Abstract
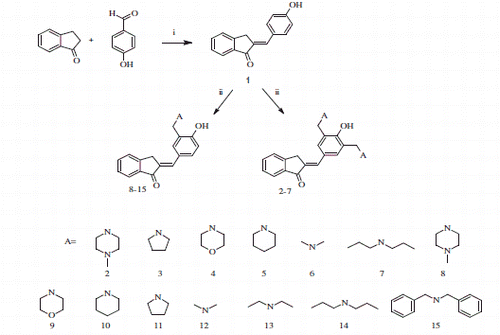
Phenolic mono Mannich bases [2-[4-hydroxy-3-(aminomethyl)benzylidene]-2,3-dihydro-1H-inden-1-one (8–15)] and bis Mannich bases [2-[4-hydroxy-3,5-bis(aminomethyl)benzylidene]-2, 3-dihydro-1H-inden-1-one (2–7)] were synthesized starting from 2-(4-hydroxybenzylidene)-2, 3-dihydro-inden-1-one (1). This study was designed in order to investigate the carbonic anhydrase (CA, EC 4.2.1.1) inhibitory properties of a library of compounds incorporating the phenol functional group. All prepared compounds showed a low inhibition percentages on both human (h) isoforms hCA I and hCA II compared to the reference sulfonamide acetazolamide. Mannich bases 2–15 had lower inhibition percentages than the compound 1 on hCA I and hCA II, except compound 14, which is a Mannich base derivative of dipropylamine, which had a similar inhibitory power as compound 1 on hCA II. All compounds synthesized 1–15 were 1.3–1.9 times more effective on hCA II comparing with the effectivenes of the compounds on hCA I.
Introduction
Carbonic anhydrases (CA, EC 4.2.1.1) are zinc metalloproteins, which catalyze a very simple reaction: the carbon dioxide (CO2) hydration reaction to bicarbonate and proton. CA isoforms are found in a variety of tissues where they participate in several important biological processes such as acid–base balance, respiration, CO2 and ion transport, bone resorption, ureagenesis, gluconeogenesis, lipogenesis, and electrolyte secretion. Many CA isozymes involved in these processes are important therapeutic targets with the potential to be inhibited/activated for the treatment of a range of disorders such as edema, glaucoma, obesity, cancer, epilepsy, and osteoporosisCitation1.
Some phenol-bearing compounds are reported with their CA inhibitory activitiesCitation2. The simple phenol molecule was reported to act as an inhibitor of the zinc enzyme CA in a different mechanism of actionCitation3. Indeed, sulfonamides coordinate to the metal ion from the enzyme active site; phenols and derivatives anchor to the water molecule/hydroxide ion coordinated to the metal ionCitation4,Citation5.
The side effects of many compounds belonging to the first-/second generation of carbonic anhydrase inhibitors (CAIs) of types 1–15 (among which metabolic acidosis, kidney stones, bone loss, and so on) are due to the potent inhibition of all mammalian CA isoforms, and not only of the target one. The main scope of the drug is to obtain isoform-selective CAIs for the various isoforms involved specifically in different pathologiesCitation5–8.
Chalcones have α-β-unsaturated ketone moiety in its structure and they are common structure in natural products such as flavonoidsCitation9. Chalcones and its analogs are excellent scaffolds for synthetic manipulations and also possess multiple biological and medicinal properties. Indanone-derived compounds were also reported with several biological activities such as cytotoxic, antioxidant, anticholinergic, antifungal, and anticancer activitiesCitation10.
Mannich bases are synthesized by Mannich reaction. It is an aminomethylation process of a compound or a drug. They are an important group of compounds in medicinal chemistry. Aminomethylation of drugs could be used to improve the delivery of a drug into the human body. Aminomethylation may increase the hydrophilic properties of drugs through the introduction of a polar function into the chemical structure. The aminomethylated drugs could act as prodrugs releasing the active substance under controlled hydrolytic conditions via deamination. There are several type of Mannich bases such as carbon Mannich bases and nitrogen Mannich basesCitation11. Phenolic Mannich bases are a group of carbon Mannich bases. It was reported CAs inhibitoryCitation12–14, cytotoxicCitation15–18, anticonvulsantCitation19–21, antiinflammatoryCitation22, diureticCitation23, and antifungalCitation24 activities of Mannich bases.
In this study, it was aimed to investigate the CA inhibitory activities of new phenolic chalcone analog compounds having indanone skeleton to make a contribution to CA research library and give some insight to the researchers who are interested in CA research. For this aim, it was planned to use some phenolic mono Mannich bases [(2-[4-hydroxy-3-(aminomethyl)benzylidene]-2,3-dihydro-1H-inden-1-one (8–15, Scheme 1)] and phenolic bis Mannich bases [(2-[4-hydroxy-3,5-bis(aminomethyl)benzylidene]-2,3-dihydro-1H-inden-1-one (2–7, Scheme 1)] synthesized according to our previous studiesCitation17,Citation25 starting from 2–(4-hydroxybenzylidene)-2,3-dihydro-inden-1-one (1, Scheme 1).
