Abstract
In the present paper, a novel series of dibenzofuran-piperazine derivatives were synthesized via the treatment of N-(2-methoxy-3-dibenzofuranyl)-2-chloroacetamide with substituted piperazine derivatives. The chemical structures of the compounds were elucidated by 1H NMR, 13C NMR, mass spectral data; elemental analysis and HPLC analysis. Each derivative was evaluated for antiplatelet activity and anticholinesterase activity. Compound 2 m with 2-furoyl moiety exhibited high percentage inhibition as much as standard drug aspirin on arachidonic acid (AA)-induced platelet aggregation. None of the compounds presented significant inhibitor effect on collagen-induced platelet aggregation. Furthermore, the anticholinesterase activity of the compounds was determined and they did not show promising inhibitor activity compared with standard drug donepezil.
Introduction
Platelets, also called trombocytes, play an essential role in hemostasis and pathological thrombosisCitation1–3. Platelet activation and aggregation are important necessities in the pathogenesis of athero thrombotic events characteristic of the acute coronary syndromes (ACSs) or due to mechanical disruption of plaque by percutaneous coronary interventions (PCIs). Hyperactivity of platelets increase the risk of various vaso-occlusive diseases, such as unstable angina, acute myocardial infarction and transient ischemic attacksCitation4,Citation5. Antiplatelet therapy is a basic tool in the prevention and treatment of thromboembolic diseases. In injured vessels, blood aggregation is generated as a physiological defense reaction by releasing biologically active compound, such as adenosine 5′-diphosphate (ADP), thrombin and prostaglandine endoperoxide. Agonists such as thrombin, arachidonic acid (AA), thromboxane A2 (TxA2), platelet-activating factor (PAF) and collagen are able to induce platelet aggregation. Among these agonists, AA is one of the most powerful agonists for platelet activationCitation6,Citation7. Currently, essential antiplatelet drugs used for the prophylaxis and treatment of thromboembolic diseases are aspirin, ridogrel, ticlopidine, clopidogrel, dipyridamole, cilostazol, tirofiban and sibrafibanCitation8–12. Nevertheless, these orally administered antiplatelet drugs have certain disadvantages, such as gastric erosion, agranulocytosis, neutropenia, thrombocytopenia, aplastic anemia and thrombotic thrombocytopenic purpura along with inefficient therapyCitation13–16. Moreover, development of resistance to these drugs is another handicap for therapyCitation17,Citation18. These limitations are among the reasons stimulating the search for new antiplatelet drugs.
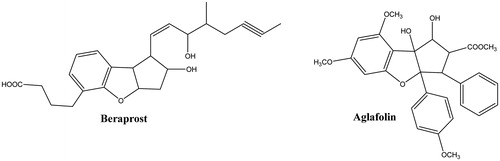
Beraprost sodium () is a stable synthetic analaogue of prostaglandin I2 (PGI2) which have both potent antiplatelet and peripheral vasodilating actions. It possesses benzofurane ring in its structureCitation19,Citation20. The drug which is in clinical trial in Europe and USA presently, inhibits platelet aggregation induced by ADP, collagen and arachidonic acidCitation21. Aglafolin () is another benzofuran including molecule, isolated from the stems of Aglaia elliptifolia, was notified with effective inhibitor activity of platelet aggregation induced by PAF both in vitro and in vivoCitation22. Recently, in a series of study Sidhu et al.Citation23–27 and Thalji et al.Citation28 have also declared different benzofuran-bearing compounds with significant antiplatelet activity. Moreover, benzofuran ring analog dibenzofuran-bearing compounds have been reported to exhibit a great variety of biological some effects, including thrombosisCitation29,Citation30 and anticholinesterase activityCitation31–33 distinct from the mentioned above. However, piperazine ring plays an important role for antiplatelet activityCitation34. By the effect of the findings about antiplatelet and anticholinesterase activities of dibenzofurans and piperazines, it was thought that it would be worthwhile to synthesize on a combination system that bears these vital moieties on the same chemical skeleton. Thus our research group synthesize dibezofuran-piperazine compounds so as to investigate probable antiplatelet and anticholinesterase activity.
Experimental
Chemistry
All chemicals were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO) and Merck Chemicals (Merck KGaA, Darmstadt, Germany). All melting points (m.p.) were determined by Electrothermal 9100 digital melting point apparatus (Electrothermal, Essex, UK) and are uncorrected. All the reactions were monitored by thin-layer chromatography (TLC) using Silica Gel 60 F254 TLC plates (Merck KGaA, Darmstadt, Germany). Spectroscopic data were recorded with the following instruments: IR, Shimadzu 8400S spectrophotometer (Shimadzu, Tokyo, Japan); NMR, Bruker DPX 500 NMR spectrometer (Bruker Bioscience, Billerica, MA, USA), in DMSO-d6, using TMS as internal standard; M + 1 peaks were determined by AB Sciex-3200 Q-TRAP LC/MS/MS system (AB Applied Biosystems Co., MA). Purity of synthesized compounds was checked by Shimadzu LC-20 A Prominence HPLC system, equipped with a Shimadzu DGU-14 A degasser, LC-20 A dual piston pump, CTO-10 ASVP column oven and SPD-MI20A PDA detector. Reodyne 7725i injection valve and a stainless steel GL Science Inertsil ODS-3 (4.6 × 250 mm) column. Solvents for the separation compounds 2a, 2i, 2 l and 2 m were: solvent A, acetonitrile (95%); solvent B, water (5%) at a flow rate of 1.0 ml/min and a sample injection volume of 20 µl. Solvents for the separation of all other compounds were: solvent A, acetonitrile (70%); solvent B, water (30%) at a flow rate of 1.2 ml/min and a sample injection volume of 20 µl. Elemental analyses were also performed on a Leco TruSpec Micro CHN/CHNS elemental analyzer (Leco, St. Joseph, MI).
