Abstract
Carbonic anhydrase (CA) inhibitors have been used for more than 60 years for therapeutic purposes in many diseases table such as in medications against antiglaucoma and as diuretics. Phenolic compounds are a new class of CA inhibitor. In our study, we tested the effects of arachidonoyl dopamine, 2,4,6-trihydroxybenzaldehyde and 3,4-dihydroxy-5-methoxybenzoic acid on esterase and the CO2-hydratase activities of CA I and II isozymes purified from in vivo to ex vivo. The Ki values of arachidonoyl dopamine, 2,4,6-trihydroxybenzaldehyde and 3,4-dihydroxy-5-methoxybenzoic acid were 203.80, 1170.00 and 910.00 μM, respectively for hCA I and 75.25, 354.00 and 1510.00 μM, respectively for hCA II. Additionally, IC50 values from in vivo studies were found to be in the range of 173.25–1360.0 μM for CA I and II, respectively, using CO2-hydratase activity methods. These results demonstrated that phenolic compounds used in in vivo studies could be used in different biomedical applications to inhibit approximately 30% of the CO2-hydratase activity of the total CA enzyme of rat erythrocytes.
Introduction
Carbonic anhydrases (EC 4.2.1.1) as a metalloenzyme family are determined to be encoded from six different independent gene families (α-, β-, γ-, ɛ-, ζ- and n-CA) and are found in archaea, eukaryotic and prokaryotic cellsCitation1,Citation2. Mammals, including humans, generally contain α-CAs. Zn2+ is located in the active site of the enzymeCitation2,Citation3. Until now, 16 different α-CA isoenzymes have been identified in various tissues and organs with different expression levels, kinetic and molecular properties, and oligomeric rearrangements as well as various abilities to respond to different inhibitory classesCitation4,Citation5.
There are 13 catalytic and three acatalytic isoforms of CA-related proteins (CARPs)Citation6. According to the known cellular localisation, these isoenzymes are cytosolic (CA I, II, III, VII V and XIII), membrane-bound (CA IV, IX, XII, XIV and XV), mitochondrial (CA VA and VB) and salivary (CA VI)Citation7. CA XV is not synthesised in humans and other primates and is abundantly found in rodents and other vertebrates as an isoformCitation8. Acatalytic CARP VIII and X appear to be cytosolic proteinsCitation9.
CAs play an important role in the respiratory system and transport of CO2/HCO3− between the lungs and other tissues. The enzyme also maintains the pH and CO2 balance by the catalytic reversible hydrationCitation10 of CO2 to produce H+ and HCO3−:
In addition, they are associated with biosynthetic reactions such as ureagenesis, glycogenesis and lipogenesis, and with other physiological and pathological processes such as tumuorigenicity, bone resorption and calcification in various tissue and organsCitation11,Citation12. Therefore, inhibitors of CAs may be able to be used as therapeutic agents in the treatment and prevention of various diseasesCitation13. For instance, sulphonamides have been used as agents in pharmacological application and diagnostics in many areas such as antiglaucoma, anti-obesity, anticonvulsants and anticancer for more than 60 years in clinical settingsCitation14.
Recently, many studies have shown that phenolic compounds and their derivatives might have a beneficial inhibitory effect on CAs that is versatile and promising. Thus, phenolic compounds have the potential to become a new class of CA inhibitor and may be an alternative for patients who are allergic to sulpha drugs and derivativesCitation15. The zinc ion (Zn2+) in CAs is critical for the inhibition profile of these enzymes. Thus far, some inhibition mechanisms have been proposed for CA isoenzymes. One involves anchoring, the inhibitor to a Zn2+ bound solvent molecule, such as water or hydroxide ion. Phenols and polyamines bind CA isoenzymes in this way. Phenolic compounds are very important in our daily lives. They are added to the content of many drugs and foods that are widely used. Natural and non-natural phenolic compounds have been reported to have anticancer, antimutagenic, antiviral, antibacterial, anti-inflammatory and anticarcinogenic effects in addition to their antioxidant propertiesCitation16.
