Abstract
This study aimed to investigate the antidiabetic, antilipidaemic and antioxidant activities of Citrus medica cv Diamante (Rutaceae) hydroalcoholic (CD) peel extract in Zucker diabetic fatty (ZDF) rats. The ability of CD to protect against oxidative stress was investigated by using different in vitro assays and in vivo by using the reactive oxygen metabolites-derived compounds (d-ROMs) test and the biological antioxidant potential test (BAP). Two different doses of CD extract (300 and 600 mg/kg/die) were administered at ZDF rats for 4 weeks. CD reduced cholesterol and triglycerides levels. A dose-dependent effect on body weight and serum glucose levels was observed. A decrease of d-ROMs and an increase of BAP were recorded by using the dose of 600 mg/kg. The extract inhibited lipid peroxidation (IC50 value of 0.23 mg/ml). These findings suggest as an efficient phytotherapeutic approach in combating hyperlipidaemic and hyperglycaemic disorders.
Introduction
A great number of epidemiological studies have shown that consumption of fruits from Citrus genus is protective in a variety of human diseases such as cardiovascular disease, cancer and metabolic syndrome. Citrus fruits are a rich source of nutrients and other bioactive components such as polyphenols which are known to be active in the prevention of diseases.
Citrus medica L. cv Diamante (CD) is the most diffused cultivar of C. medica in Italy and in particular in Calabria, Southern Italy. The ripe fruits are large, with a thin, smooth and lemon-yellow peel and the pulp is poor in juice. The most important part of the Diamante citron is the peel which is important in international trade. Peel is used for the preparation of canned products, liquors, ice cream, jams, etc.Citation1 In our previous studies, we have investigated the flowers, leaves, peel and fruits (endocarp and mesocarp) of CD for its chemical profile and we have demonstrated its promising hypoglycaemic and antioxidant activities. The influence on glucose (GLU) homeostasis and metabolic parameters of the peel extract was also investigatedCitation2,Citation3.
The metabolic syndrome is a cluster of the most dangerous cardiovascular risk factors: diabetes and raised fasting plasma GLU, abdominal obesity, high cholesterol (CHOL) and high blood pressure. In addition, people with metabolic syndrome have a five-fold greater risk of developing type 2 diabetesCitation4. International Diabetes Federation estimates that this number rises up to 552 million by 2030Citation5,Citation6. The most common form of diabetes is type 2 diabetes caused by a combination of factors, genetic susceptibility and environmental factors. Some complications, including elevated triglyceride (TG) levels, elevated small low-density lipoprotein particles, decreased high-density lipoprotein CHOL and increased mortality risk from cardiovascular diseases are associated to type 2 diabetes.
World Health Organization (WHO) has suggested the evaluation of natural products from traditional medicinal use for the treatment of diabetes as they are effective, non-toxic and with less or no side effects. The antihyperglycaemic activity of the plants is mainly due to their ability to restore the function of pancreatic tissues by causing an increase in insulin output or inhibiting the intestinal absorption of GLU or to the facilitation of metabolites in insulin-dependent processes. Oxidative stress is involved in the development and progression of diabetes-associated complications. In hyperglycaemic condition, continuous generation of reactive oxygen species (ROS) occurs and some evidence revealed diabetes-induced changes in the activities of antioxidant enzymes in various tissues. Antioxidants are important in scavenging free radicals and in protecting the human body from oxidative stress. Hence, drugs with both antioxidant effects and antidiabetic activity may be useful for the treatment of diabetes and its related complications. Antidiabetic effects were demonstrated for flavonoids, terpenoids and alkaloidsCitation7–9. However, some plant extracts showed a good hypoglycaemic activity suggesting a crucial role of synergistic effects of individual compounds.