Synthesis of the compounds
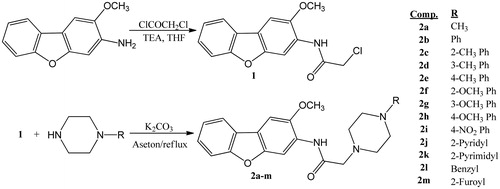
N-(2-Methoxy-3-dibenzofuranyl)-2-chloroacetamide (1)
Chloroacetyl chloride (0.2 mol, 16 ml) was added drop wise over 15 min to a magnetically stirring solution of 2-methoxy-3-aminodibenzofuran (0.2 mol) and triethylamine (0.2 mol, 28 ml) in dry THF (200 ml) at 0–5 °C and the reaction mixture was stirred for one hour at room temperature. After controlled the reaction ending by TLC, it completed and the solvent was evaporated under reduced pressure and then water was added to wash the resulting solid and the mixture was filtered, dried and recrystallized from ethanol to give compound 1.
General synthesis procedure for N-(2-methoxy-3-dibenzofuranyl)-2-(4-substituted piperazin-1-yl)acetamide derivatives (2a-m)
Compound 1 (10 mmol) was refluxed with 1-substituted piperazine derivatives (10 mmol) and potassium carbonate (10 mmol) in acetone for 5 h (200 °C). After TLC screening, the solvent was evaporated and then water was added to wash the resulting solid and the mixture was filtered and the obtained crude product was dried and then crystallized from ethanol.
N-(2-Methoxy-3-dibenzofuranyl)-2-(4-methylpiperazin-1-yl) acetamide (2a)
Yield 72–75%, m.p. 166–168 °C. HPLC: 95.2% purity. IR (KBr, cm−1): νmax 3346 (amide N–H), 1671 (C = O), 1541–1316 (C = C), 1267–926 (C–O, C–N). 1H NMR (500 MHz, DMSO-d6, ppm): δ 2.23 (s, 3H, N–CH3), 2.42 (brs, 4H, piperazine–H), 2.64 (brs, 4H, piperazine–H), 3.19 (s, 2H, –CO–CH2), 4.04 (s, 3H, O–CH3), 7.37 (t, 1H, J: 7.5 Hz, Ar–H), 7.45 (t, 1H, J: 7.5 Hz, Ar–H), 7.65 (d, 1H, J: 8 Hz, Ar–H), 7.85 (s, 1H, Ar–H), 8.08 (d, 1H, J: 7.5 Hz, Ar–H), 8.58 (s, 1H, Ar–H), 10.09 (s, 1H, N–H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 26.27, 53.16, 55.59, 57.23, 61.67, 101.93, 103.20, 111.96, 118.24, 120.24, 123.33, 124.66, 126.93, 127.76, 145.54, 150.20, 156.32, 168.77. For C20H23N3O3, calculated: 67.97% C, 6.56% H, 11.89% N; found: 67.84% C, 6.54% H, 11.81% N. MS [M + 1]+: m/z 354.
N-(2-Methoxy-3-dibenzofuranyl)-2-(4-fenilpiperazin-1-yl) acetamide (2b)
Yield 74%, m.p. 200–203 °C. HPLC: >99.9% purity. IR (KBr, cm−1): νmax 3356 (amide N–H), 1674 (C = O), 1534–1313 (C = C), 1269–926 (C–O, C–N). 1H NMR (500 MHz, DMSO-d6, ppm): δ 2.23 (s, 3H, N–CH3), 2.75 (brs, 4H, piperazine–H), 3.25 (brs, 4H, piperazine–H), 3.28 (s, 2H, –CO–CH2), 3.99 (s, 3H, O–CH3), 6.82 (t, 1H, J: 7.0 Hz, Ar–H), 6.99 (d, 1H, J: 7.5 Hz, Ar–H), 7.25 (t, 1H, J: 8.5 Hz, Ar–H), 7.37 (t, 1H, J: 7.5 Hz, Ar–H), 7.45 (t, 1H, J: 8.0 Hz, Ar–H), 7.65 (d, 1H, J: 8 Hz, Ar–H), 7.85 (s, 1H, Ar–H), 8.08 (1H, d, J: 7 Hz, Ar–H), 10.08 (s, 1H, N–H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 49.17, 53.23, 57.26, 61.76, 102.07, 103.20, 111.97, 116.02, 118.31, 119.50, 120.97, 123.34, 124.65, 126.95, 127.71, 129.46, 145.58, 150.18, 151.37, 156.32, 168.65. For C25H25N3O3, calculated: 72.27% C, 6.06% H, 10.11% N; found: 72.26% C, 6.09% H, 10.21% N. MS [M + 1]+: m/z 416.
