.
4-[1-(6-Chloropyrazin-2-yl)-4,5-dihydro-1H-imidazol-2-yl]quinoline (2c)
Yield 81%, m.p.: 138–140 °C. ES-MS m/z 311 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.98 (d, J = 4.25 Hz, 1H), 8.17 (d, J = 8.45 Hz, 1H), 7.90 (s, 1H), 7.82 (d, J = 8.4 Hz, 1H), 7.73 (t, J = 7.9 Hz, 1H), 7.51 (t, J = 7.75 Hz, 1H), 7.45 (d, J = 4.3 Hz, 1H), 7.37 (s, 1H), 4.32–4.29 (m, 4H); 13C NMR (125 MHz, CDCl3): δC 156.3, 150.1, 148.6, 147.9, 146.4, 138.1, 135.6, 130.8, 130.4, 130.2, 128.0, 125.4, 124.5, 120.7, 54.1, 49.2.
4-{1-[2-Chloro-5-(trifluoromethyl)pyrimidin-4-yl]-4,5-dihydro-1H-imidazol-2-yl}quinoline (2d)
Yield 56%, waxy solid. ES-MS m/z 378 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.95 (d, J = 4.35 Hz, 1H), 8.18–8.15 (m, 2H), 7.74–7.68 (m, 2H), 7.47 (t, J = 7.25 Hz, 1H), 7.40 (d, J = 4.35 Hz, 1H), 4.38–4.34 (m, 2H), 4.31–4.28 (m, 2H); 13C NMR (125 MHz, CDCl3): δC 159.0, 157.3, 156.8 (q, JC–F = 5.0 Hz), 156.3, 149.8, 148.1, 139.8, 130.1, 129.6, 127.4, 126.1, 124.5, 122.2 (q, JC–F = 270.0 Hz), 120.2, 115.1 (q, JC–F = 33.75 Hz), 54.3, 48.4.
4-[1-(6-Chloropyrimidin-4-yl)-4,5-dihydro-1H-imidazol-2-yl]quinoline (2e)
Yield 79%, m.p.: 138–140 °C. ES-MS m/z 311 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.99 (d, J = 4.4 Hz, 1H), 8.23 (d, J = 8.55 Hz, 1H), 8.14 (s, 1H), 7.80 (d, J = 8.35 Hz, 1H), 7.76 (t, J = 7.3 Hz, 1H), 7.54 (t, J = 7.25 Hz, 1H), 7.46 (d, J = 4.35 Hz, 1H), 6.15 (s, 1H), 4.34–4.30 (m, 2H), 4.28–4.24 (m, 2H); 13C NMR (125 MHz, CDCl3): δC 160.6, 157.8, 157.7, 156.1, 150.0, 148.4, 138.6, 130.3, 130.1, 127.8, 125.4, 124.5, 120.5, 105.9, 54.1, 48.7.
General procedure for the preparation of compounds 3–38
A sealable microwave vessel containing a stirring bar was charged with 2 (1 mmol), amine or 2-imidazolines (1.1 mmol), anhydrous Cs2CO3 (1 mmol) and toluene (2.0 mL). The mixture was set on extensive stirring at r.t., while a catalyst solution was prepared by mixing Pd(OAc)2 (0.02 mol) and BINAP (0.04 mol) in toluene (0.5 mL) at 100 °C, until a deep-red clear solution was obtained. The latter was added via a pipette into the reaction mixture, the vial was sealed and irradiated in Biotage Initiator™ microwave reactor at 90 °C for 45 min (for 2-imidazoline coupling partners) or 30 min (for primary and secondary amines). The contents of the vial were filtered through a plug of Celite (with ample ethyl acetate washing). Chromatography on silica gel using an appropriate gradient of MeOH in dichloromethane as eluent gave analytically pure title compounds.
(±)-4-[1-(6-{Octahydropyrrolo[1,2-a]piperazin-2-yl}pyridin-2-yl)-4,5-dihydro-1H-imidazol-2-yl]quinoline (3)
Waxy solid. ES-MS m/z 399 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.87 (d, J = 4.4 Hz, 1H), 8.07 (d, J = 8.4 Hz, 1H), 7.95 (d, J = 8.35 Hz, 1H), 7.64 (t, J = 7.75 Hz, 1H), 7.45–7.41 (m, 2H), 7.27 (t, J = 8.0 Hz, 1H), 5.99 (d, J = 8.35 Hz, 1H), 5.90 (d, J = 7.8 Hz, 1H), 4.23–4.19 (m, 2H), 4.16–4.11 (m, 2H), 3.00 (d, J = 11.75 Hz, 1H), 2.94 (t, J = 8.85 Hz, 1H), 2.85 (d, J = 12.5 Hz, 1H), 2.65 (d, J = 10.9 Hz, 1H), 2.20–2.15 (m, 1H), 1.94 (q, J = 8.65 Hz, 1H), 1.78–1.67 (m, 3H), 1.66–1.59 (m, 2H), 1.52–1.48 (m, 1H), 1.20–1.13 (m, 1H); 13C NMR (125 MHz, CDCl3): δC 158.6, 157.5, 150.9, 149.8, 148.3, 141.4, 139.3, 129.7, 129.5, 127.1, 126.2, 125.6, 120.0, 99.9, 98.6, 62.0, 54.0, 53.5, 51.1, 49.6, 48.7, 43.5, 27.4, 21.3.
N-[2-(Pyrrolidin-1-yl)ethyl]-6-[2-(quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyridin-2-amine (4)
Gray solid, m.p.: 132–134 °C. ES-MS m/z 387 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.87 (d, J = 4.35 Hz, 1H), 8.07 (d, J = 8.5 Hz, 1H), 7.97 (d, J = 8.35 Hz, 1H), 7.65 (t, J = 7.85 Hz, 1H), 7.46 (t, J = 8.05 Hz, 1H), 7.41 (d, J = 4.35 Hz, 1H), 7.11 (d, J = 7.9 Hz, 1H), 5.80 (d, J = 8.1 Hz, 1H), 5.74 (d, J = 7.75 Hz, 1H), 4.55–4.54 (m, 1H), 4.23–4.19 (m, 2H), 4.17–4.11 (m, 2H), 2.31–2.30 (m, 4H), 2.11–2.09 (m, 2H), 2.03–1.97 (m, 2H), 1.71–1.69 (m, 4H); 13C NMR (125 MHz, CDCl3): δC 158.8, 157.0, 151.3, 149.9, 148.3, 141.9, 138.6, 129.54, 129.5, 127.1, 126.5, 125.9, 120.2, 102.0, 97.9, 68.4, 54.7, 54.0, 53.8, 49.7, 38.5, 23.5, 21.5.
N-(Pyridin-4-ylmethyl)-6-[2-(quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyridin-2-amine (5)
Gray solid, m.p.: 102–104 °C. ES-MS m/z 381 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.77 (d, J = 4.4 Hz, 1H), 8.34 (d, J = 5.6 Hz, 2H), 7.96 (d, J = 8.45 Hz, 1H), 7.87 (d, J = 8.35 Hz, 1H), 7.56 (t, J = 7.8 Hz, 1H), 7.38 (t, J = 7.85 Hz, 1H), 7.32 (d, J = 4.3 Hz, 1H), 7.14 (t, J = 7.9 Hz, 1H), 6.74 (d, J = 5.3 Hz, 2H), 5.80 (dd, J = 15.4, 7.85 Hz, 2H), 4.37 (t, J = 5.95 Hz, 1H), 4.19–4.15 (m, 2H), 4.12–4.08 (m, 2H), 3.24 (d, J = 6.05 Hz, 2H); 13C NMR (125 MHz, CDCl3): δC 158.4, 156.5, 151.2, 149.8, 149.5 (2 carbons), 148.9, 148.1, 141.4, 139.0, 129.6, 129.5, 127.1, 126.1, 125.5, 121.8 (2 carbons), 120.1, 101.2, 98.9, 53.9, 49.5, 43.2.