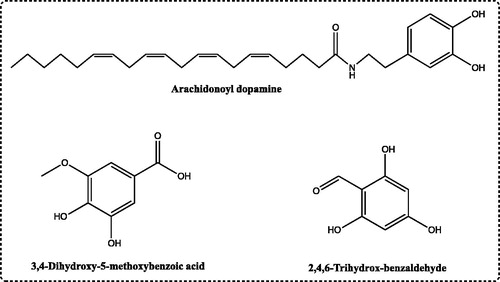
Arachidonoyl dopamine, which is used in this study, an endogenous lipid of central nervous system that provides each bidirectional transition ionotropic of the vanilloid type 1 receptorCitation17. At the same time, it is one of the best activators of cannabinoid and transient receptor potential vanilloid channel receptorsCitation18. Studies have recently demonstrated that hyperalgesia was primarily induced by endovanilloid N-arachidonoyl dopamine (NADA). In addition, environmental endovanilloid causes the contraction of smooth muscle in the guinea-pig bronchus and urinary bladder. Because of the molecular structure, NADA may also indicate the potential properties of phenolic compounds ()Citation19.
Figure 1. The chemical structure of phenolic compounds, including arachidonoyl dopamine, 3,4-dihydroxy-5-methoxybenzoic acid and 2,4,6-trihydroxybenzaldehyde as inhibitors for hCA I and II isozymes.
In recent years, a series of phenolic compounds and a phenolic acid were investigated for their in vitro inhibitory effects on CA I and II isoenzymes isolated from human erythrocytes. The significant inhibitory properties of phenolic substances such as phenolCitation20, antioxidant phenolsCitation21, phenolic acidsCitation22, natural product polyphenols and phenolic acidsCitation23, natural phenolic compoundsCitation24, dimethoxybromophenolCitation25, bromophenolsCitation26,Citation27, catechol, resorcinol, hydroquinoneCitation28, taxifolinCitation29 pyrogallol, quercetin, guaiacol and its phenolic derivativesCitation30, vanillin, caffeic acid phenethyl esterCitation31, curcumin, silymarin, resveratrol, morphine, catechin, dobutamineCitation15 and rosmarinic acidCitation32 of CA I and II isoenzymesCitation16 have been reported.
In our study, we also investigated the in vitro inhibitory effects of arachidonoyl dopamine, 2,4,6-trihydroxybenzaldehyde and 3,4-dihydroxy-5-methoxybenzoic acid on CA I and II isoenzymes from human erythrocytes. In vivo studies were performed for the phenolic compounds (). The results obtained from the study provide important new information concerning phenolic compounds.
Experimental procedures
General information
Ethics committee permission was received for in vitro and in vivo analysis from Experimental Animals Local Ethics Committee and Non-Drug Clinical Applications Local Ethics Committee of Yuzuncu Yıl University.
Chemicals
Arachidonoyl dopamine, 2,4,6-trihydroxybenzaldehyde and 3,4-dihydroxy-5-methoxybenzoic acid were commercially purchased from Sigma-Aldrich (St. Louis, MO). Cyanogen bromide (CNBr)-activated Sepharose 4B, l-tyrosine, sulphanilamide for affinity purification and other chemicals for electrophoresis and all chemicals for enzyme activity measurement were obtained from Merck (Kenilworth, NJ) or Sigma-Aldrich.
Purification of CA isoenzymes from human erythrocytes
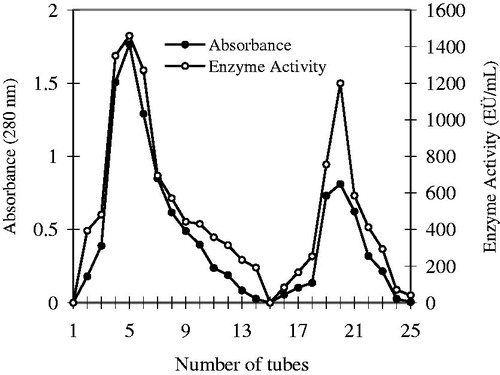
Fresh human blood was received from volunteers at the blood centre of Yuzuncu Yıl University, Faculty of Medicine Research Hospital. Blood samples placed into centrifuge tubes with heparin were centrifuged at 1500× g for 15 min and the overlying plasma and leukocyte layers were removed carefully using a dropper. There after, the samples were washed three times with a 0.9% isotonic NaCl solution. Erythrocytes were haemolysed with ice water until five times of their volumeCitation33. The haemolysate was centrifuged at +4 °C and 10 000× g for 30 min to remove cell membranes and other impurities. The supernatant was carefully removed using a dropper. A Sepharose-4B-L-tyrosine-sulphanilamide affinity column was equilibrated with a solution of 25 mM Tris-HCl/0.1 M Na2SO4 (pH 8.7). The haemolysate was adjusted to pH 8.7 with solid Tris and then they were applied to the column (1.36 × 30 cm, Sigma-Aldrich). The affinity column was then washed with a solution of 25 μM Tris-HCl/22 μM Na2SO4 (pH 8.7). Thus, the CA enzyme was bound to the column, and other impurities were removed. There after, the hCA I isoenzyme was eluted by applying a solution of 1 M NaCl/25 mM Na2HPO4 (pH 6.3). The hCA II isoenzyme was obtained using a solution of NaCH3COO 0.1/0.5 M NaClO4 (pH 5.6) from the column (flow rate: 20 mL h−1, fraction volume: 4 mL). All of the procedures were performed at +4 °CCitation34. The qualitative protein assay was performed at 280 nm in every purification step, spectrophotometrically. CO2 hydratase activity was determined for all of the eluates and the active fractions were collected ()Citation35.