N-(2-Methoxy-3-dibenzofuranyl)-2-[4-(2-methylphenyl)piperazin-1-yl]acetamide (2c)
Yield 70–73%, m.p. 168–170 °C. HPLC: >99.9% purity. IR (KBr, cm−1): νmax 3329 (amide N–H), 1670 (C = O), 1538–1325 (C = C), 1276–9265 (C–O, C–N). 1H NMR (500 MHz, DMSO-d6, ppm): δ 2.25 (s, 3H, C–CH3), 2.77 (brs, 4H, piperazine–H), 2.96 (brs, 4H, piperazine–H), 3.29 (s, 2H, –CO–CH2), 4.05 (s, 3H, O–CH3), 6.99 (t, 1H, J: 6.5 Hz, Ar–H), 7.08 (d, 1H, J: 9.0 Hz, Ar–H), 7.17–7.21 (m, 3H, Ar–H), 7.37 (t, 1H, J: 7.5 Hz, Ar–H), 7.47 (t, 1H, J: 8.0 Hz, Ar–H), 7.67 (d, 1H, J: 8.5 Hz, Ar–H), 7.87 (s, 1H, Ar–H), 8.09 (1H, d, J: 7.5 Hz, Ar–H), 10.11 (s, 1H, N–H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 18.04, 52.28, 53.75, 57.32, 61.76, 102.04, 103.23, 111.97, 118.30, 119.23, 120.97, 123.34, 123.47, 124.66, 126.95, 127.11, 127.75, 131.36, 132.33, 145.60, 150.20, 151.60, 156.32, 168.76. For C26H27N3O3, calculated: 72.71% C, 6.34% H, 9.78% N; found: 72.76% C, 6.36% H, 9.71% N. MS [M + 1]+: m/z 430.
N-(2-Methoxy-3-dibenzofuranyl)-2-[4-(3-methylphenyl)piperazin-1-yl]acetamide (2d)
Yield 78–81%, m.p. 77–82 °C. HPLC: 99.1% purity. IR (KBr, cm−1): νmax 3338 (amide N–H), 1673 (C = O), 1562–1311 (C = C), 1249–926 (C–O, C–N). 1H NMR (500 MHz, DMSO-d6, ppm): δ 2.25 (s, 3H, C–CH3), 2.73 (brs, 4H, piperazine–H), 3.23 (brs, 4H, piperazine–H), 3.28 (s, 2H, –CO–CH2), 4.05 (s, 3H, O–CH3), 6.63 (d, 1H, J: 7.5 Hz, Ar–H), 6.77 (d, 1H, J: 8.0 Hz, Ar–H), 6.81 (s, 1H, Ar–H), 7.12 (t, 1H, J: 8.0 Hz, Ar–H), 7.37 (t, 1H, J: 7.5 Hz, Ar–H), 7.43 (t, 1H, J: 8.0 Hz, Ar–H), 7.87 (s, 1H, Ar–H), 7.65 (2H, d, J: 8.0 Hz, Ar–H), 8.08 (1H, d, J: 7.5 Hz, Ar–H), 10.07 (s, 1H, N–H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 21.89, 49.21, 53.26, 57.26, 61.78, 102.07, 103.18, 111.96, 113.17, 116.70, 118.31, 120.33, 120.95, 123.32, 124.66, 126.94, 127.72, 129.27, 138.51, 145.58, 150.19, 151.39, 156.33, 168.63. For C26H27N3O3, calculated: 72.71% C, 6.34% H, 9.78% N; found: 72.82% C, 6.21% H, 9.73% N. MS [M + 1]+: m/z 430.
N-(2-Methoxy-3-dibenzofuranyl)-2-[4-(4-methylphenyl)piperazin-1-yl]acetamide (2e)
Yield 74–78%, m.p. 192–195 °C. HPLC: >99.9% purity. IR (KBr, cm−1): νmax 3258 (amide N–H), 1675 (C = O), 1589–1302 (C = C), 1242–925 (C–O, C–N). 1H NMR (500 MHz, DMSO-d6, ppm): δ 2.20 (s, 3H, C–CH3), 2.74 (brs, 4H, piperazine–H), 3.19 (brs, 4H, piperazine–H), 3.27 (s, 2H, –CO–CH2), 4.05 (s, 3H, O–CH3), 6.89 (d, 1H, J: 8.5 Hz, Ar–H), 7.05 (d, 1H, J: 8.5 Hz, Ar–H), 7.38 (t, 1H, J: 7.5 Hz, Ar–H), 7.47 (t, 1H, J: 7.5 Hz, Ar–H), 7.65 (d, 2H, J: 8.0 Hz, Ar–H), 7.85 (s, 1H, Ar–H), 8.08 (2H, d, J: 8.0 Hz, Ar–H), 8.60 (1H, s, Ar–H), 10.08 (s, 1H, N–H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 20.51, 49.67, 53.25, 57.25, 61.75, 102.06, 103.20, 111.97, 116.27, 118.30, 120.96, 123.33, 124.65, 126.95, 127.72, 128.33, 129.89, 145.58, 149.31, 150.19, 156.32, 168.66. For C26H27N3O3, calculated: 72.71% C, 6.34% H, 9.78% N; found: 72.85% C, 6.36% H, 9.78% N. MS [M + 1]+: m/z 430.
N-(2-Methoxy-3-dibenzofuranyl)-2-[4-(2-methoxyphenyl)piperazin-1-yl]acetamide (2f)
Yield 75%, m.p. 133–136 °C. HPLC: >99.9% purity. IR (KBr, cm−1): νmax 3358 (amide N–H), 1671 (C = O), 1510–1339 (C = C), 1285–926 (C–O, C–N). 1H NMR (500 MHz, DMSO-d6, ppm): δ 2.75 (brs, 4H, piperazine–H), 3.08 (brs, 4H, piperazine–H), 3.26 (s, 2H, –CO–CH2), 3.80 (s, 3H, O–CH3), 4.0 (s, 3H, O–CH3), 6.91–7.0 (m, 4H, Ar–H), 7.37 (t, 1H, J: 7.5 Hz, Ar–H), 7.38 (t, 1H, J: 7.5 Hz, Ar–H), 7.45 (t, 1H, J: 7.5 Hz, Ar–H), 7.65 (d, 1H, J: 8.0 Hz, Ar–H), 7.86 (s, 1H, Ar–H), 8.09 (1H, d, J: 7.5 Hz, Ar–H), 8.60 (1H, s, Ar–H), 10.13 (s, 1H, N–H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 50.99, 53.54, 55.85, 57.28, 61.81, 102.04, 103.21, 111.96, 112.49, 118.29, 118.53, 120.95, 121.39, 123.08, 123.33, 124.66, 126.94, 127.75, 141.53, 145.59, 150.20, 152.54, 156.32, 168.72. For C26H27N3O4, calculated: 70.09% C, 6.11% H, 9.43% N; found: 70.04% C, 6.14% H, 9.48% N. MS [M + 1]+: m/z 446.