Material and methods
CA enzyme assay
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activityCitation26. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining constant ionic strength), following the initial rates of the CA-catalyzed CO2 hydration reaction for a period of 10–100s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor, at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled–deionized water and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E–I complex. The inhibition percantage was obtained by using PRISM 3, as reported earlierCitation27, and represent the mean from at least three different determinations. All CA isoforms were recombinant ones obtained in-house as reported earlierCitation28. The cell pellets were lysed, and hCA II and hCA I were purified through affinity chromatography using pAMBS resin.
Results and discussion
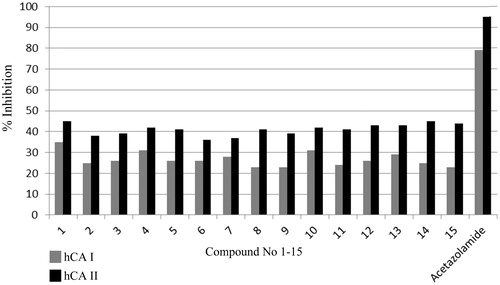
The hCA I and hCA II inhibition percentages of the compounds 1–15 are shown in and also in . According to , Mannich bases 2–15 had lower inhibition percentage on hCA I than the compound 1 from which Mannich bases were derived from. Only the compound 14 had equal inhibition percentage with the compound 1 on hCA II. All compounds had lower inhibition percentages than reference drug acetazolamide (AAZ) on both hCA I and hCA II. Accroding to the results in , bis Mannich bases compound 2 (with N-methyl piperazine), compound 3 (with pyrrolidine), compound 4 (with morpholine), and compound 7 (with dipropylamine) did more inhibition on hCA I than their corresponding mono Mannich bases.
Table 1. Inhibition percentages of compound 1–15 on hCA I and hCA II isoenzymes at 1 μM inhibitor concentration in the assay system.
The inhibition potency of the bis Mannich bases at issue in terms of times for the compound 2, 3, and 7 were 1.1 times while it was 1.3 times for the compound 4 comparing to their corresponding mono Mannich bases. On the other hand, mono Mannich base 10 (with piperidine) had shown 1.2 times more potent hCA I inhibition than its corresponding bis Mannich base analog, the compound 5.
When inhibitory activities of the compounds on hCA II were considered, mono Mannich bases were more effective than bis Mannich bases on the contrary to hCA I’s. It can be easily noticed from that mono Mannich bases 8 (with N-methyl piperazine), 11 (with pyrrolidine), 12 (with dimethylamine), and 14 (with dipropylamine) were more effective than their corresponding bis Mannich bases on hCA II. The potency ratios were 1.1 times stronger for the compounds 8 and 11, and 1.2 times stronger for the compounds 12 and 14 comparing to their corresponding bis Mannich bases.
The compound 1 was more effective on hCA I (1.1–1.5 times) and hCA II (1.1–1.3), compared with the Mannich bases 2–15. The Mannich bases reported here show poor inhibitory activity against these two CA isoforms. This is probably due to the fact that in the ortho position/s to the OH moiety there are one or two rather bulky functionalities which may interfere with the binding of the compound to the enzyme active site. In addition, the compounds 1–15 were 1.3–1.9 times more effective on hCA II than on hCA I without exception according to results in and .
Conclusion
All compounds had lower inhibition percentages on both hCA I and hCA II than reference compound AAZ. Mannich bases 2–15 had lower inhibition percentages than the compound 1 on both hCA I and hCA II. Exception was compound 14 with dipropylamine, which has equal inhibition percentage with the compound 1 on hCA II. All compounds synthesized (1–15) were 1.3–1.9 times more effective on hCA II compared with the inhibition on hCA I. The Mannich bases reported here show more poor inhibitory activity against these two CA isoforms than starting compound which is chalcone analog. This is probably due to the fact that in the ortho position to the OH moiety there are one or two rather bulky functionalities which may interfere with the binding of the compound to the enzyme active site. By this study, CA inhibitory acitivities of new phenolic compounds were reported to give some insight to the scientists focusing on CA research.
Acknowledgements
The authors H.I.G and C.T.S thank to ERASMUS Office of Ataturk University, Turkey, for their financial support to Yamali C.
Declaration of interest
The authors report no conflict of interest and are responsible for the contents and writing of the paper.