Determination of quantitative proteins
The protein quantity in all of the samples was determined using bovine serum albumin as a standard according to the Bradford methodCitation36 by measuring absorbance at 595 nm as explained previouslyCitation37.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
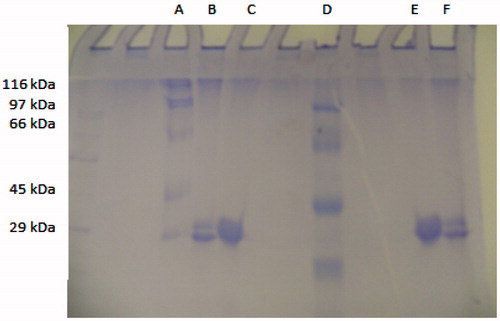
The purities of the isozymes were determined by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) according to Laemmli’s methodCitation34. Thus, samples treated with 1% SDS and 10% 2-mercaptoethanol were incubated in a boiling water bath for 5 min for denaturation. The hCA I and II isoenzyme samples were next applied to B, C, E and F wells in the electrophoresis medium (). The gel was dyed by incubating it for 1.5 h in medium containing 0.1% Coomassie Brilliant Blue R-250, 50% methanol and 10% acetic acid. The resulting electrophoresis bands were visualised ()Citation38,Citation39.
Figure 3. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of purified CA I and II isozymes from human erythrocytes. Lane A and D: standard proteins (kD): β-galactosidase from E. coli (116), phosphorylase B from rabbit muscle (97), bovine serum albumin (66), chicken ovalbumin (45) and carbonic anhydrase (29). Lanes B and F: human carbonic anhydrase I. Lanes C and E: human carbonic anhydrase II.
CO2-hydratase activity
The activity studies were performed according to the Wilbur and Anderson method as modified by Rickly et al.Citation40 CA activity was assayed with changes in the pH as non-enzymatic (to) and enzymatic (tc) reactions. An enzyme unit (EU) was calculated using the equation (to − tc/tc) in this method.
Esterase activity
Esterase activity was measured at 348 nm spectrophotometrically (Shimadzu UV mini-1240, Kyoto, Japan). The measurement was performed for 3 min at room temperature. The substrate was 4-nitrophenylacetate (4-NPA) according to the method defined by Verpoorte et alCitation41. The reaction mix contained 1.4 mL of 0.05 M (pH 7.4) Tris-SO4 buffer, 1 mL of 4-NPA (3 mM), 560 μL of distilled water and 40 μL of the enzyme solution (final volume 3 ml). Other processes are described in our previous studyCitation42,Citation43.
In vitro inhibition studies
Inhibition studies were performed on the esterase activities and CO2-hydratase activities of hCA I and II of arachidonoyl dopamine, 2,4,6-trihydroxybenzaldehyde and 3,4-dihydroxy-5-methoxybenzoic acid. The arachidonoyl dopamine concentrations were changed from 113.7 to 568.75 μM for hCA I and from 113 to 455 μM for hCA II in in vitro inhibitory studies on esterase activity. The concentrations for 2,4,6-trihydroxybenzaldehyde were from 649 to 1947 μM for hCA I and from 32 to 162 μM for hCA II. They were from 815 to 1629 μM for hCA I and from 272 to 1629 μM for hCA II of 3,4-dihydroxy-5-methoxybenzoic acid. The mathematical relationship between the CA activity and inhibitor concentrations was determined using conventional polynomial regression software (Microsoft Office 2007, Excel, Redmond, WA). The IC50 values (the inhibitor concentration that causes up to 50% inhibition) were calculated from graphs. The Ki values and inhibition types of the compounds were determined by Lineweaver–Burk graphicsCitation44.