N-(2-Methoxy-3-dibenzofuranyl)-2-[4-(3-methoxyphenyl)piperazin-1-yl]acetamide (2 g)
Yield 68–71%, m.p. 130–132 °C. HPLC: 99.3% purity. IR (KBr, cm−1): νmax 3352 (amide N–H), 1667 (C = O), 1516–1315 (C = C), 1276–926 (C–O, C–N). 1H NMR (500 MHz, DMSO-d6, ppm): δ 2.73 (brs, 4H, piperazine–H), 3.25 (brs, 4H, piperazine–H), 3.27 (s, 2H, –CO–CH2), 3.72 (s, 3H, O–CH3), 3.99 (s, 3H, O–CH3), 6.40 (d, 1H, J: 8.5 Hz, Ar–H), 6.51 (s, 1H, Ar–H), 6.75 (d, 1H, J: 8.5 Hz, Ar–H), 7.14 (t, 1H, J: 8.0 Hz, Ar–H), 7.37 (t, 1H, J: 7.5 Hz, Ar–H), 7.45 (t, 1H, J: 8 Hz, Ar–H), 7.65 (d, 1H, J: 8.0 Hz, Ar–H), 7.85 (s, 1H, Ar–H), 8.08 (d, 1H, J: 8.0 Hz, Ar–H), 8.60 (s, 1H, Ar–H), 10.07 (s, 1H, N–H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 49.12, 53.20, 55.37, 57.26, 61.75, 102.08, 102.15, 103.18, 104.82, 108.63, 111.96, 118.31, 120.96, 123.33, 124.66, 126.95, 127.71, 130.15, 145.59, 150.18, 152.72, 156.32, 160.73, 168.63. For C26H27N3O4, calculated: 70.09% C, 6.11% H, 9.43% N; found: 70.07% C, 6.18% H, 9.51% N. MS [M + 1]+: m/z 446.
N-(2-Methoxy-3-dibenzofuranyl)-2-[4-(4-methoxyphenyl)piperazin-1-yl]acetamide (2 h)
Yield 69–73%, m.p. 181–184 °C. HPLC: 99.1% purity. IR (KBr, cm−1): νmax 3349 (amide N–H), 1673 (C = O), 1519–1298 (C = C), 1275–927 (C–O, C–N). 1H NMR (500 MHz, DMSO-d6, ppm): δ 2.74 (brs, 4H, piperazine–H), 3.13 (brs, 4H, piperazine–H), 3.27 (s, 2H, –CO–CH2), 3.70 (s, 3H, O–CH3), 4.0 (s, 3H, O–CH3), 6.84 (d, 2H, J: 8.0 Hz, Ar–H), 7.38 (t, 1H, J: 7.5 Hz, Ar–H), 7.45 (t, 1H, J: 7.5 Hz, Ar–H), 7.65 (d, 2H, J: 8.5 Hz, Ar–H), 7.85 (s, 1H, Ar–H), 8.08 (2H, d, J: 7.5 Hz, Ar–H), 8.60 (1H, s, Ar–H), 10.09 (s, 1H, N–H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 50.57, 53.33, 55.68, 57.25, 61.74, 102.05, 103.19, 111.96, 114.80, 117.97, 118.30, 120.95, 123.33, 124.66, 126.95, 127.73, 145.58, 145.73, 150.19, 153.54, 156.32, 168.66. For C26H27N3O4, calculated: 70.09% C, 6.11% H, 9.43% N; found: 70.01% C, 6.15% H, 9.50% N. MS [M + 1]+: m/z 446.
N-(2-Methoxy-3-dibenzofuranyl)-2-[4-(4-nitrophenyl)piperazin-1-yl]acetamide (2i)
Yield 72–73%, m.p. 285–290 °C. HPLC: 99.2% purity. IR (KBr, cm−1): νmax 3354 (amide N–H), 1676 (C = O), 1512–1319 (C = C), 1213–927 (C–O, C–N). 1H NMR (500 MHz, DMSO-d6, ppm): δ 2.74 (brs, 4H, piperazine–H), 3.59 (brs, 4H, piperazine–H), 3.30 (s, 2H, –CO–CH2), 4.39 (s, 3H, O–CH3), 7.12 (d, 2H, J: 8.0 Hz, Ar–H), 7.35 (t, 1H, J: 7.5 Hz, Ar–H), 7.45 (t, 1H, J: 7.5 Hz, Ar–H), 7.65 (d, 2H, J: 8.0 Hz, Ar–H), 7.80 (s, 1H, Ar–H), 8.05 (2H, d, J: 8.5 Hz, Ar–H), 8.59 (1H, s, Ar–H), 10.03 (s, 1H, N–H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 50.72, 54.33, 57.25, 62.67, 103.09, 104.43, 113.61, 118.21, 121.59, 123.33, 124.66, 125.20, 126.95, 127.31, 128.82, 138.54, 146.84, 150.08, 153.73, 156.32, 168.81. For C25H24N4O5, calculated: 65.21% C, 5.25% H, 12.17% N; found: 65.25% C, 5.15% H, 12.20% N. MS [M + 1]+: m/z 461.