4-{1-[6-(4-Ethylpiperazin-1-yl)pyridin-2-yl]-4,5-dihydro-1H-imidazol-2-yl}quinoline (6)
Waxy solid. ES-MS m/z 387 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.87 (d, J = 4.35 Hz, 1H), 8.07 (d, J = 8.45 Hz, 1H), 7.94 (d, J = 8.3 Hz, 1H), 7.65 (t, J = 7.4 Hz, 1H), 7.44 (t, J = 7.45 Hz, 1H), 7.40 (d, J = 4.35 Hz, 1H), 7.25 (t, J = 8.05 Hz, 1H), 5.96 (d, J = 8.35 Hz, 1H), 5.88 (d, J = 9.65 Hz, 1H), 4.23–4.19 (m, 2H), 4.15–4.11 (m, 2H), 2.46 (t, J = 4.95 Hz, 4H), 2.25 (q, J = 7.25 Hz, 2H), 1.98 (t, J = 5.15 Hz, 4H), 0.99 (t, J = 7.25 Hz, 3H); 13C NMR (125 MHz, CDCl3): δC 158.6, 157.4, 150.9, 149.8, 148.3, 141.6, 139.3, 129.6, 129.5, 127.1, 126.2, 125.6, 120.0, 99.8, 98.6, 54.0, 52.3, 52.2 (2 carbons), 49.5, 44.0 (2 carbons), 11.9.
N-Benzyl-6-[2-(quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyridin-2-amine (7)
Viscous oil. ES-MS m/z 380 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.83 (d, J = 4.3 Hz, 1H), 8.02 (d, J = 8.45 Hz, 1H), 7.94 (d, J = 8.4 Hz, 1H), 7.61 (t, J = 7.15 Hz, 1H), 7.43 (t, J = 7.7 Hz, 1H), 7.40 (d, J = 4.3 Hz, 1H), 7.24–7.17 (m, 3H), 7.10 (t, J = 8.0 Hz, 1H), 6.92 (d, J = 7.05 Hz, 2H), 5.74 (d, J = 7.9 Hz, 2H), 4.22–4.13 (m, 5H), 3.32 (d, J = 5.6 Hz, 2H); 13C NMR (125 MHz, CDCl3): δC 158.6, 156.9, 151.3, 149.9, 148.2, 141.3, 139.2, 138.8, 129.7, 129.4, 128.4 (2 carbons), 127.2 (2 carbons), 127.01, 127.0, 126.2, 125.6, 120.2, 101.0, 98.7, 53.9, 49.6, 44.8.
N-(2-Methylpropyl)-6-[2-(quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyridin-2-amine (8)
Clear oil. ES-MS m/z 346 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.87 (d, J = 4.4 Hz, 1H), 8.07 (d, J = 8.5 Hz, 1H), 7.96 (d, J = 8.35 Hz, 1H), 7.64 (t, J = 7.65 Hz, 1H), 7.44 (t, J = 7.4 Hz, 1H), 7.40 (d, J = 4.35 Hz, 1H), 7.08 (t, J = 7.95 Hz, 1H), 5.71 (dd, J = 15.1, 8.1 Hz, 2H), 4.21–4.17 (m, 2H), 4.16–4.12 (m, 2H), 3.76 (t, J = 4.9 Hz, 1H), 2.04 (t, J = 6.1 Hz, 2H), 1.41–1.33 (m, 1H), 0.63 (d, J = Hz, 6H); 13C NMR (125 MHz, CDCl3): δC 158.7, 157.4, 151.3, 149.8, 148.2, 141.3, 138.6, 129.7, 129.4, 127.0, 126.3, 125.7, 120.2, 100.6, 98.2, 53.9, 49.6, 48.4, 28.1, 20.1 (2 carbons).
(±)-N-{6-[2-(Quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyridin-2-yl}-1-azabicyclo[2.2.2]octan-3-amine (9)
Waxy solid. ES-MS m/z 399 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.89 (d, J = 4.35 Hz, 1H), 8.10 (d, J = 8.45 Hz, 1H), 8.03 (d, J = 8.35 Hz, 1H), 7.68 (t, J = 7.6 Hz, 1H), 7.48 (t, J = 7.65 Hz, 1H), 7.39 (d, J = 4.35 Hz, 1H), 7.10 (d, J = 7.95 Hz, 1H), 5.74 (dd, J = 15.8, 8.1 Hz, 2H), 4.19–4.17 (m, 4H), 4.02 (d, J = 5.9 Hz, 1H), 5.69–5.63 (m, 5H), 2.51–5.45 (m, 1H), 2.29 (s, 1H), 2.12–2.07 (m, 1H), 1.47–1.43 (m, 2H), 1.27–1.22 (m, 1H), 1.18–1.13 (m, 1H); 13C NMR (125 MHz, CDCl3): δC 158.5, 156.6, 151.5, 149.9, 148.4, 140.8, 138.7, 129.7, 129.6, 127.2, 126.2, 125.9, 120.5, 101.1, 99.2, 57.0, 53.9, 49.9, 47.5, 47.3, 46.5, 25.8, 25.3, 20.0.
4-{1-[6-(Morpholin-4-yl)pyridin-2-yl]-4,5-dihydro-1H-imidazol-2-yl}quinoline (10)
Pale solid, m.p.: 98–100 °C. ES-MS m/z 360 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.87 (d, J = 4.35 Hz, 1H), 8.08 (d, J = 8.5 Hz, 1H), 7.95 (d, J = 8.35 Hz, 1H), 7.67 (t, J = 7.25 Hz, 1H), 7.46 (t, J = 7.75 Hz, 1H), 7.40 (d, J = 4.3 Hz, 1H), 7.29 (t, J = 8.15 Hz, 1H), 5.95 (dd, J = 13.35, 8.3 Hz, 2H), 4.25–4.20 (m, 2H), 4.18–4.13 (m, 2H), 3.27 (t, J = 4.85 Hz, 4H), 2.39 (t, J = 4.75 Hz, 4H); 13C NMR (125 MHz, CDCl3): δC 158.5, 157.5, 151.0, 149.9, 148.4, 141.6, 139.4, 129.6 (2 carbons), 127.2, 126.3, 125.6, 120.0, 99.6, 99.2, 66.3 (2 carbons), 54.1, 49.6, 44.4 (2 carbons).
4-(1-{6-[2-(Pyridin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyridin-2-yl}-4,5-dihydro-1H-imidazol-2-yl)quinoline (11)
Gray solid, m.p.: 164–166 °C. ES-MS m/z 420 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.90 (d, J = 4.35 Hz, 1H), 8.56 (d, J = 5.7 Hz, 2H), 8.08 (d, J = 8.45 Hz, 1H), 7.81 (d, J = 8.4 Hz, 1H), 7.66 (t, J = 8.1 Hz, 1H), 7.45 (t, J = 8.1 Hz, 1H), 7.38 (d, J = 4.35 Hz, 1H), 7.21 (d, J = 5.7 Hz, 2H), 6.98 (t, J = 8.2 Hz, 1H), 5.73 (dd, J = 14.1, 9.2 Hz, 2H), 4.06 (t, J = 9.6 Hz, 2H), 3.74 (quint, 9.55 Hz, 4H), 3.12 (t, J = 9.6 Hz, 2H); 13C NMR (125 MHz, CDCl3): δC 158.9, 157.6, 151.6, 150.7, 150.0, 149.8 (2 carbons), 148.3, 140.4, 139.9, 138.7, 129.8, 129.79, 127.3, 125.8, 125.2, 122.5 (2 carbons), 120.4, 105.8, 104.2, 53.6, 53.2, 49.9, 48.9.
4-(1-{6-[2-(Pyridin-3-yl)-4,5-dihydro-1H-imidazol-1-yl]pyridin-2-yl}-4,5-dihydro-1H-imidazol-2-yl)quinoline (12)
Beige solid, m.p.: 147–149 °C. ES-MS m/z 420 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.91 (d, J = 4.35 Hz, 1H), 8.58 (d, J = 4.8 Hz, 1H), 8.52–8.51 (m, 1H), 8.10 (d, J = 8.5 Hz, 1H), 7.82 (d, J = 8.4 Hz, 1H), 7.71–7.66 (m, 2H), 7.48 (t, J = 7.95 Hz, 1H), 7.40 (d, J = 4.35 Hz, 1H), 7.28–7.25 (m, 1H), 6.99 (t, J = 8.2 Hz, 1H), 5.75 (t, J = 8.45 Hz, 2H), 4.08 (t, J = 9.5 Hz, 2H), 3.76 (t, J = 9.6 Hz, 4H), 3.14 (t, J = 9.55 Hz, 2H); 13C NMR (125 MHz, CDCl3): δC 158.4, 157.7, 151.7, 150.8, 150.6, 150.0, 149.3, 148.4, 140.0, 138.7, 135.7, 129.9 (2 carbons), 128.6, 127.4, 125.9, 125.2, 123.1, 120.5, 106.0, 104.2, 53.6, 52.9, 50.0, 49.1.