We hypothesised that dietary supplementation with CD peel extract, one of the parts more consumed of this fruit, might be beneficial for ameliorating the metabolic syndrome occurring during non-insulin-dependent diabetes mellitus. This hypothesis was tested by using Zucker diabetic fatty (ZDF) rats (a genetically obese animal model of non-insulin-dependent diabetes mellitus) as an animal model. The evolution of diabetes in male leptin receptor-deficient ZDF rats (ZDF/CrlCrlj) has become a popular model for pre-clinical studies of type 2 diabetes, due to the fact that these rats exhibit disrupted islet architecture, β-cell degranulation and increased β-cell deathCitation10,Citation11. Therefore, ZDF male rats were used as a rodent model of type 2 diabetes in the present study. Herein, CD peel extract was tested for efficacy on biomarkers of metabolic syndrome in 4-week-old Zucker rats. Moreover, its antioxidant properties were investigated by different in vitro (2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), ferric reducing ability power (FRAP) and β-carotene bleaching) tests and in vivo by using reactive oxygen metabolites-derived (d-ROMs) and biological antioxidant potential (BAP) assays.
Materials and methods
Materials
Ethanol, FeSO4, FeCl2, FeCl3, chloroform, sodium carbonate, potassium persulphate, perchloric acid and HCl were obtained from VWR International s.r.l. (Milan, Italy). Tripyridyltriazine (TPTZ), ABTS solution, β-carotene, DPPH, linoleic acid, Tween-20, ferrozine, ethylenediaminetetraacetic acid (EDTA), ascorbic acid, propyl gallate, and butylated hydroxytoluene (BHT), were purchased from Sigma-Aldrich S.p.a. (Milan, Italy). All the reagents used in the study were of analytical grade. Reagents from Spinreact (Girona, Spain) were used for the determination of TG, CHOL and GLU. d-ROMs and BAP were performed by reagents from Diacron International s.r.l. (Grosseto, Italy).
Sample preparation
CD fruits were collected in September 2012 in Calabria (Italy), locality Santa Maria del Cedro (latitude 39°44'49″92 N, longitude 15°50'13″92 E and altitude 110 m over the sea level) (). The authentication was carried out at the Natural History Museum of Calabria and Botanic Garden, University of Calabria, Italy. Fruits were collected at maturity stage as used by the food industry for its production. Fruits were examined for integrity and absence of dust and insect contamination and peeled. The peels were extracted by maceration with aqueous ethanol (70%) (4 × 2 l). The obtained solutions were concentrated to obtain a dry extract with a yield of 4.8% w/w.
Chemical profile
The analysis of CD peel extract was performed as previously reportedCitation3. Naringenin, naringin, hesperetin, hesperidin, rutin, nobiletin, tangeretin, quercetin, diosmin and apigenin were selected as standards and quantified. Briefly, high performance liquid chromatography (HPLC) analyses were carried out using an HPLC system HP 1100 equipped with a UV-vis detector (280 nm), a Luna C18 RP column (5 μm, 250 × 4.60 mm) (Phenomenex, Torrance, CA), and using a mobile phase H2O/formic acid (0.1%) (A) and methanol (B). A flow rate of 1 ml/min (2 min 100% A; 8 min 80% A; 55 min 100% B; 65 min 100% A) was applied.
Animals
Eighteen male ZDF rats (5-week old) were obtained from Harlan S.r.l. (Udine, Italy). Animals were fed a standard diet (Harlan Global Diet, 2018) and had free access to drinking water throughout the study. The animal experiments were processed following the internationally accepted ethical guidelines for the care of laboratory animals (DM42/2004-A; Approval No. 78/2013-A, date 25 March 2013). Rats were housed in a temperature- and humidity-controlled facility on a 12-h-light:12-h-dark cycle.
Treatment groups
After acclimation (2 weeks from arrival), rats were randomly divided into three groups and were treated with two different doses of CD peel extract for 30 d. A control group was included in the study. For each group, six animals were used.
Group Control received only vehicle (distilled water).
Group 300: CD peel extract was given at a dose of 300 mg/kg;
Group 600: CD peel extract was given at a dose of 600 mg/kg.
The extract was diluted with distilled water in order to administer a single oral solution dose (0.25 ml/day) via gavage tube. The doses of CD extract were chosen according to a previous studyCitation3. The drinking water was changed daily at 09:00. We measured body weights, water and food intakes of rats daily. At the end of the fourth week dietary supplementation, rats were killed by decapitation and blood samples were obtained.