N-(2-Methoxy-3-dibenzofuranyl)-2-[4-(2-pyridyl)piperazin-1-yl]acetamide (2j)
Yield 74%, m.p. 177–179 °C. HPLC: >99.9% purity. IR (KBr, cm−1): νmax 3334 (amide N–H), 1678 (C = O), 1512–1303 (C = C), 1280–925 (C–O, C–N). 1H NMR (500 MHz, DMSO-d6, ppm): δ 2.63 (brs, 4H, piperazine–H), 3.25 (s, 2H, –CO–CH2), 3.79 (brs, 4H, piperazine–H), 4.0 (s, 3H, O–CH3), 6.65–6.69 (2H, m, Ar–H), 7.38 (d, 1H, J: 8.0 Hz, Ar–H), 7.42–7.45 (m, 2H, Ar–H), 7.54 (t, 1H, J: 7.5 Hz, Ar–H), 8.08 (1H, d, J: 8.5 Hz, Ar–H), 8.47 (2H, d, J: 7.5 Hz, Ar–H), 10.03 (s, 1H, N–H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 45.53, 53.05, 57.27, 61.86, 102.11, 103.19, 107.68, 111.97, 113.65, 118.32, 120.96, 123.33, 124.66, 126.95, 127.72, 138.05, 145.62, 148.06, 150.18, 156.33, 159.40, 168.64. For C23H23N5O3, calculated: 66.17% C, 5.55% H, 16.78% N; found: 66.24% C, 5.45% H, 16.80% N. MS [M + 1]+: m/z 417.
N-(2-Methoxy-3-dibenzofuranyl)-2-[4-(2-pyrimidinyl)piperazin-1-yl]acetamide (2 k)
Yield 69–71%, m.p. 183–186 °C. HPLC: 99.4% purity. IR (KBr, cm−1): 3358 (amide N–H), 1669 (C = O), 1542–1316 (C = C), 1288–926 (C–O, C–N). 1H NMR (500 MHz, DMSO-d6, ppm): δ 2.65 (brs, 4H, piperazine–H), 3.27 (s, 2H, –CO–CH2), 3.86 (brs, 4H, piperazine–H), 4.04 (s, 3H, O–CH3), 6.67 (t, 1H, J: 4.5 Hz, Ar–H), 7.38 (t, 1H, J: 7.5 Hz, Ar–H), 7.46 (t, 1H, J: 7.5 Hz, Ar–H), 7.66 (d, 1H, J: 8.0 Hz, Ar–H), 7.86 (s, 1H, Ar–H), 8.08 (2H, d, J: 8.5 Hz, Ar–H), 8.39 (2H, d, J: 5 Hz, Ar–H), 8.60 (s, 1H, Ar–H), 10.03 (s, 1H, N–H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 44.16, 53.03, 57.27, 61.85, 102.12, 103.18, 110.79, 111.97, 118.33, 120.96, 123.33, 124.66, 126.95, 127.71, 145.65, 150.18, 156.33, 158.44, 161.66, 168.61. For C23H23N5O3, calculated: 66.17% C, 5.55% H, 16.78% N; found: 66.24% C, 5.45% H, 16.80% N. MS [M + 1]+: m/z 418.
N-(2-Methoxy-3-dibenzofuranyl)-2-(4-benzylpiperazin-1-yl) acetamide (2l)
Yield 70–73%, m.p. 102–105 °C. HPLC: 98.8% purity. IR (KBr, cm−1): νmax 3348 (amide N–H), 1673 (C = O), 1536–1312 (C = C), 1284–927 (C–O, C–N). 1H NMR (500 MHz, DMSO-d6, ppm): δ 2.62 (brs, 4H, piperazine–H), 3.20 (s, 2H, –CO–CH2), 3.60 (brs, 2H, C–CH2), 3.86 (brs, 4H, piperazine–H), 3.99 (s, 3H, O–CH3), 7.31–7.38 (m, 5H, Ar–H), 7.38 (t, 1H, J: 8.5 Hz, Ar–H), 7.64 (d, 1H, J: 8.0 Hz, Ar–H), 7.85 (s, 1H, Ar–H), 7.08 (d, 1H, J: 7.5 Hz, Ar–H), 8.08 (d, 1H, J: 8.5 Hz, Ar–H), 8.57 (s, 1H, Ar–H), 10.06 (s, 1H, N–H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 53.14, 57.20, 61.59, 102.00, 103.20, 111.96, 118.27, 120.94, 123.33, 124.65, 126.94, 127.51, 127.72, 128.70, 129.55, 145.57, 150.18, 156.31, 168.66. For C26H27N3O3, calculated: 72.71% C, 6.34% H, 9.78% N; found: 72.74% C, 6.45% H, 9.82% N. MS [M + 1]+: m/z 430.
