Abstract
The rapid dilution of the enzyme-inhibitor complex assay to monitor the recovery of enzyme activity is a well-established assay to determine the reversibility of inhibition. Our laboratory has previously employed this method to ascertain the reversibility of known glutamate carboxypeptidase II (GCPII)-targeting agents. Due to the tedious and time-consuming nature of the assay, we sought to develop a facile method to determine the reversibility of well-characterized GCPII inhibitors using bio-layer interferometry (BLI). The results from the BLI assay are in agreement with the rapid dilution method. Herein, we report for the first time, a rapid, novel real-time BLI method to determine reversibility of inhibition.
Introduction
The expression of glutamate carboxypeptidase II (GCP II) in human prostate epithelium is known as prostate-specific membrane antigen (PSMA). GCP II is a glycosylated cell-surface zinc metallopeptidase uniquely expressed on prostate tumor cells and the neovasculature of non-prostatic malignanciesCitation1,Citation2. As a consequence, GCP II has attracted significant attention over the past several years as a target for the delivery of imaging and therapeutic agents, and continues to serve as an important clinically-relevant biomarker.
GCP II is reported to possess two predominant, yet poorly understood, enzymatic activities: the hydrolytic cleavage and liberation of glutamate from γ-glutamyl derivatives of folatesCitation3 and the proteolysis of the neuropeptide N-acetylaspartylglutamate (NAAG)Citation4. Although its role in the progression of prostate cancer remains conjectural, there is emerging evidence that GCP II plays a regulatory role in angiogenesisCitation5. Various chemical scaffolds have been developed as inhibitors of this enzyme to selectively deliver imaging and therapeutic agentsCitation6–8.
We previously reported a series of phosphoramidate peptidomimetic inhibitors of PMSA and classified their reversibility of inhibition by monitoring the recovery of enzyme activity following rapid dilution of the enzyme-inhibitor complexCitation9. In addition, the correlation between reversibility of inhibition and GCP II internalization in LNCaP cells has been determined; we found that pseudo-irreversible inhibitors induced internalization to a greater extent than slowly reversible or reversible inhibitorsCitation9. Furthermore, we recently demonstrated that reversibility of inhibition has an effect on internalization and percent uptake of GCP II-targeted SPECT agents with the irreversible-targeting agent, demonstrating superior uptake and internalization in GCP II-positive (GCP II+) cellsCitation10. These results confirm the significance of pseudo-irreversible inhibitors as targeting molecules in the development of targeted imaging and therapeutic agents and provide rationale for establishing a convenient and rapid method for ascertaining mode of inhibition. The focus of the work described herein was aimed at developing an efficient method to determine the mode of inhibition for inhibitors of GCP II; our current benchmark method (a rapid dilution, HPLC-based assay) is tediousCitation9. An attractive aspect of a bio-layer interferometry (BLI)-based assay is the small quantities of reagents required, which can also be reused for subsequent assays. BLI also allows for real-time monitoring of on- and off-rates of small enzyme-small molecule interactions. In addition, multiple inhibitors can be evaluated simultaneously for dissociation constants and reversibility of inhibition.
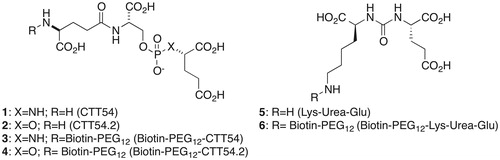
In prior studies, we reported the IC50 values and reversibility of inhibition for CTT54 (1, IC50 = 14 nM)Citation11 and CTT54.2 (2, IC50 = 144 nM)Citation12 (), utilizing a commonly employed method which involves monitoring the recovery of enzyme activity following rapid dilution of the enzyme-inhibitor complex by HPLCCitation9. The results from these rapid dilution assays were used to validate the real-time BLI assay employed in this study as a qualitative method to assess the mode of inhibition.