Biochemical analysis
At the end of the experimental period, the ZDF rats were anesthetised with thiopenthal sodium (120 mg/kg IP) after withholding food for 12 h. After decapitation, blood was collected, put in plastic tubes and centrifuged at 1400 × g for 15 min at 4 °C. Serum was stored at −70 °C and later assayed for GLU, CHOL and TGCitation12.
DPPH radical scavenging activity assay
The DPPH radical scavenging activity was determined as previously reportedCitation13. A mixture of 1.5 ml of 0.25 mm DPPH solution in ethanol and 12 μl of CD extract at different concentrations was prepared and allowed to reach a steady state at 25 °C for 30 min. The bleaching of DPPH was measured at 517 nm with UV-vis Jenway 6003 spectrophotometer (Milan, Italy). Ascorbic acid was used as positive control. The DPPH radicals scavenging activity was calculated according to the following equation: = [(A0 − A1/A0) × 100].
ABTS radical scavenging activity assay
This assay was based on the method previously describedCitation13. ABTS radical cation (ABTS+) was produced by the reaction of a 7 mm ABTS solution with 2.45 mm potassium persulphate. The mixture was stored in the dark at room temperature for 12 h before use. The ABTS+ solution was diluted with ethanol to an absorbance of 0.70 ± 0.05 at 734 nm. The scavenging ability of the extract was calculated according to the following equation: ABTS scavenging activity (%) = [(A0–A)/A0] × 100, where A0 is the absorbance of the control reaction and A is the absorbance in the presence of the sample. Ascorbic acid was used as positive control.
β-carotene bleaching test
The β-carotene bleaching test was performed as previously describedCitation13. A mixture of 1 ml of β-carotene solution (0.2 mg/ml), 0.02 ml of linoleic acid and 0.2 ml of 100% Tween-20 was prepared. After evaporation of solvent and dilution with water, 5 ml of the emulsion were added into tubes containing 0.2 ml of CD at different concentrations. The tubes were placed at 45 °C in a water bath for 60 min. The measurement (at 470 nm) was carried out at an initial time (t = 0) and after 60 min. The antioxidant activity was measured by using the following equation: [1 − (A0 − At)/(A°0 − A°t) × 100, where A0 and A°0 are the absorbance values measured at the initial incubation time for samples/standard and control, respectively, while At and A°t are the absorbance values measure in the samples/standard and control respectively at t = 60 min. Propyl gallate was used as positive control.
Ferric reducing ability power (FRAP) assay
The FRAP method measures the absorption change that appears when the TPTZ (2,4,6-tripyridyl-s-triazine)-Fe3+ complex is reduced to the TPTZ-Fe2+ form in the presence of antioxidant compoundsCitation14. Briefly, the FRAP reagent contained 2.5 ml of 10 mm TPTZ solution in 40 mm HCl plus 2.5 ml of 20 mm FeCl3 and 25 ml of 0.3 M acetate buffer (pH 3.6) was freshly prepared. CD extract was dissolved at a concentration of 1 μg/ml. An aliquot of 0.2 ml of solution was mixed with 1.8 ml of FRAP reagent and the absorption of the reaction mixture was measured at 595 nm. Solutions of known Fe (II) concentration, in the range of 50–500 μm (FeSO4), were used for obtaining the calibration curve. The FRAP value represents the ratio between the slope of the linear plot for reducing Fe3+-TPTZ reagent by CD extract compared to the slope of the plot for FeSO4. BHT was used as positive control.
Assessment of oxidative stress (d-ROMs and BAP tests)
Oxidative stress assessment was performed by means of an integrated analytical system composed of a photometer (work conditions: wavelength 505 or 546 nm, optical path 1 cm, temperature 37 °C, kinetic or endpoint mode) and a mini-centrifuge (FRAS4, H&D s.r.l., Parma, Italy). Samples of whole blood were centrifuged at 6000 × g for 5 min and 10 μl of plasma tested for total oxidant capacity and for the biological antioxidant potential (BAP) by using the d-ROMs and BAP test kits according to manufacturer instructions (Diacron International s.r.l., Grosseto, Italy).