References
- Isik S, Vullo D, Durdagi S, et al. Interaction of carbonic anhydrase isozymes I, II, and IX with some pyridine and phenol hydrazinecarbothioamide derivatives. Bioorg Med Chem Lett 2015;25:5636–41
- Innocenti A, Gulcin I, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Antioxidant polyphenols effectively inhibit mammalian isoforms I-XV. Bioorg Med Chem Lett 2010;20:5050–3
- Nair SK, Ludwig PA, Christianson DW. Site binding of phenol in the active-site of human carbonic anhydraseII - structural implications for substrate association. J Am Chem Soc 1994;116:3659–60
- De Simone G, Supuran CT. (In)organic anions as carbonic anhydrase inhibitors. J Inorg Biochem 2012;111:117–29
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Smith KS, Jakubzick C, Whittam TS, Ferry JG. Carbonic anhydrase is an ancient enzyme widespread in prokaryotes. Proc Natl Acad Sci USA 1999;96:15184–9
- Xu Y, Feng L, Jeffrey PD, et al. Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms. Nature 2008;452:56–61
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77
- Saxena HO, Faridi U, Kumar JK, et al. Synthesis of chalcone derivatives on steroidal framework and their anticancer activities. Steroids 2007;72:892–900
- Fadare OA, Akinpelu DA, Ejemubu H, Obafemi CA. 1-Indanone chalcones and their 2,4-dinitrophenylhydrazone derivatives: synthesis, physicochemical properties and in vitro antibacterial activity. Afr J Pur Appl Chem 2014;8:68–77
- Roman G. Mannich bases in medicinal chemistry and drug design. Eur J Med Chem 2015;89:743–816
- Bilginer S, Unluer E, Gul HI, et al. Carbonic anhydrase inhibitors. Phenols incorporating 2- or 3-pyridyl-ethenylcarbonyl and tertiary amine moieties strongly inhibit Saccharomyces cerevisiae beta-carbonic anhydrase. J Enzyme Inhib Med Chem 2014;29:495–9
- Sieger GM, Barringe Wc Krueger JE. Mannich derivatives of medicinals. 2. Derivatives of some carbonic anhydrase inhibitors. J Med Chem 1971;14:458–60
- Buyukkidan N, Buyukkidan B, Bulbul M, et al. Synthesis and characterization of phenolic Mannich bases and effects of these compounds on human carbonic anhydrase isozymes I and II. J Enzyme Inhib Med Chem 2013;28:337–42
- Gul HI, Cizmecioglu M, Zencir S, et al. Cytotoxic activity of 4′-hydroxychalcone derivatives against Jurkat cells and their effects on mammalian DNA topoisomerase I. J Enzyme Inhib Med Chem 2009;24:804–7
- Gul HI, Yerdelen KO, Das U, et al. Synthesis and cytotoxicity of novel 3-aryl-1-(3-dibenzylaminomethyl-4-hydroxyphenyl)-propenones and related compounds. Chem Pharm Bull 2008;56:1675–81
- Tugrak M, Yamali C, Sakagami H, Gul HI. Synthesis of mono Mannich bases of 2-(4-hydroxybenzylidene)-2,3-dihydroinden-1-one and evaluation of their cytotoxicities. J Enzyme Inhib Med Chem 2015. [Epub ahead of print]. doi: 10.3109/14756366.2015.1070263
- Yerdelen KO, Gul HI, Sakagami H, Umemura N. Synthesis and biological evaluation of 1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one and its aminomethyl derivatives. J Enzyme Inhib Med Chem 2015;30:383–8
- Gul HI, Calis U, Vepsalainen J. Synthesis and evaluation of anticonvulsant activities of some bis Mannich bases and corresponding piperidinols. Arzneimittelforschung 2002;52:863–9
- Gul HI, Calis U, Vepsalainen J. Synthesis of some mono-Mannich bases and corresponding azine derivatives and evaluation of their anticonvulsant activity. Arzneimittelforschung 2004;54:359–64
- Gul HI, Calis U, Ozturk Z, et al. Evaluation of anticonvulsant activities of bis(3-aryl-3-oxo-propyl)ethylamine hydrochlorides and 4-aryl-3-arylcarbonyl-1-ethyl-4-piperidinol hydrochlorides. Arzne-imittelforschung 2007;57:133–6
- Gul HI, Suleyman H, Gul M. Evaluation of the anti-inflammatory activity of N,N′-bis(3-dimethylamino-1-phenyl-propylidene) hydrazine dihydrochloride. Pharm Biol 2009;47:968–72
- Koechel DA, Rankin GO. Diuretic activity of Mannich base derivatives of ethacrynic acid and certain ethacrynic acid analogues. J Med Chem 1978;21:764–9
- Mete E, Ozelgul C, Kazaz C, et al. Synthesis and antifungal activity of 1-aryl-3-phenethylamino-1-propanone hydrochlorides and 3-aroyl-4-aryl-1-phenethyl-4-piperidinols. Arch Pharm (Weinheim) 2010;343:291–300
- Tugrak M, Gul HI, Sakagami H. Synthesis and cytotoxicities of 2-[4-hydroxy-(3,5-bis-aminomethyl)-benzylidene]-indan-1-ones. Lett Drug Des Discov 2015;12:806–12
- RG K. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73
- Maresca A, Temperini C, Pochet L, et al. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem 2010;53:335–44
- D’Arnbrosio K, Smaine FZ, Carta FD, et al. Development of potent carbonic anhydrase inhibitors incorporating both sulfonamide and sulfamide groups. J Med Chem 2012;55:6776–83