N-(2-Methoxy-3-dibenzofuranyl)-2-[4-(2-furoyl)piperazin-1-yl]acetamide (2 m)
Yield 75%, m.p. 150–154 °C. HPLC: 95.9% purity. IR (KBr, cm−1): νmax 3334 (amide N–H), 1678 (C = O), 1512–1303 (C = C), 1280–925 (C–O, C–N). 1H NMR (500 MHz, DMSO-d6, ppm): δ 2.65 (brs, 4H, piperazine–H), 3.28 (s, 2H, –CO–CH2), 3.78 (brs, 4H, piperazine–H), 4.05 (s, 3H, O–CH3), 6.64–6.65 (m, 2H, Ar–H), 7.04 (d, 1H, J: 3.5 Hz, Ar–H), 7.36 (t, 1H, J: 8.0 Hz, Ar–H), 7.42 (t, 1H, J: 7.5 Hz, Ar–H), 7.65 (d, 1H, J: 8.5 Hz, Ar–H), 7.86 (s, 1H, Ar–H), 8.08 (d, 1H, J: 7.5 Hz, Ar–H), 8.58 (s, 1H, Ar–H), 10.03 (s, 1H, N–H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 43.97, 53.26, 57.28, 61.55, 102.24, 103.17, 111.79, 111.97, 116.17, 118.40, 120.97, 123.33, 124.64, 126.97, 127.65, 145.24, 145.71, 147.41, 150.15, 156.34, 158.56, 168.44. For C24H23N3O5, calculated: 66.50% C, 5.35% H, 9.69% N; found: 66.54% C, 5.32% H, 9.72% N. MS [M + 1]+: m/z 434.
Antiplatelet activity
Platelet aggregation was measured with the APACT aggregometer with software APACT professional version 1.1. (Automated Platelet Aggregation and Coagulation Tracer, Biochemica GmbH, Flacht, Germany) according to the turbidimetric method described by Born et al.Citation35. Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were obtained at the Blood Center of the Gazi University Hospital, Ankara. For collagen-induced aggregation studies, PRP and PPP were prepared from the venous blood from healthy volunteers who had not taken any drugs with antiplatelet activity for 15 d. In brief, freshly drawn venous human citrated blood (3.2% sodium citrate, 9:1) was centrifuged at 800 rpm and then at 3500 rpm for 10 min to obtain PRP and PPP, respectively. Platelet numbers were determined with a cell counter (Swelab Alfa, × Boule Medical AB, Stockholm, Sweden) and adjusted to 3.6 × 108 platelets/ml with PPP. The test compound (or the reference inhibitor) is dissolved in dimethylsulfoxide (DMSO). To eliminate solvent effects, the final concentration of DMSO was fixed at <1%. 199 µl sample of PRP was placed in the cuvette of aggregometer and incubated for 5 min at 37 °C with 0.5 µl of test compound (or reference inhibitor, or DMSO) before addition of 10 µl inducer (arachidonic acid, AA, 350 µM collagen, 5 µg/ml final concentration). The percentage of aggregation is determined as the ratio of heights of the aggregation curves with and without the test compound. Each curve is corrected automatically for the light absorption of PPP of the same donor. All experiments were performed in triplicate. Inhibition of platelet aggregation was expressed as percentage of inhibition using the following equation:
AChE inhibition
All compounds were subjected to a slightly modified method of Ellman’s testCitation36 in order to evaluate their potency to inhibit the AChE. The spectrophotometric method is based on the reaction of released thiocholine to give a colored product with a chromogenic reagent 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB). AChE (E.C.3.1.1.7 from Electric Eel, 500 units) and donepezil hydrochloride were purchased from Sigma Aldrich (Steinheim, Germany). Potassium dihydrogen phosphate, DTNB, potassium hydroxide, sodium hydrogen carbonate, gelatine and acetylthiocholine iodide (ATC) were obtained from Fluka (Buchs, Switzerland). Spectrophotometric measurements were performed on a 1700 Shimadzu UV-1700 UV–Vis spectrophotometer. Cholinesterase activity of the compounds (1–30) was measured in 100 mM phosphate buffer (pH 8.0) at 25 °C, using ATC as substrates, respectively. DTNB (10 mM) was used in order to observe absorbance changes at 412 nm. Donepezil hydrochloride was used as a positive controlCitation37. Enzyme solutions were prepared in gelatin solution (1%), at a concentration of 2.5 units/ml. AChE and compound solution (50 µL) which is prepared in 2% DMSO at a concentration range of 0.001–100 µM were added to 3.0 ml phosphate buffer (pH 8 ± 0.1) and incubated at 25 °C for 5 min. The reaction was started by adding DTNB (50 µL) and ATC (10 µL) to the enzyme–inhibitor mixture. The production of the yellow anion was recorded for 10 min at 412 nm. As a control, an identical solution of the enzyme without the inhibitor is processed following the same protocol. The blank reading contained 3.0 ml buffer, 50 μL 2% DMSO, 50 μL DTNB and 10 μL substrate. All processes were assayed in triplicate. The inhibition rate (%) was calculated by the following equation:
where AI is the absorbance in the presence of the inhibitor, AC is the absorbance of the control and AB is the absorbance of blank reading. Both of the values are corrected with blank-reading value. SPSS for Windows 15.0 was used for statistical analysis. Data were expressed as mean ± SD.
Results and discussion
Chemistry
The synthesis of the N-(2-methoxy-3-dibenzofuranyl)-2-(4-substituted piperazin-1-yl)acetamide derivatives (2a-m) were carried out as shown in Scheme 1. The starting material 2-methoxy-3-aminodibenzofuran was first acetylated with chloroacetyl chloride in tetrahidrofuran and trietilamin. The obtained intermediate product N-(2-methoxy-3-dibenzofuranyl)-2-chloroacetamide (1) was then refluxed with piperazine derivatives in acetone with the presence of potassium carbonate to give final compounds (2a-m). The structures of the obtained compounds were elucidated using spectral data. In the IR spectra, the characteristic N–H bands and amide carbonyl functions were observed at 3329–3358 cm−1. In the NMR spectra, protons of piperazine ring were seen as broad singlets at about 2.42–3.86 ppm range, changing different substituents bonded to the ring. For all compounds, peaks at about 3.19–3.30 ppm, 3.99–4.39 ppm and 10.03–10.13 ppm were seen belonging to CH2CO, OCH3 and NH protons, respectively. M + 1 peaks in MS spectra were in agreement with the calculated molecular weight of the title compounds (2a-m) and elemental analysis results for C, H and N elements were satisfactory with calculated values of the compounds. According to HPLC analysis, purity ratio was found greater than 95% for all compounds. Peak purity index of all compounds were also checked and no impurity was determined. Some of the results were given as supplementary material.