(±)-4-[1-(2-{Octahydropyrrolo[1,2-a]piperazin-2-yl}pyrimidin-4-yl)-4,5-dihydro-1H-imidazol-2-yl]quinoline (13)
Waxy solid. ES-MS m/z 400 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.90 (d, J = 4.35 Hz, 1H), 8.08 (d, J = 8.45 Hz, 1H), 7.96 (d, J = 5.55 Hz, 1H), 7.82 (d, J = 8.35 Hz, 1H), 7.66 (t, J = 8.2 Hz, 1H), 7.46 (t, J = 7.95 Hz, 1H), 7.39 (d, J = 4.35 Hz, 1H), 5.72 (d, J = 5.65 Hz, 1H), 4.25–4.21 (m, 2H), 4.13–4.09 (m, 2H), 2.93 (t, J = 8.4 Hz, 1H), 2.64 (bs, 1H), 2.21 (s, 1H), 1.92 (q, J = 8.7 Hz, 1H), 1.75–1.57 (m, 4H), 1.40 (bs, 1H), 1.17–1.15 (m, 1H); 13C NMR (125 MHz, CDCl3): δC 160.4, 158.3, 157.5, 157.2, 149.8, 148.2, 141.0, 129.9, 129.7, 127.4, 126.0, 125.2, 119.9, 95.4, 62.1, 54.1 (2 carbons), 53.5, 51.2, 48.2, 27.2, 21.2 (2 carbons).
4-{1-[2-(4-Ethylpiperazin-1-yl)pyrimidin-4-yl]-4,5-dihydro-1H-imidazol-2-yl}quinoline (14)
Yellowish solid, m.p.: 113–115 °C. ES-MS m/z 388 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.91 (d, J = 4.3 Hz, 1H), 8.09 (d, J = 8.5 Hz, 1H), 7.95 (d, J = 5.75 Hz, 1H), 7.81 (d, J = 8.35 Hz, 1H), 7.68 (t, J = 8.15 Hz, 1H), 7.47 (t, J = 7.85 Hz, 1H), 7.40 (d, J = 4.35 Hz, 1H), 5.71 (d, J = 5.7 Hz, 1H), 4.33–4.10 (m, 5H), 2.27–2.22 (m, 4H), 1.95 (bs, 4H), 1.01–0.96 (m, 4H); 13C NMR (125 MHz, CDCl3): δC 160.3, 158.4, 157.5, 157.2, 149.9, 148.2, 141.1, 129.8, 129.7, 127.4, 126.0, 125.2, 119.9, 95.4, 54.1 (2 carbons), 52.3, 52.2 (2 carbons), 48.2, 42.8, 11.9.
1-{4-[2-(Quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyrimidin-2-yl}piperidin-4-ol (15)
Glassy solid, m.p.: 88–90 °C. ES-MS m/z 375 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.89 (d, J = 4.4 Hz, 1H), 8.09 (d, J = 8.5 Hz, 1H), 7.96 (d, J = 5.7 Hz, 1H), 7.84 (d, J = 8.2 Hz, 1H), 7.68 (t, J = 8.35 Hz, 1H), 7.48 (t, J = 8.1 Hz, 1H), 7.39 (d, J = 4.4 Hz, 1H), 5.71 (d, J = 5.7 Hz, 1H), 4.26–4.22 (m, 2H), 4.15–4.11 (m, 2H), 3.57–3.52 (m, 1H), 2.37 (bs, 3H), 2.19 (s, 2H), 1.37–1.36 (m, 2H), 0.90 (bs, 2H); 13C NMR (125 MHz, CDCl3): δC 160.2, 158.4, 157.6, 157.3, 149.8, 148.2, 141.2, 129.8, 129.7, 127.4, 126.0, 125.2, 119.9, 95.2, 67.6, 54.1 (2 carbons), 48.2, 40.6, 33.8 (2 carbons).
(1-{4-[2-(Quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyrimidin-2-yl}piperidin-4-yl)methanol (16)
White solid, m.p.: 172–174 °C. ES-MS m/z 389 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.87 (d, J = 4.35 Hz, 1H), 8.08 (d, J = 8.55 Hz, 1H), 7.95 (d, J = 5.5 Hz, 1H), 7.83 (d, J = 8.35 Hz, 1H), 7.67 (t, J = 8.0 Hz, 1H), 7.47 (t, J = 7.8 Hz, 1H), 7.39 (d, J = 4.4 Hz, 1H), 5.69 (d, J = 5.6 Hz, 1H), 4.25–4.21 (m, 2H), 4.13–4.10 (m, 2H), 3.39 (s, 2H), 3.25 (d, J = 6.2 Hz, 2H), 2.41 (bs, 1H), 2.08 (bs, 2H), 1.40–1.34 (m, 1H), 1.30–1.28 (m, 2H), 0.52 (bs, 2H); 13C NMR (125 MHz, CDCl3): δC 160.1, 158.4, 157.6, 157.2, 149.7, 148.1, 141.2, 129.8, 129.6, 127.4, 126.0, 125.2, 119.9, 95.0, 67.3, 54.1, 50.5, 48.2, 42.9, 38.9 (2 carbons), 28.2.
2-(1-{4-[2-(Quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyrimidin-2-yl}piperidin-4-yl)ethan-1-ol (17)
Off-white solid, m.p.: 155–157 °C. ES-MS m/z 403 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.88 (d, J = 4.35 Hz, 1H), 8.07 (d, J = 8.45 Hz, 1H), 7.93 (d, J = 5.55 Hz, 1H), 7.81 (d, J = 8.3 Hz, 1H), 7.66 (t, J = 8.25 Hz, 1H), 7.46 (t, J = 7.85 Hz, 1H), 7.39 (d, J = 4.35 Hz, 1H), 5.67 (d, J = 5.65 Hz, 1H), 4.24–4.20 (m, 2H), 4.12–4.08 (m, 2H), 3.52 (t, J = 6.35 Hz, 2H), 2.51 (bs, 2H), 2.07 (bs, 2H), 1.30–1.23 (m, 6H), 0.50 (bs, 2H); 13C NMR (125 MHz, CDCl3): δC 160.2, 158.3, 157.6, 157.2, 149.8, 148.1, 141.2, 129.7, 129.6, 127.4, 126.0, 125.2, 119.8, 94.9, 59.9 (2 carbons), 54.0, 48.2 (2 carbons), 43.2, 39.3, 32.6, 31.7.
4-{1-[6-(Morpholin-4-yl)pyrazin-2-yl]-4,5-dihydro-1H-imidazol-2-yl}quinoline (18)
White solid, m.p.: 132–134 °C. ES-MS m/z 361 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.89 (d, J = 4.35 Hz, 1H), 8.09 (d, J = 8.5 Hz, 1H), 7.88 (d, J = 8.25 Hz, 1H), 7.69 (t, J = 7.25 Hz, 1H), 7.49–7.47 (m, 2H), 7.39 (d, J = 4.3 Hz, 1H), 7.36 (s, 1H), 4.29 (t, J = 9.05 Hz, 2H), 4.18 (t, J = 10.55 Hz, 2H), 3.27 (t, J = 4.9 Hz, 4H), 2.43 (t, J = 4.8 Hz, 4H); 13C NMR (125 MHz, CDCl3): δC 157.9, 152.2, 149.9, 148.3, 146.3, 141.0, 129.8, 127.4, 126.1, 125.4, 123.0, 120.0, 119.8, 110.1, 66.1 (2 carbons), 54.6, 48.7, 43.8 (2 carbons).