Briefly, for d-ROMs assay, plasma was diluted in an acidic buffered solution (pH 4.8). A compound (chromogen) that have the ability to change its colour when is oxidised by hydroperoxyl and alkoxyl radicals is then added to this solution. The chromogenic substrate used in the d-ROMs test was N, N,-diethyl paraphenylenediamine, whose quantity was assessed by means of the photometer. Results of the d-ROMs test are expressed as Carratelli Units (CARR U), where 1 U CARR corresponds to 0.08 mg/100 ml H2O2. For BAP assay, plasma was dissolved in the coloured solution, previously prepared by mixing a FeCl3 reagent with a thiocyanate derivative reagent. After 5 min of incubation at 37 °C, the solution underwent photometric readingCitation15.
Statistical analysis
Results are expressed as means ± SD. Differences among the groups were analysed by one-way ANOVA. All statistical analyses were performed using the SAS software (SAS Institute Inc., Cary, NC). p < 0.05 was considered significant.
Results
The HPLC analysis of CD peel extract revealed the presence of apigenin (62.8 mg/kg fresh weight), hesperetin (50.4 mg/kg fresh weight) as main flavonoids. Naringin and quercetin were also identified with a content of 18.6 and 18.2 mg/kg of fresh weight. Among selected markers, nobiletin and rutin are present in trace. The comparison of these results (fruits collected in September) with those obtained in our previous studyCitation3 (fruits collected in October) shows that at the maturity stage the flavonoids content (referred to these markers) does not undergo significant changes.
Water intake over the 4-week period of the study did not differ among ZDF rats supplemented with CD peel extract and the control group. Similarly, feed intake decreased after 7 d of extract treatment until the end of the experiment but no significant difference was detected (data not shown). Body weights differed among the three groups of ZDF rats. The net gain in body weight during the experimental period was lower (p < 0.05) in ZDF rats supplemented with CD peel extract compared with the control group ().
Table 1. Body weights recorded weekly in rats supplemented with C. Medica cv Diamante extract (300 and 600 mg/kg).
A significant difference between weight gain was detected in relation with the dose of CD administered thus suggesting a dose-dependent effect. Levels of GLU, CHOL and TGs are reported in . The 600 mg/kg CD supplementation resulted in a significant lowering of blood GLU levels compared to those detected in the control group. The serum TG and total CHOL concentration were also significantly lower in both CD supplemented groups compared to the control group.
Table 2. Levels of glucose, cholesterol and triglycerides in rats supplemented with C. medica cv Diamante extract (300 and 600 mg/kg) for 4 weeks.
Additionally, a significant difference between CHOL and TG concentration was also detected between 300 and 600 mg/kg groups.
The current study demonstrates that CD peel extract is an effective hypolipidaemic and hypoglycaemic agent in db/db rat, obese-diabetic animals with insulin resistance. Insulin resistance profoundly contributes to the pathophysiology of type-2 diabetes and induces reduced GLU utilisation and increased GLU production in the liver, leading to hyperglycaemiaCitation16. Insulin resistance, in both human and animal models, is commonly associated with several abnormalities in the lipid metabolism, including increased plasma free fatty acid levels, hypertriglyceridaemia, hypercholesterolaemia and enhanced lipogenesis in the liver.
Antioxidants may act in vivo through different mechanisms. In evaluating antioxidant activity, the source of ROS and the target substrate must be considered. Therefore, choosing an adequate assay is critical and in practise, several assays were developedCitation17. In this work, we have evaluated in vitro the radical scavenging activity by using DPPH and ABTS test and the ability of peel extract to protect linoleic acid by oxidation and reduce iron (FRAP assay). This ion is one of the most common oxidative initiators in both biological and food systems.
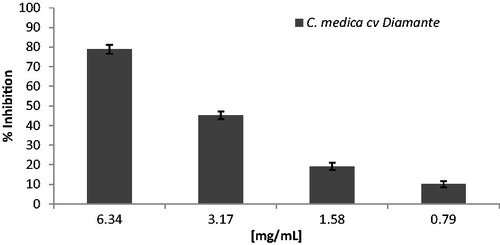
CD peel extract was investigated in vivo by using d-ROM’s and BAP tests. The d-ROMs test provides a measure of the whole oxidant capacity of plasma against the N, N-diethyl paraphenylenediamine in acidic buffer. Such oxidant capacity is mainly due to hydroperoxides, with the contribution of other minor oxidant factors. The BAP test measures the reduction of Fe3+ to Fe2+, as it occurs by adding a reducing/antioxidant system, in such a way, it allows an effective measurement of reducing ability or antioxidant potential of tested blood plasmaCitation18. CD peel extract inhibited both DPPH and ABTS radical in a concentration-dependent manner () with IC50 values of 0.81 and 3.48 mg/ml ().
