Abstract
A series of new benzimidazolium salts (1a–g) were synthesized from the reaction of 1-(4-vinylbenzyl)benzimidazole with various alkyl halides. These salts were used to synthesize silver N-heterocyclic carbene (Ag-NHC) complexes (2a–f). The thirteen compounds were characterized by FT-IR, NMR (1H and 13C) spectroscopic methods and an elemental analysis technique. These selected candidates were tested for their in vitro antimicrobial activities. Antibacterial and antifungal results indicated that the new salts, and particularly their silver complexes, were found to be strongly effective against seven Gram (−) bacterial strains, three Gram (+) bacterial strains and one yeast (Candida albicans).
Introduction
Heterocyclic compounds such as benzimidazole, imidazole, triazole, benzodiazepine and indazole are very important for both pharmacological and industrial applicationsCitation1–4. Different pharmacological and biological properties of many heteroaromatic compounds, which are stable, safe, and have biological activity, have been investigated for these two significant applications by different research groupsCitation5–12. The rapid development of microbial resistance to currently used antibiotics has become a serious healthcare problemCitation13,Citation14. Hence, there is an immediate need for research on novel antimicrobial agents with potent activity against resistant microorganismsCitation13,Citation15. The benzimidazole ring has a significant heterocyclic pharmacophore in drug discoveryCitation16. In particular, this nucleus is a constituent of vitamin-B12Citation17. Benzimidazoles are regarded as a promising class of bioactive heterocyclic compounds, which have attracted considerable interest in medicinal chemistry because of their various range of biological activities including antiviral, anticancerCitation16,Citation18,Citation19, antimicrobialCitation1,Citation16,Citation18–20, anti-inflammatoryCitation16,Citation19, anthelmintic, antihistaminic, proton pump inhibitor, antioxidant, antihypertensive and anticoagulant activitiesCitation16. The aim of this study was to investigate the in vitro antimicrobial activity of new synthesized benzimidazolium salts (1a–f) and Ag-NHC complexes (2a–g), which were formed in situ deprotonation with silver oxide of 1a–f. The structures of these compounds were characterized by spectroscopic and analytical methods. All of them were more effective against both Gram (−) and Gram (+) bacteria compared to yeast. In general, silver complexes showed better results than NHC precursors.
Experimental
General considerations
All reactions for the synthesis of 1-(4-vinylbenzyl) substituted benzimidazolium salts (1a–g) and their silver complexes (2a–f) were made under argon. Chemical reagents and solvents were purchased from Sigma-Aldrich (Interlab, St Charles, MO) and Merck (Darmstadt, Germany). NMR (1H and 13C NMR) studies were performed in CDCl3 and DMSO-d6. The 1H NMR (300 MHz) and 13C NMR (75 MHz) spectra were recorded with a Bruker AC300P FT spectrophotometer (Coventry, UK). Chemical shifts (δ) were given in ppm relative to tetramethylsilane as an internal reference. Coupling constants (J) were given in hertz (Hz). 1H NMR signals were labeled as singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), pentet (p), hextet (hex) or heptet (hept). Melting points (m.p.) were measured in glass capillary tubes using an Electrothermal-9200 melting point device. Elemental analyses were performed with the CHNS-932 LECO apparatus (Isomass Scientific Inc., Calgary, Canada). The FT-IR spectra of the synthesized new compounds were recorded in the 450–4000 cm−1 region using a Shimadzu FT-IR 8400 spectrophotometer (Shimadzu Middle East & Africa FZE, Japan).
Synthesis of new compounds
General preparation of 1-(4-vinylbenzyl)-3-alkylbenzimidazolium salts, (1)
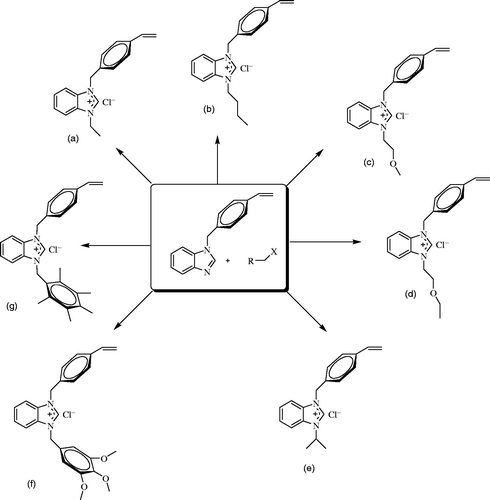
The synthesis of benzimidazolium salts were prepared according to literatureCitation1,Citation21. 1-(4-Vinylbenzyl)benzimidazole was synthesized from 4-vinylbenzyl chloride and benzimidazole in ethyl alcohol. Alkyl halides (1 mmol) were added slowly to a solution of 1-(4-vinylbenzyl)benzimidazole (1 mmol) in N,N-dimethylformamide (DMF) (4 ml) at 25 °C and the mixture was mixed at 80 °C for 12 h. The formed salt was washed with diethyl ether (3 × 15 ml) and dried under vacuum. The product was crystallized from ethyl alcohol/diethyl ether mixture (2:1) at room temperature.