Establishing a BLI-based binding assay for GCP II required baiting a BLI biosensor tip with a small molecule, which inhibits the enzymatic activity of GCP II, in order to capture GCP II from a buffered solution. We chose to prepare biotinylated derivatives of known GCP II inhibitors linked through a PEG12 linker; we previously found biotinylated GCP II inhibitor with a PEG linker did not alter the reversibility of inhibitionCitation13. CTT54 (1), CTT54.2 (2), and biotin-PEG12-CTT-54 (3) were available from previous studiesCitation12,Citation13. Biotin-PEG12-CTT54.2 (4) and Biotin-PEG12-Lys-Urea-Glu (6) were prepared from compounds 2 and 5 (see Supplementary material for synthesis) and biotin-PEG12-NHS using the same protocol to prepare biotin-PEG12-CTT-54Citation14.
Methods and material
BLI assay
The reversibility of inhibition studies (pseudo-irreversible, slowly reversible, and rapidly reversible) for the biotinylated derivatives (3, 4, and 6) of known GCPII inhibitors on the Octet Red (ForteBio, Menlo Park, CA) system were all carried out in 96-well plates (Greiner) at 30 °C and shaken at 1000 rpm for each step. For each reversibility of inhibition experiment, compound 3, 4, or 6 (100 μM, 250 μL), were added to individual wells of a 96-well plate. Streptavidin-coated biosensor tips (ForteBio) were mounted in the instrument and dipped into respective wells containing compound 3, 4, or 6 and incubated for 600 s to saturate the tips with each compound. Unoccupied streptavidin binding-sites were blocked by incubating the loaded biosensor tips in wells containing biocytin (1 mM, 250 μL) for 600 s. Unbound biocytin was then removed by dipping and incubating the biosensor tips in well containing Tris buffer (250 μL, 50 mM) for 600 s. Purified GCP IICitation15 was then loaded onto the biosensor tips by dipping and incubating the inhibitor-coated tips in wells containing a solution of the protein (25 μg/mL, 250 μL) for 600 s. To obtain a clean dissociation signal, the biosensor tips loaded with the GCP II-inhibitor complex were then dipped into wells with 50 mM Tris buffer (250 μL, 50 mM) containing 1% triton X-100 and compound 1 (14 μM; 1000-fold greater than the IC50 value of 1) for 28 800 s.
Results and discussions
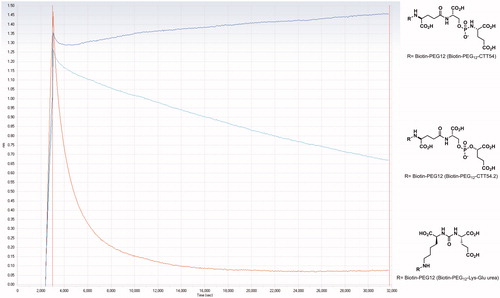
The dissociation of the GCP II-inhibitor complexes with 3, 4 and 6 was observed for 8 h. In the case of reversible inhibitors, a rapid exponential decay curve would be observed for the dissociation of the enzyme-inhibitor complexCitation16,Citation17. For an irreversible inhibitor, the enzyme-inhibitor complex dissociation progress curve should be flat except for instrumental drift. The progress curve for a slowly reversible inhibitor in the time scale of the activity assay, should be similar to that of a reversible competitive inhibitor but with a slower decay. The observed dissociation curves for the biotinylated derivatives 3, 4 and 6 were consistent with the known modes of binding for compounds 1,Citation11 2,Citation12 5, and their biotinylated derivatives 3, 4, and 6. Compound 3 showed little or no change in the experimental time frame while GCP II dissociated slowly from the enzyme-inhibitor complex with 4 (). These observations are distinct from what is normally observed for classical competitive inhibitorsCitation16,Citation17. On the other hand, compound 6 showed a rapid exponential decay, which is in agreement with the results from the HPLC assay that the urea compounds 5 and 6 are reversible.
Figure 2. Dissociation of the irreversible GCP II inhibitor 3 (Biotin-PEG12-CTT54), slowly-reversible inhibitor 4 (Biotin-PEG12-CTT54.2), and reversible inhibitor 6 (Biotin-PEG12-Lys-Glu urea), compound structures adjacent to the curves, in (50 mM TRIS, pH 7.4 with 1% X-100 trition) buffer containing 14 μM CTT54 (1).