(±)-4-[1-(6-{Octahydropyrrolo[1,2-a]piperazin-2-yl}pyrazin-2-yl)-4,5-dihydro-1H-imidazol-2-yl]quinoline (19)
White flakes, m.p.: 112–114 °C. ES-MS m/z 400 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.88 (d, J = 4.35 Hz, 1H), 8.08 (d, J = 8.5 Hz, 1H), 7.88 (d, J = 8.4 Hz, 1H), 7.66 (t, J = 7.55 Hz, 1H), 7.50 (s, 1H), 7.45 (t, J = 7.5 Hz, 1H), 7.39 (d, J = 4.35 Hz, 1H), 7.32 (s, 1H), 4.29–4.25 (m, 2H), 4.19–4.15 (m, 2H), 2.96–2.93 (m, 2H), 2.86 (d, J = 12.5 Hz, 1H), 2.67 (d, J = 10.85 Hz, 1H), 2.25 (t, J = 10.25 Hz, 1H), 2.13 (s, 1H), 1.95 (q, J = 8.7 Hz, 1H), 1.80 (t, J = 11.6 Hz, 1H), 1.75–1.61 (m, 3H), 1.48–1.43 (m, 1H), 1.20–1.14 (m, 1H); 13C NMR (125 MHz, CDCl3): δC 158.1, 152.3, 149.8, 148.3, 146.3, 141.0, 129.9, 129.7, 127.3, 126.1, 125.3, 123.4, 120.0, 119.1, 61.8, 54.5, 53.5, 50.9, 48.8, 48.1, 43.0, 27.4, 21.2.
4-{1-[6-(4-Ethylpiperazin-1-yl)pyrazin-2-yl]-4,5-dihydro-1H-imidazol-2-yl}quinoline (20)
Gray solid, m.p.: 144–146 °C. ES-MS m/z 388 [M + H]+.1H NMR (500 MHz, CDCl3): δH 8.87 (d, J = 4.35 Hz, 1H), 8.07 (d, J = 8.45 Hz, 1H), 7.85 (d, J = 8.4 Hz, 1H), 7.65 (t, J = 7.35 Hz, 1H), 7.45–7.42 (m, 2H), 7.38 (d, J = 4.3 Hz, 1H), 7.29 (s, 1H), 4.25 (t, J = 9.15 Hz, 2H), 4.14 (t, J = 9.15 Hz, 2H), 2.48 (t, J = 4.8 Hz, 4H), 2.23 (q, J = 7.2 Hz, 2H), 1.96 (t, J = 4.85 Hz, 4H), 0.97 (t, J = 7.15 Hz, 3H); 13C NMR (125 MHz, CDCl3): δC 158.0, 152.1, 149.8, 148.2, 146.2, 141.0, 129.7, 129.66, 127.3, 126.1, 125.3, 123.2, 119.9, 119.0, 54.5, 52.2, 51.9 (2 carbons), 48.6, 43.4 (2 carbons), 11.8.
N,N-Dimethyl-6-[2-(quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyrazin-2-amine (21)
White solid, m.p.: 156–158 °C. ES-MS m/z 319 [M + H]+.1H NMR (500 MHz, CDCl3): δH 8.90 (d, J = 4.3 Hz, 1H), 8.09 (d, J = 8.5 Hz, 1H), 7.91 (d, J = 8.4 Hz, 1H), 7.68 (t, J = 7.9 Hz, 1H), 7.47 (t, J = 7.25 Hz, 1H), 7.42 (t, J = 4.25 Hz, 1H), 7.39 (s, 1H), 7.26 (s, 1H), 4.31–4.27 (m, 2H), 4.22–4.18 (m, 2H), 2.21 (s, 6H); 13C NMR (125 MHz, CDCl3): δC 158.3, 152.6, 150.0, 148.4, 146.5, 141.1, 129.8, 129.7, 127.3, 126.3, 125.4, 122.8, 120.1, 118.2, 54.5, 48.9, 36.7 (2 carbons).
4-{1-[6-(4-Phenylpiperazin-1-yl)pyrazin-2-yl]-4,5-dihydro-1H-imidazol-2-yl}quinoline (22)
Beige solid, m.p.: 178–180 °C (decomp.). ES-MS m/z 436 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.94 (d, J = 4.3 Hz, 1H), 8.14 (d, J = 8.5 Hz, 1H), 7.90 (d, J = 8.3 Hz, 1H), 7.70 (t, J = 7.5 Hz, 1H), 7.54 (s, 1H), 7.49 (t, J = 7.35 Hz, 1H), 7.43 (d, J = 4.3 Hz, 1H), 7.36 (s, 1H), 7.27–7.24 (m, 2H), 6.87 (t, J = 7.3 Hz, 1H), 6.80 (d, J = 8.1 Hz, 2H), 4.32–4.28 (m, 2H), 4.21–4.17 (m, 2H), 2.74–2.72 (m, 4H), 2.65–2.63 (m, 4H); 13C NMR (125 MHz, CDCl3): δC 157.9, 152.0, 150.9, 150.0, 148.3, 146.3, 141.2, 129.8 (2 carbons), 129.2 (2 carbons), 127.4, 126.2, 125.4, 123.2, 120.3, 120.0, 119.4, 116.4 (2 carbons), 54.6, 48.7, 48.5 (2 carbons), 43.5 (2 carbons).
4-{1-[6-(Piperidin-1-yl)pyrazin-2-yl]-4,5-dihydro-1H-imidazol-2-yl}quinoline (23)
Beige solid, m.p.: 136–138 °C (decomp.). ES-MS m/z 359 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.90 (d, J = 4.35 Hz, 1H), 8.09 (d, J = 8.5 Hz, 1H), 7.91 (d, J = 8.4 Hz, 1H), 7.68 (t, J = 7.2 Hz, 1H), 7.48–7.46 (m, 2H), 7.40 (d, J = 4.35 Hz, 1H), 7.26 (s, 1H), 4.30–4.26 (m, 2H), 4.20–4.16 (m, 2H), 2.51 (t, J = 5.5 Hz, 4H), 1.37–1.32 (m, 2H), 1.09–1.04 (m, 4H); 13C NMR (125 MHz, CDCl3): δC 158.2, 152.2, 149.9, 148.4, 146.5, 141.1, 129.8, 129.6, 127.3, 126.1, 125.4, 123.4, 120.0, 118.3, 54.5, 48.8, 44.7 (2 carbons), 25.1 (2 carbons), 24.3.
N-Benzyl-N-ethyl-6-[2-(quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyrazin-2-amine (24)
Oil, ES-MS m/z 409 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.80 (d, J = 4.35 Hz, 1H), 8.00 (d, J = 8.45 Hz, 1H), 7.89 (d, J = 8.3 Hz, 1H), 7.61 (t, J = 7.25 Hz, 1H), 7.43 (t, J = 7.15 Hz, 1H), 7.38 (d, J = 4.35 Hz, 1H), 7.36 (s, 1H), 7.25 (s, 1H), 7.19–7.18 (m, 3H), 6.80–6.79 (m, 2H), 4.29–4.25 (m, 2H), 4.22–4.18 (m, 2H), 3.83 (s, 2H), 2.68 (q, J = 7.05 Hz, 2H), 0.62 (t, J = 7.05 Hz, 3H); 13C NMR (125 MHz, CDCl3): δC 158.2, 151.7, 149.9, 148.3, 146.7, 140.7, 137.6, 129.9, 129.6, 128.5 (2 carbons), 127.3, 127.1, 126.7 (2 carbons), 125.9, 125.2, 122.8, 120.1, 118.7, 54.4, 49.9, 49.1, 41.9, 12.0.