Table 3. In vitro radical scavenging and antioxidant capacities of C. medica cv Diamante extract.
In the β-carotene bleaching test, a model system made of β-carotene and linoleic acid undergoes a discolouration in the absence of an antioxidant. The free linoleic acid radical formed upon the abstraction of a hydrogen atom from one of its methylene groups attacked the β-carotene molecules, which lost the double bonds and therefore, its characteristic orange colour. In this test, CD peel extract exhibited a better activity (IC50 value of 0.23 mg/ml) than those found by using the radical scavenging assays. This difference is probably the consequence of a higher specificity of the assay for lipophilic compounds. These properties are also very much needed by the food industry in order to find possible alternatives to synthetic preservatives such as BHT. The FRAP test evaluate the capacity of CD to donate an electron to Fe (III) resulting in the reducing of Fe3+/ferricyanide complex to Fe2+ complex. In this assay, extract exhibited a limited ability with FRAP value of 3.88 μM Fe(II)/g at 1 mg/ml.
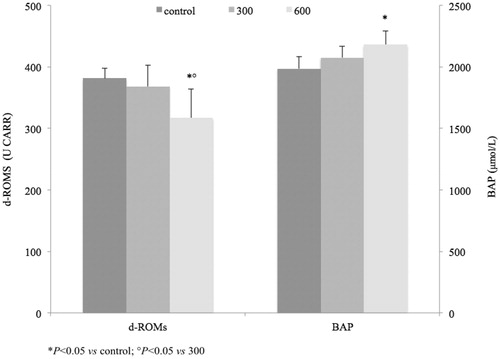
CD exerted an antioxidant activity at the dose of 600 mg/kg in both d-ROMs and BAP tests (). A significant decrease of d-ROMs was detected for group 600 versus control as well as versus group 300. No differences were seen for group 300 versus control. Accordingly, significantly higher levels of BAP were detected in group 600, thus showing an improvement of the anti-oxidant barrier after CD supplementation. Therefore, the lower d-ROMs level in group B is probably due to an improvement of the BAP, as showed by the increase of BAP level. Such a BAP is attributed to the major component of plasma barrier to oxidation (vitamin C, vitamin E, uric acid, bilirubin and so on)Citation19. The d-ROMs and BAP tests were proven useful in patients suffering from oxidative stress-related diseasesCitation20.
Discussion
Citrus peels are largely used in many traditional medicines. However, the systematic in-depth investigation of their healthy properties embarks the last decades, when their flavonoid profiles were recognised. Citrus flavonoids showed several bioactivities, including antioxidant, anti-inflammatory, neuroprotective, hypolipidaemia, regulation of metabolic syndrome and antitumor activityCitation21–23.
In this study, the CD peel extract was investigated for its potential antidiabetic, antilipidaemic and antioxidant activities. Peel extract administration (600 mg/kg) significantly decreased the serum GLU compared to that in the control rat. In a previous study on normal mice, we showed that CD was able to reduce serum GLU and we hypothesised that such effect was due to an inhibitory activity against both α-amylase and α-glycosidase. Moreover, a direct stimulatory effect on the exocytotic release of insulin on the mouse insulinoma MIN6 β-cells was also showedCitation3. The results of the present study showed that CD extract is also effective in insulin-resistant diabetes.
Among identified flavonoids, apigenin and hesperetin were the most abundant followed by quercetin and naringin. Different works investigated the effect of these flavonoids on diabetes and metabolic syndrome. The antihyperglycaemic effect of apigenin was analysed on streptozotocin (STZ)-induced diabetic Wistar ratsCitation24. The administration of this flavonoid at the dose of 4 mg/kg bw/day after 1 week determined the reduction of blood GLU levels and demonstrated an efficient protection on liver and kidney. Moreover, apigenin improved GLU tolerance through inhibition of microRNA maturation in miRNA103 transgenic miceCitation25. Escande et al.Citation26 described the ability of apigenin and quercetin to increase intracellular nicotinamide adenine dinucleotide (NAD+) levels by the inhibition of enzyme CD38. This mechanism protects against diet-induced obesity through silent mating information regulation 2 homolog 1 (SIRT1) activation.