In vivo inhibition studies
Permission from the ethics committee for the in vivo studies was obtained from Yuzuncu Yıl University Experimental Animals Local Ethics Committee. Forty-eight albino rats test animals of (250 ± 20 g) were selected and separated into eight equal groups (n = 6). 2,4,6-Trihydroxybenzaldehyde and 3,4-dihydroxy-5-methoxybenzoic acid were applied at a dose of 10 mg/kg, and arachidonoyl dopamine was also applied at a dose of 5 mg/kg, intraperitoneally. The blood samples were drawn into the anticoagulant-containing tubes at 1st and 4th hours for each group from the heart under anaesthesia (10 mg/kg ketamine) according to the Gülçin et al.Citation33 Additionally, there were control groups for the 1st and 4th hours. Blood samples were centrifuged at +4 °C and 2500× g for 15 min. Plasma and leucocytes were removed carefully and erythrocytes were haemolysed with ice-waterCitation43. Next, CO2-hydratase activities were assayed colorimetrically according to the Wilbur and Anderson methodCitation40 as described above.
Measurement of haemoglobin (Hb)
The determination of haemoglobin was performed using the cyanmethamoglobin method in haemolysates. This method is based on a spectrophotometric measurement at 540 nm of the cyanomethemoglobin concentration formed after oxidation to Fe3+ from Fe2+ ions. The experimental procedure was conducted according to that in our previous studiesCitation43. The results of the CA activities were given as the EU/gHb for in vivo studies. The time to decrease to half of the enzyme activity (t50) was calculated using % Activity-Time charts.
Statistical analysis
The obtained results were expressed as the mean and standard deviation. Tukey’s range test to compare between groups was used for post-hoc analysis. Student’s t-test was performed for intra-group comparisons with respect to time within the same group. Significance was taken as 5% (p < 0.05) in statistical calculations, and SPSS 15 statistical software package (SPSS Inc., Chicago, IL) was used for calculations.
Results and discussion
Sulphonamides and their derivatives are classic inhibitors of CAsCitation45. Sulphamates and sulphonamides are used in medications associated with different clinical applications such as in anticonvulsant, anti-obesity, anticancer and anti-infectiveCitation46. However, many of these compounds inhibit 16 different isoforms of the CAs at different levels in mammals. Researchers have recently identified new sulphamides and sulphonamides compounds as inhibitors. Presently, variety inhibitors have been discovered, such as coumarins, polyamines and phenols. They have different features from sulphonamides, including interesting inhibition mechanismCitation47. For instance, it has been demonstrated that mammalian isoforms I–XIV of CA are inhibited at low concentrations by ferulic acid, p-hydroxybenzoic acid, p-coumaric acid, gallic acid, quercetin, ellagic acid and syringic acidCitation23. In addition, Innocenti and co-workers have reported that a series of phenolic compounds such as resveratrol, silymarin, dobutamine, curcumin and catechins inhibited the esterase activity of mammalian CA isoenzymes. They state that these molecules have different molecular structures from other CA inhibitors and exhibited different inhibitory profiles with various CA isozymes. The obtained data can lead to drug design studies with different inhibition mechanismCitation48.
In the present study, we purified the hCA I and II isozymes in one simple step by Sepharose-4B-L-tyrosine-sulphanilamide affinity chromatography, one of the best protein purification methods. To prepare the affinity gel, Sepharose 4B was used with CNBr-activated as a matrix. L-tyrosine was inserted covalently into the matrix as a long arm and sulphanilamide was used as a ligand in the affinity gelCitation49. Enzyme purity was checked by SDS-PAGE (). The hCA I and II isoenzymes was purified with yields of 56 and 14%, specific activities of 4709.5 and 11057.1 and fold purifications of at 365 and 856, respectively (). The activities of the isozymes were measured using the esterase and CO2-hydratase methods as in our previous studiesCitation43,Citation50. Arachidonoyl dopamine, 2,4,6-trihydroxybenzaldehyde and 3,4-dihydroxy-5-methoxybenzoic acid were used as inhibitors for the hCA I and II isozymes. The IC50 values, the concentration with a 50% decrease of the enzyme activity, were determined for each compound. For this purpose, the CA activities were measured at five different concentrations of arachidonoyl dopamine, 2,4,6-trihydroxybenzaldehyde and 3,4-dihydroxy-5-methoxybenzoic acid ( and ). The IC50 values were calculated graphically from measurements at five different inhibitor concentrations for the esterase activity of the hCA I and II isoenzymes (). Next, the average Ki values were also calculated from Lineweaver–Burk plots. Arachidonoyl dopamine has the best inhibition effect on esterase activity of the hCA I and II isoenzymes. The IC50 and Ki values were determined to be 346.50–203.80 and 231.00–75.25 μM for arachidonoyl dopamine in vitro, respectively (). In addition, 2,4,6-trihydroxybenzaldehyde for the hCA I isoenzyme and 3,4-dihydroxy-5-methoxybenzoic acid for the hCA II isoenzyme showed the best inhibitory effect on the CO2-hydaratase activities according to arachidonoyl dopamine. The IC50 values were in the range of 115.50–542.88 μM and 1360.00–693.00 μM on the hCA I and II isoenzymes for the CO2 hydratase activities, respectively (). Indeed, three compounds also demonstrated the inhibitory effect at low concentrations under in vitro conditions ().