4-{1-[2-(4-Ethylpiperazin-1-yl)-5-(trifluoromethyl)pyrimidin-4-yl]-4,5-dihydro-1H-imidazol-2-yl}quinoline (25)
Beige solid, m.p.: 143–145 °C. ES-MS m/z 456 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.90 (d, J = 4.3 Hz, 1H), 8.24 (s, 1H), 8.08 (d, J = 8.45 Hz, 1H), 7.85 (d, J = 8.35 Hz, 1H), 7.68 (t, J = 8.15 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.39 (d, J = 4.2 Hz, 1H), 4.33 (t, J = 9.4 Hz, 2H), 4.19 (t, J = 9.45 Hz, 2H), 2.55 (s, 4H), 2.21 (q, J = 7.2 Hz, 2H), 1.87 (s, 4H), 0.96 (t, J = 7.2 Hz, 3H); 13C NMR (125 MHz, CDCl3): δC 159.2, 158.4 (q, JC-F = 5.0 Hz), 157.1, 157.0, 149.9, 148.3, 141.1, 129.83, 129.8, 127.5, 126.1, 125.2, 124.6 (q, JC-F = 268.75 Hz), 120.0, 102.1 (q, JC-F = 32.5 Hz), 54.1, 52.0, 51.9 (2 carbons), 48.7, 46.5, 46.48, 11.6.
(±)-4-[1-(2-{Octahydropyrrolo[1,2-a]piperazin-2-yl}-5-(trifluoromethyl)pyrimidin-4-yl)-4,5-dihydro-1H-imidazol-2-yl]quinoline (26)
Waxy solid. ES-MS m/z 468 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.90 (d, J = 4.35 Hz, 1H), 8.25 (s, 1H), 8.08 (d, J = 8.5 Hz, 1H), 7.85 (d, J = 8.25 Hz, 1H), 7.68 (t, J = 8.15 Hz, 1H), 7.48 (t, J = 7.85 Hz, 1H), 7.40 (d, J = 4.35 Hz, 1H), 4.36–4.32 (m, 2H), 4.19 (t, J = 9.15 Hz, 2H), 2.93–2.88 (m, 2H), 2.82 (d, J = 13.25 Hz, 1H), 2.51 (d, J = 10.3 Hz, 1H), 2.26 (t, J = 10.95 Hz, 1H), 1.98–1.93 (m, 1H), 1.84 (bs, 1H), 1.75–1.68 (m, 1H), 1.63–1.53 (m, 4H), 1.14–1.11 (m, 1H); 13C NMR (125 MHz, CDCl3): δC 159.8, 158.5 (q, JC-F = 6.25 Hz), 157.2, 157.0, 149.8, 148.2, 141.0, 129.9, 129.8, 127.4, 126.1, 125.2, 124.6 (q, JC-F = 268.75 Hz), 120.1, 102.4 (q, JC-F = 31.25 Hz), 61.9, 54.1, 53.0, 51.0, 50.7, 48.7, 46.5, 27.0, 21.1.
1-{4-[2-(Quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]-5-(trifluoromethyl)pyrimidin-2-yl}piperidin-4-ol (27)
Gray solid, m.p.: 163–165 °C. ES-MS m/z 443 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.89 (d, J = 4.35 Hz, 1H), 8.24 (s, 1H), 8.08 (d, J = 8.5 Hz, 1H), 7.85 (d, J = 8.3 Hz, 1H), 7.69 (t, J = 7.25 Hz, 1H), 7.50 (t, J = 7.85 Hz, 1H), 7.42 (d, J = 4.35 Hz, 1H), 4.35 (t, J = 9.5 Hz, 2H), 4.20 (t, J = 9.55 Hz, 2H), 3.56–3.51 (m, 1H), 2.82 (s, 1H), 2.74–2.71 (m, 2H), 2.22 (s, 2H), 1.34–1.30 (m, 2H), 1.01–0.94 (m, 2H); 13C NMR (125 MHz, CDCl3): δC 159.4, 158.4 (q, JC-F = 6.25 Hz), 157.3, 156.9, 149.7, 148.0, 141.2, 130.0, 129.5, 127.6, 126.2, 125.2, 124.6 (q, JC-F = 268.75 Hz), 120.1, 102.4 (q, JC-F = 32.5 Hz), 66.2, 54.0, 48.7, 44.2, 44.19, 33.6 (2 carbons).
(1-{4-[2-(Quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]-5-(trifluoromethyl)pyrimidin-2-yl}piperidin-4-yl)methanol (28)
Gray solid, m.p.: 118–120 °C. ES-MS m/z 457 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.88 (d, J = 4.35 Hz, 1H), 8.23 (s, 1H), 8.08 (d, J = 8.45 Hz, 1H), 7.85 (d, J = 8.35 Hz, 1H), 7.68 (t, J = 7.8 Hz, 1H), 7.49 (t, J = 7.65 Hz, 1H), 7.42 (d, J = 4.35 Hz, 1H), 4.35 (t, J = 9.4 Hz, 2H), 4.19 (t, J = 9.5 Hz, 2H), 3.23 (d, J = 6.25 Hz, 2H), 2.88 (d, J = 13.1 Hz, 2H), 2.73 (s, 2H), 2.03 (s, 2H), 1.25–1.23 (m, 2H), 0.58 (s, 2H); 13C NMR (125 MHz, CDCl3): δC 159.3, 158.3 (q, JC-F = 6.25 Hz), 157.4, 156.9, 149.8, 148.1, 141.2, 129.9, 129.6, 127.5, 126.2, 125.2, 124.7 (q, JC-F = 268.75 Hz), 120.1, 102.3 (q, JC-F = 31.25 Hz), 66.8, 54.0, 48.8, 46.91, 46.9, 45.9, 38.1, 28.1.
2-(1-{4-[2-(Quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]-5-(trifluoromethyl)pyrimidin-2-yl}piperidin-4-yl)ethan-1-ol (29)
Oil. ES-MS m/z 471 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.89 (d, J = 4.3 Hz, 1H), 8.22 (s, 1H), 8.08 (d, J = 8.5 Hz, 1H), 7.85 (d, J = 8.35 Hz, 1H), 7.68 (t, J = 8.15 Hz, 1H), 7.49 (t, J = 7.95 Hz, 1H), 7.41 (d, J = 4.4 Hz, 1H), 4.34 (t, J = 9.5 Hz, 2H), 4.19 (t, J = 9.55 Hz, 2H), 3.51 (t, J = 6.35 Hz, 2H), 2.84 (d, J = 12.1 Hz, 2H), 2.54 (s, 2H), 2.02 (t, J = 12.6 Hz, 2H), 1.27–1.18 (m, 4H), 0.54 (s, 2H); 13C NMR (125 MHz, CDCl3): δC 159.3, 158.3 (q, JC-F = 6.25 Hz), 157.4, 157.0, 149.8, 148.2, 141.2, 129.8, 129.6, 127.5, 126.2, 125.3, 124.7 (q, JC-F = 268.75 Hz), 120.1, 102.3 (q, JC-F = 32.5 Hz), 59.8, 54.0, 48.7, 47.3, 47.26, 45.9, 38.9, 32.1, 31.6.
N-(Pyridin-4-ylmethyl)-4-[2-(quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]-5-(trifluoromethyl)-pyrimidin-2-amine (30)
Brown solid, m.p.: 99–101 °C. ES-MS m/z 450 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.75 (d, J = 4.35 Hz, 1H), 8.35 (d, J = 5.35 Hz, 2H), 8.15 (s, 1H), 7.91 (d, J = 8.5 Hz, 1H), 7.74 (d, J = 8.35 Hz, 1H), 7.54 (t, J = 8.2 Hz, 1H), 7.39 (t, J = 7.85 Hz, 1H), 7.29 (d, J = 4.3 Hz, 1H), 6.60 (d, J = 5.2 Hz, 2H), 5.25–5.23 (m, 1H), 4.31 (t, J = 9.4 Hz, 2H), 4.17 (t, J = 9.5 Hz, 2H), 3.03–3.02 (m, 2H); 13C NMR (125 MHz, CDCl3): δC 158.0, 157.0, 155.2 (q, JC-F = 5.0 Hz), 149.8, 149.7 (3 carbons), 147.9, 146.5, 141.2, 129.8, 129.7, 127.4, 126.0, 125.0, 124.4 (q, JC-F = 268.75 Hz), 121.4 (2 carbons), 120.0, 100.7 (q, JC-F = 31.25 Hz), 54.0, 48.5, 41.9.