Biology
The antiplatelet activity of the N-(2-methoxy-3-dibenzofuranyl)-2-(4-substituted piperazin-1-yl)acetamide derivatives (2a-m) was studied according to Born test. The arachidonic acid or collagen were used as inducer of platelet aggregation on citrated platelet rich human plasma and aspirin was used as standard drug. The synthesized 13 compounds were studied at 100 µM concentration as initial dose and the inhibition percentages were shown in . Compound 2m bearing 2-furoyl moiety exhibited the highest inhibition percentage of 100% which is nearly about standard drug’s inhibition value (100%). The other compounds (2a-l) displayed inhibition values below 10%. IC50 was calculated only for compound 2 m as 26.1 µM, whereas the IC50 was calculated as 3.9 µM for standard drug aspirin (). The inhibitor effect of the compounds on the collagen-induced aggregation was observed insuffiecient which was found less than 8.9%. The final compounds possess an acetamide bearing 4-substituted piperazine skeleton which is also present in drug named ranolazine that is used to treat a cardiovascular disease, chronic angina. In a recent study, to develop the activity potential, diazabicyclic ranolazine analogs were also investigated and they have been determined with higher activityCitation38. Besides the final compounds differ from each other with the aromatic structure bonded to the fourth position piperazine ring. The high activity of compound 2 m shows that heterocyclic aromatic structure connected to piperazine is important for activity of the N-(2-methoxy-3-dibenzofuranyl)-2-(4-substituted piperazin-1-yl)acetamide derivatives. But same high activity was not seen for the compounds 2j with 2-pyridyl, 2k with 2-pyrimidyl and 2 l with benzyl substitutions. At this point, it can be claimed that distance with a carboyl function or any other groups between piperazine and heterocyclic or any aromatic ring is important for the antiplatelet activity of the studied compounds. This is an interesting finding for further studies.
Table 1. Inhibitory data (100 µM) for compounds 2a-m against arachidonic acid (AA, 350 µM) and collogen (5 µg/ml) induced platelet aggregation.
Table 2. IC50 (µM) of the compound 2m on 350 µM AA-induced platelet aggregation.
The anticholinesterase activities were also determined by slightly modified Ellman’s assay. All final compounds (2a-m) were tested for their inhibition potency against AChE (). Among these compounds, compound 2a with 4-methyl substitution at the fourth position of piperazine ring and compound 2i with 4-nitro phenyl substitution were found as the most active compounds. The inhibition percentages were calculated 42.0 and 41.12% at 1 and 0.1 mM concentrations for compound 2a and the inhibition percentages were calculated 41.35 and 30.22% at 1 and 0.1 mM concentrations for compound 2i. The IC50 values could not be defined none of all compounds. The inhibition percentages were not determined for compounds 2 h and 2m and these compounds were evaluated as inactive at two tested concentrations. Compounds 2e bearing 4-methyl phenyl moiety and compound 2k bearing 2-pyrimidyl moiety exhibited anticholinesterase activity with nearly 40% inhibition value at 1 mM concentration, but both of them did not exhibited similar inhibition value at 0.1 mM which were found below 4%. The other compounds showed relatively weak activity and the inhibition values were found less than 27.37%. Standard drug Donepezil was studied at lower concentrations for the purpose of finding IC50 value and it was determined as 0.054 µM. None of the compounds showed comparable activity with Donepezil and significant anticholinesterase activity contrary to expectations.
Table 3. % AChE inhibition of the compounds and IC50 values.
Conclusion
Thirteen new N-(2-methoxy-3-dibenzofuranyl)-2-(4-substituted piperazin-1-yl)acetamide derivatives were synthesized and evaluated for their antiplatelet and anticholinesterase activity. Compound 2m bearing furoyl moiety was determined as a strong antiplatelet active molecule as inhibitors of AA-induced platelet aggregation. Inversely, none of the compounds showed sufficient acetylcholinesterase inhibitory activity.
Supplementary material available online
IENZ_1108971_Supp.zip
Download Zip (514.7 KB)Declaration of interest
The authors report no conflicts of interest.