Table 1. Summary scheme of hCA I and II isoenzymes purified using Sepharose-4B-L-tyrosine affinity chromatography.
Table 2. The IC50, Ki and inhibition type of phenolic compounds for the hCA I and II isozymes as ex vivo using the esterase activity method.
Table 3. The IC50 values of substances for the hCA I and II isozymes ex vivo using the CO2-hydratase activity method.
In recent years, inhibition studies of many phenolic compounds have been performed extensively. These studies were conducted on various enzymes, including CA isozymes glucose 6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, glutathione reductase and lactoperoxidaseCitation51–54. Particularly, it was observed that the inhibition effects of phenolic compounds on CA isozymes are variable, and the inhibition can be in the millimolar to the submicromolar range. Researchers have determined that phenolic compounds are generally bound to the enzyme active site by Ki studies on CA isozymes. CA is a Zn2+-related enzyme, and this Zn2+-bound water molecule interacts with Thr199 in the active siteCitation55. This structure is a ligand for inhibitors such as phenolic compoundsCitation56,Citation57. On the other hand, some inhibitors may interact with the active site of the CA.
The in vivo CA activity was assayed using the CO2-hydratase activity method and represented the physiological activity of the CA enzyme. In vivo results from the enzyme activity studies are crucial to recognise the physiological role of the enzyme. Particularly, drug-enzyme or any chemical compound-enzyme interaction studies are important to understand the toxicological mechanisms. Thus, in vivo studies concerning various enzymes are available in the literature. For instance, Çoban and co-workers reported the in vivo effects of morphine on rat CA enzyme. They provided a single dose of 5 mg/kg to the rats intraperitoneally and found an in vivo inhibitory effect with 4.39 ± 0.63% activity values after 3 h on the CO2-hydratase activity of rat erythrocyte CACitation58. In addition, it was demonstrated that the inhibitory levels were approximately 52% with 42% activity values after 1 h in rats treated with dantrolene (10 mg/kg)Citation33. Additionally, Beydemir and colleagues revealed that gentamicin sulphate (3.2 mg/kg) inhibits the rat CA enzyme to approximately at 65% of the level at 1 h under in vivo conditionsCitation59. In the present study, we studied 48 albino rats (250 ± 20 g) separated into eight equal groups (n = 6). The dose of 2,4,6-trihydroxybenzaldehyde and 3,4-dihydroxy-5-methoxybenzoic acid was 10 mg/kg, intraperitoneally. The dose of arachidonoyl dopamine was 5 mg/kg, intraperitoneally. We observed approximately 30% inhibition levels as 73, 71 and 68% after 4 h for arachidonoyl dopamine, 3,4-dihydroxy-5-methoxybenzoic acid and 2-4-6-trihydroxybenzaldehyde, respectively. The inhibition effects were almost similar among the three chemicals at 4 h (). The half-life (t50) values of the substances were calculated by activity%-time graphs in in vivo studies ( and ). The half-life (t50) values were 8.34, 7.70 and 6.79 h for arachidonoyl dopamine, 3,4-dihydroxy-5-methoxybenzoic acid and 2,4,6-trihydroxybenzaldehyde, respectively. According to the results obtained from this study, 2,4,6-trihydroxybenzaldehyde in vivo inhibited the CA enzyme to a greater amount in a shorter time in vivo. The inhibition order was determined as 2,4,6-trihydroxybenzaldehyde > 3,4-dihydroxy-5-methoxybenzoic acid > arachidonoyl dopamine according to the activity and t50 values. These results indicate that there is a significant correlation in between the half-life and CO2-hydratase activity under in vivo conditions.
Figure 4. The half-life (t50) values of the substances were calculated by activity%-elapsed time graphs in in vivo studies. The half-life (t50) values were found for arachidonoyl dopamine, 3,4-dihydroxy-5-methoxybenzoic acid and 2,4,6-trihydroxybenzaldehyde. These experiments were performed using CO2-hydratase activity assays.