4-(1-{2-[2-(2-Methylpropyl)-4,5-dihydro-1H-imidazol-1-yl]-5-(trifluoromethyl)pyrimidin-4-yl}-4,5-dihydro-1H-imidazol-2-yl)quinoline (31)
White solid, m.p.: 151–153 °C. ES-MS m/z 468 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.94 (d, J = 4.3 Hz, 1H), 8.21 (s, 1H), 8.17 (d, J = 8.45 Hz, 1H), 7.74 (d, J = 8.35 Hz, 1H), 7.70 (t, J = 7.15 Hz, 1H), 7.46 (t, J = 7.95 Hz, 1H), 7.41 (d, J = 4.3 Hz, 1H), 4.38–4.34 (m, 2H), 4.31–4.27 (m, 2H), 3.65 (t, J = 8.75 Hz, 2H), 3.38–3.36 (m, 1H), 2.84 (s, 1H), 2.02 (d, J = 6.95 Hz, 2H), 1.71–1.63 (m, 1H), 0.77 (d, J = 6.6 Hz, 6H); 13C NMR (125 MHz, CDCl3): δC 163.3, 158.3 (q, JC-F = 5.0 Hz), 157.4, 156.9, 149.9, 148.1, 140.1, 130.2, 129.6, 127.2, 126.1, 124.7, 123.4 (q, JC-F = 300.0 Hz), 120.3, 110.1, 107.8 (q, JC-F = 32.5 Hz), 54.1, 53.4, 51.5, 48.7, 38.4, 26.6, 22.3 (2 carbons).
4-{1-[2-(Morpholin-4-yl)-5-(trifluoromethyl)pyrimidin-4-yl]-4,5-dihydro-1H-imidazol-2-yl}quinoline (32)
Gray solid, m.p.: 127–129 °C. ES-MS m/z 429 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.91 (d, J = 4.35 Hz, 1H), 8.27 (s, 1H), 8.11 (d, J = Hz, 1H), 7.85 (d, J = 8.3 Hz, 1H), 7.71 (t, J = 8.15 Hz, 1H), 7.51 (t, J = 8.0 Hz, 1H), 7.40 (t, J = 4.3 Hz, 1H), 4.35 (t, J = 9.45 Hz, 2H), 4.21 (t, J = 9.3 Hz, 2H), 3.11 (t, J = 4.55 Hz, 4H), 2.47 (s, 4H); 13C NMR (125 MHz, CDCl3): δC 159.5, 158.5 (q, JC-F = 6.25 Hz), 157.1, 157.0, 149.8, 148.2, 141.1, 130.0, 129.8, 127.6, 126.1, 125.2, 124.5 (q, JC-F = 268.75 Hz), 120.0, 102.3 (q, JC-F = 32.5 Hz), 66.1 (2 carbons), 54.0, 48.7, 47.2, 47.2.
4-{1-[6-(4-Ethylpiperazin-1-yl)pyrimidin-4-yl]-4,5-dihydro-1H-imidazol-2-yl}quinoline (33)
Off-white solid, m.p.: 87–89 °C. ES-MS m/z 388 [M + H]+.1H NMR (500 MHz, CDCl3): δH 8.95 (d, J = 4.25 Hz, 1H), 8.11 (d, J = 8.5 Hz, 1H), 8.06 (s, 1H), 7.82 (d, J = 8.4 Hz, 1H), 7.70 (t, J = 8.25 Hz, 1H), 7.49 (t, J = 8.0 Hz, 1H), 7.46 (d, J = 4.25 Hz, 1H), 4.95 (s, 1H), 4.30–4.26 (m, 2H), 4.20–4.16 (m, 2H), 2.98 (s, 4H), 2.30 (q, J = 7.25 Hz, 2H), 2.16 (t, J = 4.85 Hz, 4H), 1.01 (t, J = 7.2 Hz, 3H); 13C NMR (125 MHz, CDCl3): δC 162.1, 157.6, 157.4, 157.2, 150.1, 148.3, 139.4, 130.2, 129.9, 127.9, 125.6, 125.0, 120.8, 87.4, 53.8, 52.3, 51.9 (2 carbons), 48.9, 43.6 (2 carbons), 11.8.
(±)-4-[1-(6-{octahydropyrrolo[1,2-a]piperazin-2-yl}pyrimidin-4-yl)-4,5-dihydro-1H-imidazol-2-yl]quinoline (34)
Off-white solid, m.p.: 106–108 °C. ES-MS m/z 400 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.97 (d, J = 4.25 Hz, 1H), 8.14 (d, J = 8.45 Hz, 1H), 8.09 (s, 1H), 7.85 (d, J = 8.3 Hz, 1H), 7.72 (t, J = 8.1 Hz, 1H), 7.52 (t, J = 8.0 Hz, 1H), 7.48 (d, J = 4.3 Hz, 1H), 4.98 (s, 1H), 4.33–4.28 (m, 2H), 4.23–4.19 (m, 2H), 3.49 (s, 1H), 2.99 (t, J = 8.35 Hz, 1H), 2.82 (d, J = 11.05 Hz, 1H), 2.63–2.57 (m, 1H), 2.37 (s, 1H), 2.21 (t, J = 11.7 Hz, 1H), 2.02 (q, J = 8.7 Hz, 1H), 2.87–2.82 (m, 1H), 1.79–1.68 (m, 3H), 1.59 (s, 1H), 1.30–1.23 (m, 1H); 13C NMR (125 MHz, CDCl3): δC 162.1, 157.6, 157.4, 157.2, 150.1, 148.3, 139.4, 130.1, 130.0, 127.9, 125.6, 125.0, 120.8, 87.6, 61.7, 53.8, 53.4, 50.9, 48.9, 48.4, 43.2, 27.3, 21.2.
1-{6-[2-(Quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyrimidin-4-yl}piperidin-4-ol (35)
White solid, m.p.: 135–137 °C. ES-MS m/z 375 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.93 (d, J = 4.3 Hz, 1H), 8.10 (d, J = 8.4 Hz, 1H), 8.05 (s, 1H), 7.83 (d, J = 8.25 Hz, 1H), 7.70 (t, J = 8.05 Hz, 1H), 7.50 (t, J = 7.8 Hz, 1H), 7.46 (d, J = 4.3 Hz, 1H), 4.98 (s, 1H), 4.30–4.26 (m, 2H), 4.21–4.16 (m, 2H), 3.67–3.62 (m, 1H), 3.28 (s, 1H), 2.65 (t, J = 10.3 Hz, 2H), 2.54 (s, 2H), 1.51–1.47 (m, 2H), 1.11–1.04 (m, 2H); 13C NMR (125 MHz, CDCl3): δC 161.7, 157.7, 157.4, 157.3, 150.0, 148.1, 139.4, 130.2, 129.8, 128.0, 125.6, 125.0, 120.8, 87.4, 66.9, 53.6, 48.9, 41.4 (2 carbons), 33.3 (2 carbons).
(1-{6-[2-(Quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyrimidin-4-yl}piperidin-4-yl)methanol (36)
White solid, m.p.: 128–130 °C. ES-MS m/z 389 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.96 (d, J = 4.3 Hz, 1H), 8.14 (d, J = 8.45 Hz, 1H), 8.09 (s, 1H), 7.87 (d, J = 8.35 Hz, 1H), 7.73 (t, J = 8.0 Hz, 1H), 7.54 (t, J = 7.8 Hz, 1H), 7.49 (d, J = 4.3 Hz, 1H), 4.99 (s, 1H), 4.34–4.30 (m, 2H), 4.23–4.20 (m, 2H), 3.53 (s, 2H), 3.32 (d, J = 6.2 Hz, 2H), 2.47–2.41 (m, 3H), 1.58–1.52 (m, 1H), 1.47 (d, J = 13.9 Hz, 2H), 0.77–0.69 (m, 2H); 13C NMR (125 MHz, CDCl3): δC 162.1, 161.8, 157.7, 157.4, 150.0, 148.2, 139.5, 130.3, 129.8, 128.0, 125.6, 125.0, 120.9, 87.7, 67.0, 53.7, 48.9, 44.1 (2 carbons), 38.8, 27.9 (2 carbons).