References
- McNicol A, Israels SJ. Platelets and anti-platelet therapy. J Pharmacol Sci 2003;93:381–96
- Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes. Part I. N Engl J Med 1992;326:242–50
- Braunwald E, Angiolillo D, Bates E, et al. Antiplatelet therapy and platelet function testing. Clin Cardiol (Suppl. 1) 2008;31:I36
- Youssef KM, Al-Omar MA, El-Subbagh HI, et al. Synthesis, antiplatelet aggregation activity, and molecular modeling study of novel substituted-piperazine analogues. Med Chem Res 2011;20:898–911
- Nacak Baytas S, Inceler N, Ozkan Y, et al. Novel 1,3-diarylpyrazole acrylamides: synthesis, antiplatelet activity screening, and in silico evaluation studies. Med Chem Res 2013;22:5922–33
- Weksler BB. Antiplatelet agents in stroke prevention. Combination therapy: present and future. Cerebrovasc Dis 2000;10:41–8
- Mills DC. ADP receptors on platelets. Thromb Haemost 1996;76:835–56
- Kunapuli SP. Multiple P2 receptor subtypes on platelets: a new interpretation of their function. Trends Pharmacol Sci 1998;19:391–4
- Gachet C. ADP receptors of platelets and their inhibition. Thromb Haemost 2001;8:222–32
- Van De Graaff E, Steinhubl SR. Antiplatelet medications and their indications in preventing and treating coronary thrombosis. Ann Med 2000;32:561–71
- Patrono C, Coller B, Dalen JE, et al. Platelet-active drugs: the relationships among dose, effectiveness, and side effects. Chest 2001;119:39S–63S
- Dogne J-M, de Leval X, Benoit P, et al. Recent advances in antiplatelet agents. Curr Med Chem 2001;9:577–89
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Br Med J 2002;324:71–86
- Storey F. The P2Y12 receptor as a therapeutic target in cardiovascular disease. Platelets 2001;12:197–209
- Berger PB. The thienopyridines in coronary artery disease. Curr Cardiol Rep 1999;1:192–8
- McKee SA, Sane DC, Deliargyris EN. Aspirin resistance in cardiovascular disease: a review of prevalence, mechanisms, and clinical significance. Thromb Haemost 2002;88:711–5
- Bennett JS. Novel platelet inhibitors. Annu Rev Med 2001;52:161–84
- Schros K. Antiplatelet drugs: a comparative review. Drugs 1995;50:7–28
- Hirano T, Yamori Y, Kanai N, et al. The effects of beraprost Na, a stable prostacyclin analog, on animal models of stroke. Mol Chem Neuropathol 1992;17:91–102
- Murai T, Muraoka K, Saga K, et al. Effect of beraprost sodium on peripheral circulation insufficiency in rats and rabbits. Arzneimittelforschung 1989;39:856–9
- Nishio S, Nagase H, Kanou K, et al. Research and development of beraprost sodium, a new stable PGI2 analogue. Zasshi Yakugaku 1997;117:509–21
- Wu TS, Liou MJ, Kuoh CS, et al. Cytotoxic and antiplatelet aggregation principles from Aglaia elliptifolia. J Nat Prod 1997;60:606–8
- Verghese J, Liang A, Sidhu PS, et al. First steps in the direction of synthetic, allosteric, direct inhibitors of thrombin and factor Xa. Bioorg Med Chem Lett 2009;19:4126–9
- Sidhu PS, Liang A, Mehta AY, et al. Rational design of potent, small, synthetic allosteric inhibitors of thrombin. J Med Chem 2011;54:5522–31
- Abdel Aziz MH, Sidhu PS, Liang A, et al. Designing allosteric regulators of thrombin. Monosulfated benzofuran dimers selectively interact with arg173 of exosite II to induce inhibition. J Med Chem 2011;55:6888–97
- Sidhu PS, Mosier PS, Desai UR. On scaffold hopping: challenges in the discovery of sulfated small molecules as mimetics of glycosaminoglycans. Bioorg Med Chem Lett 2013;23:355–9
- Sidhu PS, Hamdy MH, Sarkar A, et al. Designing allosteric regulators of thrombin. Exosite 2 features multiple sub-sites that can be targeted by sulfated small molecules for inducing inhibition. J Med Chem 2013;56:5059–70
- Thalji RK, Aiyar N, Davenport EA, et al. Benzofuran-substituted urea derivatives as novel P2Y(1) receptor antagonists. Bioorg Med Chem Lett 2010;20:4104–7
- Kikumoto R, Tamao Y, Ohkubo K, et al. Crystal structure of two new biofunctional nonsubstrate type thrombin inhibitors complexed with human α-thrombin. J Med Chem 1980;23:1293–9
- Wang TC, Chen IL, Kuo DH, Liao CH. Synthesis and cytoxic and antiplatelet activities of dibenzofuran and carbazole-substituted oximes. Helvetica Chim Acta 2004;87:983–90
- Sadashiva CT, Narendra Sharath Chandra JN, Ponnappa KC, et al. Synthesis and efficacy of 1-[bis(4-fluorophenyl)-methyl]piperazine derivatives for acetylcholinesterase inhibition, as a stimulant of central cholinergic neurotransmission in Alzheimer's disease. Bioorg Med Chem Lett 2006;16:932–6
- Yurttaş L, Özkay Y, Kaplancıklı ZA. Design, synthesis and evaluation of new thiazole-piperazines as acetylcholinesterase inhibitors. J Enzyme Inhib Med Chem 2013;28:1040–7
- Mohammadi-Farani A, Ahmadi A, Nadri H, Aliabadi A. Synthesis, docking and acetylcholinesterase inhibitory assessment of 2-(2-(4-benzylpiperazin-1-yl)ethyl)isoindoline-1,3-dione derivatives with potential anti-Alzheimer effects. DARU J Pharm Sci 2013;21:47
- Darias V, Bravo L, Tabares de Nava B, Fraile C. Study of the antiaggregating activity of new phenacyl-piperazine derivatives. Farmaco Sci 1986;41:684–8
- Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962;194:927–9
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95
- Perry NSL, Houghton PJ, Theobald AE, et al. In-vitro inhibition of human erythrocyte acetylcholine esterase by Salvia lavandulaefolia essential oil and constituent terpenes. J Pharm Pharmacol 2000;52:895–902
- Lopez-Ortiz M, Monsalvo I, Demare P, et al. Synthesis of ranolazine derivatives containing the (1S,4S)-2,5-diazabicyclo[2.2.1]heptane moiety and their evaluation as vasodilating agents. Chem Biol Drug Des 2014;83:710–20