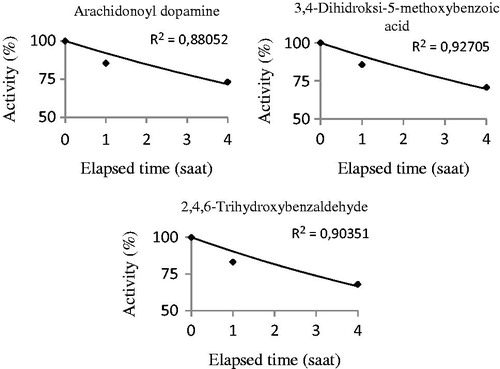
Table 4. The activity values of substances for in vivo rat erythrocyte CA enzyme as IU/gHb using the CO2-hydratase activity method.
Table 5. The half-life (t50) values of substances for rat erythrocytes CA enzyme using the CO2-hydratase activity method.
It is understood from the results that phenolic compounds may generally show inhibition effects with their OH group on CA isoenzymes. Particularly, the CA enzyme has a Zn2+ in its active site. Zn2+ may be a ligand for the OH group of the phenolic compound. On the other hand, some groups of the structural parts of the enzyme may interact with the OH group of the phenolic compound. Thus, many phenolic compounds and their synthesised derivatives can act as novel inhibitors for CA isoenzymes.
Consequently, we reported that arachidonoyl dopamine, 3,4-dihydroxy-5-methoxybenzoic acid and 2,4,6-trihydroxybenzaldehyde were effective CA inhibitors both in vitro and also in vivo. CA inhibitors are crucial for different biomedical applications, such as antiglaucoma, metabolic acidosis, epilepsy, acute mountain sickness, anti-obesity and anti-inflammation. However, the use of phenolic compounds in patients with metabolic acidosis may also generate adverse effects. To minimise of the side effects of arachidonoyl dopamine, 2,4,6-trihydroxybenzaldehyde and 3,4-dihydroxy-5-methoxybenzoic acid should be used carefully and their doses should be adjusted correctly.
Declaration of interest
There is no conflict among the authors concerning the article. The authors are solely accountable for the content and writing of this article.
References
- Singh S, Supuran CT. 3D-QSAR CoMFA studies on sulfonamide inhibitors of the Rv3588c β-carbonic anhydrase from Mycobacterium tuberculosis and design of not yet synthesized new molecules. J Enzyme Inhib Med Chem 2014;29:449–55
- Akbaba Y, Bastem E, Topal F, et al. Synthesis and carbonic anhydrase inhibitory effects of novel sulfonamides derived from 1-aminoaindanes and anilines. Arch Pharm (Weinheim) 2014;347:950–7
- Çetinkaya Y, Göçer H, Göksu S, Gülçin İ. Synthesis and carbonic anhydrase isoenzymes I and II inhibitory effects of novel benzylamine derivatives. J Enzyme Inhib Med Chem 2014;29:168–74
- Akıncıoğlu A, Topal M, Gülçin İ, Göksu S. Novel sulphamides and sulphonamides incorporating the tetralin scaffold as carbonic anhydrase and acetylcholine esterase inhibitors. Arch Pharm (Weinheim) 2014;347:68–76
- Güney M, Coşkun A, Topal F, et al. Oxidation of cyanobenzocycloheptatrienes: synthesis, photooxygenation reaction and carbonic anhydrase isoenzymes inhibition properties of some new benzotropone derivates. Bioorg Med Chem 2014;22:3537–43
- Chegaev K, Lazzarato L, Tamboli Y, et al. Furazan and furoxan sulfonamides are strong α-carbonic anhydrase inhibitors and potential antiglaucoma agents. Bioorg Med Chem 2014;22:3913–21
- Çetinkaya Y, Göçer H, Gülçin İ, Menzek A. Synthesis and carbonic anhydrase isoenzymes inhibitory effects of brominated diphenylmethanone and its derivatives. Arch Pharm (Weinheim) 2014;347:354–9
- Nar M, Çetinkaya Y, Gülçin İ, Menzek A. (3,4-Dihydroxyphenyl)(2,3,4-trihydroxphenyl)methanone and its derivatives as carbonic anhydrase isoenzymes inhibitors. J Enzyme Inhib Med Chem 2013;28:402–6
- Yıldırım A, Atmaca U, Keskin A, et al. N-Acylsulfonamides strongly inhibit human carbonic anhydrase isoenzymes I and II. Bioorg Med Chem 2015;23:2598–605
- Ż olnowska B, Slawiński J, Pogorzelska A, et al. Carbonic anhydrase inhibitors. Synthesis, and molecular structure of novel series N-substitued N'-(2-arylmethylthio-4-chloro-5-methylbenzenesulfonyl) guanidines and their inhibition of human cytosolic isozymes I and II and the transmembrane tumor-associated isozymes IX and XII. Eur J Med Chem 2014;71:135–47