2-(1-{6-[2-(Quinolin-4-yl)-4,5-dihydro-1H-imidazol-1-yl]pyrimidin-4-yl}piperidin-4-yl)ethan-1-ol (37)
Oil. ES-MS m/z 403 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.94 (d, J = 4.35 Hz, 1H), 8.11 (d, J = 8.45 Hz, 1H), 8.05 (s, 1H), 7.83 (d, J = 8.35 Hz, 1H), 7.70 (t, J = 8.2 Hz, 1H), 7.50 (t, J = 8.1 Hz, 1H), 7.47 (d, J = 4.3 Hz, 1H), 4.96 (s, 1H), 4.29 (t, J = 9.2 Hz, 2H), 4.18 (t, J = 9.15 Hz, 2H), 3.55 (t, J = 6.6 Hz, 2H), 3.46 (bs, 1H), 3.42–3.35 (m, 4H), 1.52–1.45 (m, 1H), 1.41 (d, J = 13.9 Hz, 2H), 1.31 (q, J = 6.55 Hz, 2H), 0.71–0.64 (m, 2H); 13C NMR (125 MHz, CDCl3): δC 161.8, 157.7, 157.4, 157.35, 150.0, 148.2, 139.4, 130.2, 129.9, 127.9, 125.6, 125.0, 120.8, 87.5, 59.9, 53.6, 48.9, 44.4 (2 carbons), 39.1, 32.6, 31.4 (2 carbons).
4-{1-[6-(Morpholin-4-yl)pyrimidin-4-yl]-4,5-dihydro-1H-imidazol-2-yl}quinoline (38)
Gray solid, m.p.: 147–149 °C. ES-MS m/z 361 [M + H]+. 1H NMR (500 MHz, CDCl3): δH 8.95 (d, J = 4.35 Hz, 1H), 8.12 (d, J = 8.45 Hz, 1H), 8.06 (s, 1H), 7.82 (d, J = 8.4 Hz, 1H), 7.71 (t, J = 8.15 Hz, 1H), 7.50 (t, J = 8.1 Hz, 1H), 7.47 (d, J = 4.35 Hz, 1H), 4.96 (s, 1H), 4.31–4.27 (m, 2H), 4.22–4.18 (m, 2H), 3.43 (t, J = 4.85 Hz, 4H), 2.93 (s, 4H); 13C NMR (125 MHz, CDCl3): δC 162.3, 157.6, 157.4, 157.2, 150.0, 148.2, 139.3, 130.2, 129.9, 127.9, 125.6, 124.9, 120.8, 87.3, 66.1 (2 carbons), 53.7, 48.9, 44.0 (2 carbons).
Determination of antitubercular activity
Mycobacterium tuberculosis H37Rv strain (originated from the Institute of Hygiene and Epidemiology at Prague, 1976) was obtained on 7 August 2013 from the Federal Scientific Center for Expertise of Medical Products (RF Ministry of Health Care). The lyophilized strain was seeded on Löwenstein–Jensen growth medium. The 3-week culture was suspended in physiological solution containing glycerol (15%) was transferred into cryotubes and kept at −80 °C. Three weeks in advance of the experiment, the culture was brought to ambient temperature and re-seeded into Löwenstein–Jensen growth medium. Thus, the second generation of the original M. tuberculosis culture was used in present study.
The minimal inhibitory concentration (MIC) of the compounds was determined using the REMA (resazurin microtitre plate assay)Citation15. A 3-week M. tuberculosis culture was transferred into a dry, sterile tube containing 8–9 3-mm glass beads. The tube was placed on a Vortex shaker for 30–40 s and then 5 mL Middlebrook 7H9 Broth (Becton Dickinson, Franklin Lakes, NJ, catalog No. 271310) was introduced. The turbidity of bacterial suspension was adjusted to 1.0 McFarland units (corresponding to approximately 3 × 108 bacteria/mL) and diluted 20-fold with Middlebrook 7H9 Broth containing OADC enrichment (Becton Dickinson, catalog No. 245116). The same culture medium was used to prepare the 1:100 M. tuberculosis (1% population) control. The stock solutions of the compounds in DMSO (10 mg/mL) were diluted with Middlebrook 7H9 Broth (containing OADC enrichment) to a concentration of 800 µg/mL. Two hundred microliters of the solution thus obtained was introduced into the second row of a 96-well microtiter plate. This raw was used to perform twofold serial dilutions using and eight-channel pipette to obtain final concentrations of 1.6, 3.1, 6.2, 12.5, 25, 50, 100, 200 and 400 µg/mL concentrations of the compound in rows 2–9 (accounting for 100 µL of bacterial suspension introduced for testing). Row 10 – MTb suspension control, row 11 – same culture diluted 10-fold (the 1% control). Row 12 was used as a blank control for optical density reading (200 µL of the grown medium). The bacterial suspension (100 µL) was introduced into each well except rows 11 (1% population control) and 12 (blank culture medium), to the total volume of 200 µL in each well. The plates were incubated at 35 °C for 7 days. At that point, 0.01% aqueous solution (30 µL) of resazurin (Sigma, St Louis, MO, product No. R7017) was introduced in each well and the incubation continued for 18 h at 35 °C. The fluorescence reading was performed using FLUOstar Optima (BMG Labtech, Offenburg, Germany) plate reader operating at λex = 520 nm and λem = 590 nm. The bacterial viability was determined by comparing the mean values (±SD at p = 0.05) of fluorescence in the control wells (row 12, blank and row 11, 1% control) and the wells containing the compound tested.
The MIC was determined as the compound concentration at which the fluorescence reached a plateau or was statistically (t criterion) similar to that of 1% control.
Determination of antimalarial activity and cytotoxicity
Compound activity against drug-sensitive 3D7 strain of Plasmodium falciparum was performed using a previously described 3-day SYBR green I growth and proliferation assay in a 96-well formatCitation16. Host cell cytotoxicity was determined in a 96-well format with a starting HepG2 cell density of 10 000 cells/well grown in Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies, Waltham, MA). The cells were incubated with serial dilutions of test compounds for 3 days, and the resulting cell viability was quantified using Promega CellTiterBlue.
Results and discussion
Commercially available quinoline-4-carboxaldehyde was converted on a 10 -g scale into the known 2-imidazoline 1Citation17. This pharmacophoric moiety was further elaborated into key haloazine building blocks 2a–2e by reacting 1 with 2,6-dibromopyridine, 2,4-dichloropyrimidine, 2,6-dichloropyrazine, 2,4-dichloro-5-trifluoropyrimidine and 4,5-dichloropyrimidine using the previously described Buchwald–Hartwig-type protocol under conventional heatingCitation1. These cores were further decorated with various primary and secondary amine appendages, as well as some knownCitation1 2-imidazolines, using the recently developed protocol for Pd-catalyzed imidazoline arylation under microwave irradiation (Scheme 1)Citation18.
Scheme 1. Synthesis of the 2-(quinolin-4-yl)imidazoline library (3–39) employing various heteroarene linkers and periphery amines.
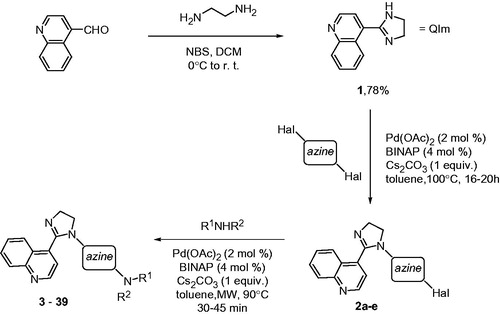
As expected, unsymmetrical dihalo azines (2,4-dichloropyrimidine and 2,4-dichloro-5-trifluoropyrimidine) regiospecifically reacted at the more reactive position 4 in the first step. 2-ImidazolinesCitation19, as well as amidines in generalCitation20,Citation21 have a high propensity to undergo transamination reactions with reactive amines under conventional heating. This complication (which is even further exacerbated for microwave-assisted processesCitation22) would potentially create an obstacle to the present library synthesis. Notably, neither under conventional heating (first Buchwald–Hartwig step) nor under microwave irradiation (second Buchwald–Hartwig step) was the imidazoline linkage affected via the transamination reaction and the target compounds 3–39 were obtained in good to excellent yields ().
Table 1. Compounds 3–38 synthesized in this work.