Other studies showed that hesperidin and naringin alter the expression of the genes encoding the regulatory enzymes of glycolysis and gluconeogenesis in the liver of the db/db mice, and significantly upregulate the mRNA level of hepatic glucokinase (GK), a key enzyme in the regulation of GLU utilisation in the liverCitation27. As such, hesperidin and/or naringin contained in the CD peel extract likely improved the hyperglycaemia mainly via the restoration of hepatic GK in the db/db rat. Hepatic GK has a major effect on GLU homeostasis and is a potential target for the pharmacological treatment of type-2 diabetes, as evidenced by the fact that liver-specific GK-knockout mice exhibited mild hyperglycaemiaCitation28. More recently, the administration of hesperidin and naringin (at a dose level of 50 mg/kg bw for 30 d) in high-fat diet/streptozotocin (HFD/STZ) type 2 diabetic rats reduced the level of GLU, glycosylated haemoglobin, aspartate aminotransferase, lactate dehydrogenase and creatine kinase muscle-brain, and ameliorated the lowered serum insulin level and hepatic and muscle glycogen content of insulin resistant. Both flavonoids were also able to improve lipid profile and serum adiponectin and resistin (adipose tissue-specific secretory factor, ADSF) levelsCitation29.
The serum TG concentration was significantly lowered by the Citrus extract also at the dose of 300 mg/kg, which is supported by other findings, where the flavonoid naringenin was found to increase low density lipoprotein (LDL) receptor expression and inhibit apoB secretion in HepG2 cells, possibly explaining the insulin-like effects of naringenin in vivoCitation30. Furthermore, hesperidin and naringin appeared to facilitate TG excretion into the faeces.
The present study revealed that CD administration (300 and 600 mg/kg) lowered not only the TG levels but also the CHOL levels in type-2 diabetic animals.
Jung et al.Citation27 showed that similar results were accompanied by the inhibition of hepatic 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase and CHOL acyltransferases (ACAT) activities, and an increase in the faecal CHOL. Inhibitors of hepatic HMG-CoA reductase, a key enzyme in CHOL synthesis, are well-established drugs for the treatment of hypercholesterolemia and decrease the incidence of dyslipidaemia in diabetic subjectsCitation31. ACAT is another key CHOL-regulating enzyme involved in the esterification and absorption of CHOL, secretion of hepatic LDL-CHOL and CHOL accumulation in the arterial wallCitation32.
Citrus flavonoids have emerged as promising agents for the treatment of metabolic deregulationCitation33. Some epidemiological studies report that intake of Citrus flavonoid-containing foods attenuates cardiovascular diseases. In animal models, Citrus flavonoid supplements prevent hepatic steatosis, dyslipidaemia and insulin sensitivity primarily through inhibition of hepatic fatty acid synthesis and increased fatty acid oxidation. Citrus flavonoids blunt the inflammatory response in metabolically important tissues, including liver, adipose tissue, kidney and the aorta. The mechanisms underlying flavonoid-induced metabolic regulation have not been completely established. In mouse models, Citrus flavonoids show marked suppression of atherogenesis through improved metabolic parameters and also through direct impact on the vessel wall. Previous data showed that Citrus peel extract causes lowering of CHOL levels by modulating hepatic HMG-CoA levelsCitation34. Overall, the scientific evidences obtained with fruits from the Citrus genus provide explanations for the beneficial effects of CD extract in hyperlipidaemic and hyperglycaemic disorders.
Among identified flavonoids, particular attention was addressed to naringin. Its supplementation lowered plasma lipids in different hyperlipidaemia and obesity experimental modelsCitation22. This flavonoid lowered elevated plasma lipid concentrations and decreased plasma lipids and CHOL. The CHOL-lowering effect was related to the reduction of hepatic 3-hydroxy-3-methyl CoA (HMG-CoA) reductase activity, whereas ACAT activity was unaffected.