- Slawiński J, Pogorzelska A, Ż olnowska B, et al. Carbonic anhydrase inhibitors. Synthesis of novel series of 5-substituted 2,4-dichlorobenzenesulfonamides and their inhibition of human cytosolic isozymes I and II and the transmembrane tumor-associated isozymes IX and XII. Eur J Med Chem 2014;82:47–55
- Göksu S, Naderi A, Akbaba Y, Kalın P, et al. Carbonic anhydrase inhibitory properties of novel benzylsulfamides using molecular modeling and experimental studies. Bioorg Chem 2014;56:75–82
- Gocer H, Akıncıoğlu A, Göksu S, et al. Carbonic anhydrase and acetylcholinesterase inhibitory effects of carbamates and sulfamoylcarbamates. J Enzyme Inhib Med Chem 2015;30:316–20
- Supuran CT. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnological use for CO2 capture. J Enzyme Inhib Med Chem 2013;28:229–30
- Gülçin İ, Beydemir Ş. Phenolic compounds as antioxidants: carbonic anhydrase isoenzymes inhibitors. Mini Rev Med Chem 2013;13:408–30
- Öztürk Sarikaya SB, Topal F, Şentürk M, et al. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–62
- Marinelli S, Di Marzo V, Florenzano F, et al. N-aranhydonoyl-dopamine tunes synaptic transmission onto dopaminergic neurons by activating both cannabinoid and vanilloid receptors. Neuropsychopharmacology 2007;32:298–308
- Sharkey KA, Cristino L, Oland LD, et al. Arvanil, anandamide and N-arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur J Neurosci 2007;25:2773–82
- O’Sullivan SE, Kendall DA, Randall MD. Characterisation of the vasorelaxant properties of the novel endocannabinoid N-arachidonoyl-dopamine (NADA). Br J Pharmacol 2004;141:803–12
- Gülçin İ, Daştan A. Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities. J Enzyme Inhib Med Chem 2007;22:685–95
- Şentürk M, Gülçin İ, Daştan A, et al. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–11
- Sarıkaya SB, Gülçin İ, Supuran CT. Carbonic anhydrase inhibitors: inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem Biol Drug Des 2010;75:515–20
- Innocenti A, Öztürk Sarikaya SB, Gülçin İ, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg Med Chem 2010;18:2159–64
- Şentürk M, Gülçin İ, Beydemir Ş, et al. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–9
- Boztaş M, Çetinkaya Y, Topal M, et al. Synthesis and carbonic anhydrase isoenzymes I, II, IX, and XII inhibitory effects of dimethoxybromophenol derivatives incorporating cyclopropane moieties. J Med Chem 2015;58:640–50
- Balaydın HT, Gülçin İ, Menzek A, et al. Synthesis and antioxidant properties of diphenylmethane derivative bromophenols including a natural product. J Enzyme Inhib Med Chem 2010;25:685–95
- Taslimi P, Gülçin İ, Öztaşkın N, et al. The effects of some bromophenol derivatives on human carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem. doi: 10.3109/14756366.2015.1054820
- Scozzafava A, Passaponti M, Supuran CT, Gülçin İ. Carbonic anhydrase inhibitors: guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J Enzyme Inhib Med Chem 2015;30:586–91
- Gocer H, Topal F, Topal M, et al. Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin. J Enzyme Inhib Med Chem. doi: 10.3109/14756366.2015.1036051
- Scozzafava A, Kalın P, Supuran CT, et al. The impact of hydroquinone on acetylcholine esterase and certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J Enzyme Inhib Med Chem 2015;30:941–6
- Gocer H, Gülçin İ. Caffeic acid phenethyl ester (CAPE): correlation of structure and antioxidant properties. Int J Food Sci Nutr 2011;62:821–5
- Topal M, Gülçin İ. Rosmarinic acid: a potent carbonic anhydrase isoenzymes inhibitor. Turk J Chem 2014;38:894–902
- Gülçin İ, Beydemir Ş, Büyükokuroğlu ME. In vitro and in vivo effects of dantrolene on carbonic anhydrase enzyme activities. Biol Pharm Bull 2004;27:613–6
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5