When evaluated for antimalarial activity against chloroquine-sensitive P. falciparum 3D7 strain, compounds 3–39 were found to be completely devoid of any activity at concentrations as high as 10 µM, which did not justify their further scrutiny at higher concentrations (cf. IC50 values for artemisinin and chloroquine which have been reported as 4.0 nMCitation23 and 9.8 nMCitation24, respectively). This result was somewhat surprising in light of the apparent similarity of the basic scaffold in 3–39 to that of some potent synthetic quinoline-based antimalarials reported recentlyCitation25. In parallel with antimalarial testing, the compounds were evaluated for non-specific cytotoxicity against HepG2 cells and showed no appreciable cytotoxicity at concentrations as high as 100 µM. This result was encouraging in light of our intention to evaluate these compounds as antitubercular agents. Due to the known permeability issues with respect to bacterial cell wall, higher compound concentrations are often required to achieve appreciable efficacy in M. tuberculosis cultures. This hampers the development of potentially cytotoxic compounds as therapeutic agents.
Compounds 3–39 were tested for the ability to inhibit the bacterial growth of H37Rv strain at 100 µg/mL concentrations. Of that number, two compounds – 16 and 18 – showed >50% inhibition of the bacterial culture growth. These compounds were further evaluated for the activity in 1.6–400 µg/mL range in order to determine the MIC. To our delight, these experiments confirmed that closely related compounds 16 and 18 possessed a specific antimycobacterial activity with MIC values of 200 and 50 µg/mL (MICMTb = 520 and 140 µM), respectively (see Supplemental material for growth inhibition curves and plate images). This level of potency is comparable to that of the recently reported quinoline-2-carboxamide antitubercular agents (MICMTb = 109–552 µM)Citation26 and, combined with the non-cytotoxic profile of compounds 16 and 18, clearly defines a promising starting point for further potency optimization ().
Table 2. The antitubercular lead compounds identified in this work.
Conclusion
The compounds described in this article were designed around a quinolin-4-ylinidazoline core that was expected to endow compounds with a broad-spectrum antimicrobial activity, in particular, antiplasmodial and antimycobacterial. Although the absence of antimalarial activity in these compounds is somewhat puzzling, the chemotype described herein clearly represents a novel starting point for the development of new-generation, potent antitubercular agents devoid of cytotoxic effects. In the context of today’s battle with drug-resistant forms of tuberculosis, this chemotype represents a timely addition to the portfolio of small-molecule candidates for further optimization.
Supplementary material available online
Copies of 1H and 13C NMR spectra (compounds 1, 2a–e, 3–38) and graphic information related to antibacterial testing of the lead compounds (16 and 18) are available online.
Supplementaryinformation.pdf
Download PDF (2.4 MB)Acknowledgements
The authors are indebted to Professor Vicky Avery of Griffith University for evaluating cytotoxicity and antimalarial activity of compounds 3–38. This research was supported by the Russian Scientific Fund (project grant 1450-00069).
Declaration of interest
The authors declare no conflict of interest. The authors are solely responsible for the content and results presented in this paper.
References
- Krasavin M. Novel diversely substituted 1-heteroaryl-2-imidazolines for fragment-based drug discovery. Tetrahedron Lett 2012;53:2876–80
- Krasavin M. Biologically active compounds based on the privileged 2-imidazoline scaffold: the world beyond adrenergic/imidazoline receptor modulators. Eur J Med Chem 2015;97:525–37
- Krasavin M. Pd-catalyzed N-arylation of 2-imidazolines provides convenient access to selective cyclooxygenase-2 inhibitors. Lett Org Chem 2013;10:235–9
- Sarnpitak P, Mujumdar P, Morisseau C, et al. Potent, orally available, selective COX-2 inhibitors based on 2-imidazoline core. Eur J Med Chem 2014;84:160–72
- Sarnpitak P, Mujumdar P, Taylor P, et al. Panel docking of small-molecule libraries—prospects to improve efficiency of lead compound discovery. Biotechnol Adv 2015;33:941–7
- Schrader FC, Barho M, Steiner I, et al. The antimalarial pipeline – an update. Int J Med Microbiol 2012;302:165–71
- Chhabria M, Patel S, Jani M. Recent development and future perspective of antitubercular therapy. Antiinfect Agents Med Chem 2010;9:59–103
- Welsch ME, Snyder SA, Stockwell BR. Privileged scaffolds for library design and drug discovery. Curr Opin Chem Biol 2010;14:347–61
- Kumar D, Khan SI, Tekwani BL, et al. 4-Aminoquinoline-pyrimidine hybrids: synthesis, antimalarial activity, heme binding and docking studies. Eur J Med Chem 2015;89:490–502
- Shibi IG, Aswathy L, Jisha RS, et al. Molecular docking and QSAR analyses for understanding the antimalarial activity of some 7-substituted-4-aminoquinoline derivatives. Eur J Pharm Sci 2015;77:9–23
- Keri RS, Patil SA. Quinoline: A promising antitubercular target. Biomed Pharmacother 2014;68:1161–75
- Lilienkampf A, Mao J, Wan B, et al. Structure–activity relationships for a series of quinoline-based compounds active against replicating and nonreplicating Mycobacterium tuberculosis. J Med Chem 2009;52:2109–18
- Ahirwar S, Shrivastava A, Pathak AK. 2D QSAR study of novel quinoline derivatives as potent antitubercular agents. J Comp Meth Mol Des 2014;4:6–13
- Fujioka H, Murai K, Ohba Y, et al. A mild and efficient one-pot synthesis of 2-dihydroimidazoles from aldehydes. Tetrahedron Lett 2005;46:2197–9
- Martin A, Camacho M, Portaels F, Palomino CC. Resazurin microtiter assay plate testing of Mycobacterium tuberculosis susceptibilities to second-line drugs: rapid, simple, and inexpensive method. Antimicrob Agents Chemother 2003;47:3616–19
- Smilkstein M, Sriwilaijaroen N, Kelly JX, et al. Simple and inexpensive fluorescence-based technique for highthroughput antimalarial drug screening. Antimicrob Agents Chemother 2004;48:1803–6
- Mujumdar P, Grkovic T, Krasavin M. A simple two-step access to diversely substituted imidazo[4,5-b]pyridines and benzimidazoles from readily available 2-imidazolines. Tetrahedron Lett 2013;54:3336–40
- Mujumdar P, Sarnpitak P, Shetnev A, et al. Pd-catalyzed amination of imidazolin-1-yl azines: toward a new kinase-inhibitory chemotype. Tetrahedron Lett 2015;56:2827–31
- Butler RN, Fitzgerald KJ. 12- and 1,3-Diamine exchange between substituted 4,5-dihydroimidazoles and 1,4,5,6-tetrahydropyrimidines: routes to benzimidazoles, dihydroimidazoles, and tetrahydropyrimidines. J Chem Soc Perkin Trans 1989;1:155–7
- Vincent S, Mons S, Lebeau L, Mioskowski C. N,N-Dibenzyl formamidine as a new protective group for primary amines. Tetrahedron Lett 1997;38:7527–30
- Furth PS, Reitman MS, Cook AF. A novel formamidine linker for use in solid-phase synthesis. Tetrahedron Lett 1997;38:5403–6
- Pereira MF, Thiery V, Besson T. Microwave-assisted regioselective N-alkylation of cyclic amidines. Tetrahedron Lett 2007;48:7657–9
- Bhattacharya A, Mishra LC, Sharma M, et al. Antimalarial pharmacodynamics of chalcone derivatives in combination with artemisinin against Plasmodium falciparum in vitro. Eur J Med Chem 2009;44:3388–93
- Reiter C, Frohlich T, Zeino M, et al. New efficient artemisinin derived agents against human leukemia cells, human cytomegalovirus and Plasmodium falciparum: 2nd generation 1,2,4-trioxane-ferrocene hybrids. Eur J Med Chem 2015;97:164–72
- Kumar A, Srivastava K, Kumar SR, et al. 4Anilinoquinoline triazines: a novel class of hybrid antimalarial agents. Eur J Med Chem 2011;46:676–90
- Gonec T, Bobal P, Sujan J, et al. Investigating the spectrum of biological activity of substituted quinoline-2-carboxamides and their isosteres. Molecules 2012;17:613–44