Several experimental and clinical studies evidenced that oxidative stress plays a role in the pathogenesis of diabetes. In diabetes, ROS are produced by GLU oxidation and degradation of glycated proteins. This condition leads to cell damage, increased lipid peroxidation and development of insulin resistance. For this reason, substances that can act not only as hypoglycaemic agents but also as an antioxidant are extensively searched. Previously, Menichini et al.Citation2 evaluated the in vitro antioxidant activity of CD flowers, leaves and fruits at two maturity stages. Mesocarp of immature fruits showed the most promising DPPH radical scavenging activity with IC50 value of 0.38 mg/ml followed by flowers and leaves extracts (IC50 values of 0.42 and 0.50 mg/ml, respectively). Both mesocarp and endocarp of immature CD fruits were able to inhibit the discolouration of β-carotene with IC50 values of 0.004 and 0.005 mg/ml, respectively at 60 min of incubation). As previously cited CD peel extract is a rich source of flavonoids that showed antioxidant activity by a different mechanism of actionCitation35. Both apigenin and hesperetin exhibited mild antioxidant potency. Quercetin, one of the main abundant identified compounds, is known as potent ROS scavenger and able to empower the endogenous antioxidant shield due to its contribution to the total plasma antioxidant capacity, which is 6.24 times higher than the reference antioxidant troloxCitation36.
The antioxidant activity of C. medica was recently confirmedCitation37. Powder of Citrus was extracted by using ultrasonator and 50% methanol as solvent. C. medica extract exhibited an antioxidant activity with values of 1.48, 0.92, and 0.38 μm Fe(II)/g in DPPH, ABTS and FRAP test, respectively.
Our results confirm such hypothesis suggesting that free radicals investigation may be important for early detection of oxidative stress. Confirming previous in vitro observations, CD peel extract seems to possess, also in vivo, a significant antioxidant activity.
Conclusion
In this study, CD supplement was found to be effective for improving the lipid metabolism in ZDF rats. In addition, at the higher dose, the extract was beneficial for lowering the blood GLU level and for improving oxidative status. Both effects will be probably due to the effects of flavonoids hesperidin and naringin, which are present in the CD peel extract.
Accordingly, CD exhibited antidiabetic properties and were able to reduce certain diabetic complications related to hyperlipidaemia. Further works are required to determine the dose-related changes associated with CD and its possible use as putative antidiabetic agents. To the best of our knowledge, the present study demonstrated for the first time that CD extract showed significant antidiabetic and antioxidant activity.
Declaration of interest
The authors declare that they have no conflict of interest.
References
- Cutuli GD, Martino E, Lo Giudice V, et al. Trattato di agrumicoltura. Bologna, Italy: Edagricole; 1985
- Menichini F, Loizzo MR, Bonesi M, et al. Phytochemical profile, antioxidant, anti-inflammatory and hypoglycemic potential of hydroalcoholic extracts from Citrus medica L. cv Diamante flowers, leaves and fruits at two maturity stages. Food Chem Toxicol 2011;49:1549–55
- Menichini F, Tundis R, Loizzo MR, et al. C. medica cv Diamante peel chemical composition and influence on glucose homeostasis and metabolic parameters. Food Chem 2011;124:1083–9
- Stern MP, Williams K, González-Villalpando C, et al. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care 2004;27:2676–81
- Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2–7 million participants. Lancet 2011;378:31–40
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311–21
- Castellano JM, Guinda A, Delgado T, et al. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes 2013;62:1791–9
- Chen J, Mangelinckx S, Adams A, et al. Natural flavonoids as potential herbal medication for the treatment of diabetes mellitus and its complications. Nat Prod Commun 2015;10:187–200
- Derosa G, Maffioli P. Alkaloids in the nature: pharmacological applications in clinical practice of berberine and mate tea. Curr Top Med Chem 2014;14:200–6