- Segel IH. Biochemical calculations: enzyme kinetics. New York: John Wiley and Sons, Inc; 1968: 213
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54
- Akıncıoğlu A, Akıncıoğlu H, Gülçin I, et al. Discovery of potent carbonic anhydrase and acetylcholine esterase inhibitors: novel sulfamoylcarbamates and sulfamides derived from acetophenones. Bioorg Med Chem 2015;23:3592–602
- Hisar O, Beydemir Ş, Gulcin I, et al. The effects of melatonin hormone on carbonic anhydrase enzyme activity in rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. Turk J Vet Anim Sci 2005;29:841–5
- Ekinci D, Beydemir Ş. Purification of PON1 from human serum and assessment of enzyme kinetics against metal toxicity. Biol Trace Elem Res 2010;135:112–20
- Rickly EE, Ghazanfar SA, Gibbons BH, Edsall JT. Carbonic anhydrases from human erythrocytes. Preparation and properties of two enzymes. J Biol Chem 1964;239:1065–78
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–9
- Soyut H, Beydemir Ş, Hisar O. Effects of some metals on carbonic anhydrase from brains of rainbow trout. Biol Trace Elem Res 2008;123:179–90
- Beydemir Ş, Ciftci M, Ozmen I, et al. Effects of some medical drugs on enzyme activities of carbonic anhydrase from human erythrocytes in vitro and from rat erythrocytes in vivo. Pharmacol Res 2000;42:187–91
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–66
- Akbaba Y, Akıncıoğlu A, Göçer H, et al. Carbonic anhydrase inhibitory properties of novel sulfonamide derivatives of aminoindanes and aminotetralins. J Enzyme Inhib Med Chem 2014;29:35–42
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74
- Carta F, Vullo D, Maresca A, et al. Mono-/dihydroxybenzoic acid esters and phenol pyridinium derivatives as inhibitors of the mammalian carbonic anhydrase isoforms I, II, VII, IX, XII and XIV. Bioorg Med Chem 2013;21:1564–9
- Innocenti A, Gülçin İ, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Antioxidant polyphenol natural products effectively inhibit mammalian isoforms I-XV. Bioorg Med Chem Lett 2010;20:5050–3
- Atasaver A, Özdemir H, Gülçin İ, Irfan Küfrevioğlu O. One-step purification of lactoperoxidase from bovine milk by affinity chromatography. Food Chem 2013;136:864–70
- Ekinci D, Beydemir Ş, Alım Ş. Some drugs inhibit in vitro hydratase and esterase activities of human carbonic anhydrase-I and II. Pharmacol Rep 2007;59:580–7
- Gülçin I, Beydemir Ş, Çoban TA, Ekinci D. The inhibitory effect of dantrolene sodium and propofol on 6-phosphogluconate dehydrogenase from rat erythrocyte. Fresen Environ Bull 2008;17:1283–7
- Şişecioğlu M, Çankaya M, Gülçin I, Özdemir M. The inhibitory effect of propofol on lactoperoxidase. Protein Peptide Lett 2009;16:46–9
- Şentürk M, Gülçin I, Çiftci M, Küfreviolu OI. Dantrolene inhibits human erythrocyte glutathione reductase. Biol Pharm Bull 2008;31:2036–9
- Beydemir Ş, Gülçin I, Küfreviolu OI, Çiftçi M. Glucose 6-phosphate dehydrogenase: in vitro and in vivo effects of dantrolene sodium. Pol J Pharmacol 2003;55:787–92
- Akıncıoğlu A, Akbaba Y, Göçer H, et al. Novel sulfamides as potential carbonic anhydrase isoenzymes inhibitors. Bioorg Med Chem 2013;21:1379–85
- Arabaci B, Gülçin İ, Alwasel S. Capsaicin: a potent inhibitor of carbonic anhydrase isoenzymes. Molecules 2015;19:10103–14
- Aksu K, Nar M, Tanç M, et al. The synthesis of sulfamide analogues of dopamine related compounds and their carbonic anhydrase inhibitory properties. Bioorg Med Chem 2013;21:2925–31
- Çoban TA, Beydemir Ş, Gülçin İ, Ekinci D. Morphine inhibits erythrocyte carbonic anhydrase in vitro and in vivo. Biol Pharm Bull 2007;30:2257–61
- Beydemir Ş, Çiftçi M, Küfrevioğlu Öİ, Büyükokuroğlu ME. Effects of gentamicin sulfate on enzyme activities of carbonic anhydrase from human erythrocytes in vitro and from rat erythrocytes in vivo. Biol Pharm Bull 2002;25:966–69