Conclusion
These results support the validation of BLI as a qualitative method to rapidly assess the reversibility of inhibition in real-time, which should be broadly applicable to various small-molecule enzyme inhibitors and receptor ligands. We anticipate the development of this BLI method will allow our lab and others to rapidly identify pseudo-irreversible inhibitors, and to advance them as targeting molecule candidates for both imaging and therapeutic applications for prostate cancer. To the best of our knowledge, this is the first BLI assay developed to qualitatively identify reversibility of inhibition. Subsequent studies will be aimed at developing a more universal BLI assay to determine mode of inhibition, kon/koff rates, or IC50 values without the need to biotinylate each compound of interest.
Supplementary material available online
IENZ_1132208_Supplementary_Information.pdf
Download PDF (289.7 KB)Acknowledgements
The authors extend their gratitude to both Prof. Jeffrey P. Jones for access to the OctetRED system and ForteBio for technical assistance.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This work was supported in part by the National Institutes of Health (R01CA140617).
References
- Haffner MC, Kronberger IE, Ross JS, et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum Pathol 2009;40:1754–61
- Chang SS, O'Keefe DS, Bacich DJ, et al. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res 1999;5:2674–81
- Heston WD. Characterization and glutamyl preferring carboxypeptidase function of prostate specific membrane antigen: a novel folate hydrolase. Urology 1997;49:104–12
- Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc Natl Acad Sci USA 1996;93:749–53
- Conway RE, Petrovic N, Li Z, et al. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol Cell Biol 2006;26:5310–24
- Tsukamoto T, Wozniak KM, Slusher BS. Progress in the discovery and development of glutamate carboxypeptidase II inhibitors. Drug Discov Today 2007;12:767–76
- Zhou J, Neale JH, Pomper MG, Kozikowski AP. NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat Rev Drug Discov 2005;4:1015–26
- Wu LY, Anderson MO, Toriyabe Y, et al. The molecular pruning of a phosphoramidate peptidomimetic inhibitor of prostate-specific membrane antigen. Bioorg Med Chem 2007;15:7434–43
- Liu T, Toriyabe Y, Kazak M, Berkman CE. Pseudoirreversible inhibition of prostate-specific membrane antigen by phosphoramidate peptidomimetics. Biochemistry 2008;47:12658–60
- Nedrow-Byers JR, Moore AL, Ganguly T, et al. PSMA-targeted SPECT agents: mode of binding effect on in vitro performance. Prostate 2012;73:355–62
- Nedrow-Byers JR, Jabbes M, Jewett C, et al. A phosphoramidate-based prostate-specific membrane antigen-targeted SPECT agent. Prostate 2011;72:904–12
- Liu T, Wu LY, Hopkins MR, et al. A targeted low molecular weight near-infrared fluorescent probe for prostate cancer. Bioorg Med Chem Lett 2010;20:7124–6
- Liu T, Nedrow-Byers JR, Hopkins MR, et al. Targeting prostate cancer cells with a multivalent PSMA inhibitor-guided streptavidin conjugate. Bioorg Med Chem Lett 2012;22:3931–4
- Wu, LY, Liu, T, Hopkins, MR, et al. Chemoaffinity capture of pre-targeted prostate cancer cells with magnetic beads. Prostate 2012;72:1532–41
- Liu T, Toriyabe Y, Berkman CE. Purification of prostate-specific membrane antigen using conformational epitope-specific antibody-affinity chromatography. Protein Expr Purif 2006;49:251–5
- Maun HR, Wen X, Lingel A, et al. Hedgehog pathway antagonist 5E1 binds hedgehog at the pseudo-active site. J Biol Chem 2010;285:26570–80
- Paek SH, Cho IH, Kim DH, et al. Label-free, needle-type biosensor for continuous glucose monitoring based on competitive binding. Biosens Bioelectron 2012;40:38–44