- Clark JB, Palmer CJ, Shaw WN. The diabetic Zucker fatty rat. Proc Soc Exp Biol Med 1983;173:68–75
- Mega C, de Lemos ET, Vala H, et al. Diabetic nephropathy amelioration by a low-dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat). Exp Diabetes Res 2011;2011:162092–103
- Bhasin MK, Chahal SMS. A laboratory manual for human blood analysis. Delhi: Kamal raj Enterprises; 1996
- Loizzo MR, Tundis R, Bonesi M, et al. Radical scavenging, antioxidant and metal chelating activities of Annona cherimola Mill. (cherimoya) peel and pulp in relation to their total phenolic and total flavonoid contents. J Food Comp Anal 2012;25:179–84
- Benzie IF, Strains JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996;239:70–6
- Inchingolo F, Marrelli M, Annibali S, et al. Influence of endodontic treatment on systemic oxidative stress. Int J Med Sci 2013;11:1–6
- McGarry JD. Disordered metabolism in diabetes: have we underemphasized the fat component? J Cell Biochem 1994;55Suppl:29–38
- Antolovich M, Prenzler PD, Patsalides E, et al. Methods for testing antioxidant activity. Analyst 2002;127:183–98
- Pasquini A, Luchetti E, Marchetti V, et al. Analytical performances of d-ROMs test and BAP test in canine plasma. Definition of the normal range in healthy Labrador dogs. Vet Res Commun 2008;32:137–43
- Hetyey CS, Manczur F, Dudás-Györki Z, et al. Plasma antioxidant capacity in dogs with naturally occurring heart diseases. J Vet Med A Physiol Pathol Clin Med 2007;54:36–9
- Palmieri B, Sblendorio V. Oxidative stress tests: overview on reliability and use. Part I. Eur Rev Med Pharmacol Sci 2007;11:309–42
- Gosslau A, Chen KY, Ho CT, Li S. Anti-inflammatory effects of characterized orange peel extracts enriched with bioactive polymethoxyflavones. Food Sci Hum Wellness 2014;3:26–35
- Alam MA, Subhan N, Rahman MM, et al. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr 2014;5:404–17
- Chanet A, Milenkovic D, Manach C, et al. Citrus flavanones: what is their role in cardiovascular protection? J Agric Food Chem 2012;60:8809–22
- Rauter AP, Martins A, Borges C, et al. Antihyperglycaemic and protective effects of flavonoids on streptozotocin-induced diabetic rats. Phytother Res 2010;24:S133–8
- Ohno M, Shibata C, Kishikawa T, et al. The flavonoid apigenin improves glucose tolerance through inhibition of microRNA maturation in miRNA103 transgenic mice. Sci Rep 2013;3:2553–9
- Escande C, Nin V, Price NL, et al. Flavonoid apigenin is an inhibitor of the NAD + ase CD38: implications for cellular NAD + metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 2013;62:1084–93
- Jung UJ, Lee MK, Park YB, et al. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol 2006;38:1134–45
- Postic C, Shiota M, Niswender KD, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem 1999;274:305–15
- Osama MA, Ayman MM, Abdel M et al. Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetol Croat 2012;412:53–67
- Borradaile NM, de Dreu LE, Huff MW. Inhibition of net HepG2 cell apolipoprotein B secretion by the citrus flavonoid naringenin involves activation of phosphatidylinositol 3-kinase, independent of insulin receptor substrate-1 phosphorylation. Diabetes 2003;52:2554–61
- Raz I, Eldor R, Cernea S, Shafrir E. Diabetes: insulin resistance and derangements in lipid metabolism. Cure through intervention in fat transport and storage. Diabetes Metab Res Rev 2005;21:3–14
- Suckling KE, Stange EF. Role of acyl-CoA: cholesterol acyltransferase in cellular cholesterol metabolism. J Lipid Res 1985;26:647–71
- Assini JM, Mulvihill EE, Huff MW. Citrus flavonoids and lipid metabolism. Curr Opin Lipidol 2013;24:34–40
- Bok SH, Lee SH, Park YB, et al. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J Nutr 1999;129:1182–5
- Yu J, Wang L, Walzem RL, et al. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J Agric Food Chem 2005;53:2009–14
- Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 2008;585:325–37
- Zhao P, Duan L, Guo L, et al. Chemical and biological comparison of the fruit extracts of Citrus wilsonii Tanaka and Citrus medica L. Food Chem 2015;173:54–60