1-(4-Vinylbenzyl)-3-ethylbenzimidazolium chloride, 1a
Yield: 85%, m.p.: 280–281 °C, IR(CN): 1561.0 cm−1. 1H NMR (300 MHz, DMSO-d6), δ: 1.59 (t, 3H, CH2CH3, J = 7.2 Hz); 4.57 (q, 2H, CH2CH3, J = 7.2 Hz); 5.28 (d, 1H, CH2C6H4CH=CH2, J = 10.2 Hz); 5.85 (s, 2H, CH2C6H4CH=CH2); 5.88 (d, 1H, CH2C6H4CH=CH2, J = 17.7 Hz); 6.73 (m, 1H, CH2C6H4CH=CH2); 7.52–8.13 (m, 8H, Ar–H); 10.46 (s, 1H, NCHN). 13C NMR (75 MHz, DMSO-d6), δ: 14.6 and 42.6 (CH2CH3); 49.9 (CH2C6H4CH=CH2); 114.3, 114.6, 115.5, 126.9, 127.1, 129.2, 131.6, 134.0, 134.1, 136.4, 137.9 and 141.5 (Ar–C and CH2C6H4CH=CH2); 142.8 (2-CH). Anal. calc. for C18H19N2Cl (298.81 g/mol): C, 72.35%; H, 6.41%; N, 9.37%. Found: C, 72.50%; H, 6.30%; N, 9.41%.
1-(4-Vinylbenzyl)-3-butylbenzimidazolium chloride, 1b
Yield: 78%, m.p.: 141–142 °C, IR(CN): 1556.2 cm−1. 1H NMR (300 MHz, DMSO-d6), δ: 0.94 (t, 3H, CH2CH2CH2CH3, J = 7.2 Hz); 1.35 (hex., 2H, CH2CH2CH2CH3, J = 7.2 Hz); 1.93 (p, 2H, CH2CH2CH2CH3, J = 7.2 Hz); 4.55 (t, 2H, CH2CH2CH2CH3, J = 6.9 Hz); 5.27 (d, 1H, CH2C6H4CH=CH2, J = 11.1 Hz); 5.82 (s, 2H, CH2C6H4CH=CH2); 5.89 (d, 1H, CH2C6H4CH=CH2, J = 19.8 Hz); 6.71 (m, 1H, CH2C6H4CH=CH2); 7.38–7.99 (m, 8H, Ar–H); 10.35 (s, 1H, NCHN). 13C NMR (75 MHz, DMSO-d6), δ: 13.9, 19.6, 30.9 and 47.0 (CH2CH2CH2CH3); 50.1 (CH2C6H4CH=CH2); 114.4, 115.7, 127.1, 129.2, 131.3, 131.8, 134.0, 136.4 and 137.9 (Ar–C and CH2C6H4CH=CH2); 143.0 (2-CH). Anal. calc. for C20H23N2Cl (326.86 g/mol): C, 73.49%; H, 7.09%; N, 8.57%. Found: C, 73.55%; H, 7.17%; N, 8.52%.
1-(4-Vinylbenzyl)-3–(2-methoxyethyl)benzimidazolium chloride, 1c
Yield: 83%, m.p.: >355 °C, IR(CN): 1557.9 cm−1. 1H NMR (300 MHz, DMSO-d6), δ: 2.52 (s, 3H, CH2CH2OCH3); 3.81 (t, 2H, CH2CH2OCH3, J = 4.8 Hz); 4.75 (t, 2H, CH2CH2OCH3, J = 4.8 Hz); 5.29 (d, 1H, CH2C6H4CH=CH2, J = 10.8 Hz); 5.83 (s, 2H, CH2C6H4CH=CH2); 5.85 (d, 1H, CH2C6H4CH=CH2, J = 18.0 Hz); 6.73 (m, 1H, CH2C6H4CH=CH2); 7.51–8.14 (m, 8H, Ar–H); 10.05 (s, 1H, NCHN). Anal. calc. for C19H21N2OCl (328.84 g/mol): C, 69.40%; H, 6.44%; N, 8.52%. Found: C, 69.29%; H, 6.55%; N, 8.56%.
1-(4-Vinylbenzyl)-3-(2-ethoxyethyl)benzimidazolium chloride, 1d
Yield: 88%, m.p.: 127–128 °C, IR(CN): 1562.7 cm−1. 1H NMR (300 MHz, DMSO-d6), δ: 1.07 (t, 3H, CH2CH2OCH2CH3, J = 4.2 Hz); 3.47 (q, 2H, CH2CH2OCH2CH3, J = 4.2 Hz); 3.95 (t, 2H, CH2CH2OCH2CH3, J = 4.8 Hz); 4.86 (t, 2H, CH2CH2OCH2CH3, J = 4.8 Hz); 5.25 (d, 1H, CH2C6H4CH=CH2, J = 11.1 Hz); 5.70 (d, 1H, CH2C6H4CH=CH2, J = 17.7 Hz); 5.87 (s, 2H, CH2C6H4CH=CH2); 6.63 (m, 1H, CH2C6H4CH=CH2); 7.28–7.99 (m, 8H, Ar–H); 11.41 (s, 1H, NCHN). Anal. Calc. for C20H23N2OCl (342.86 g/mol): C, 70.06%; H, 6.76%; N, 8.17%. Found: C, 70.15%; H, 6.67%; N, 8.21%.
1–(4-vinylbenzyl)-3-isopropylbenzimidazolium chloride, 1e
Yield: 78%, m.p.: >355 °C, IR(CN): 1554.8 cm−1. 1H NMR (300 MHz, DMSO-d6), δ: 1.65 [d, 6H, CH(CH3)2, J = 6.3 Hz]; 5.04 [hept., 1H, CH(CH3)2, J = 6.9 Hz]; 5.28 (d, 1H, CH2C6H4CH=CH2, J = 10.8 Hz); 5.73 (s, 2H, CH2C6H4CH=CH2); 5.83 (d, 1H, CH2C6H4CH=CH2, J = 17.4 Hz); 6.71 (m, 1H, CH2C6H4CH=CH2); 7.25–8.46 (m, 8H, Ar–H); 10.05 (s, 1H, NCHN). 13C NMR (75 MHz, DMSO-d6), δ: 21.9 and 50.3 [CH(CH3)2]; 51.3 (CH2C6H4CH=CH2); 114.3, 114.6, 115.8, 127.1, 127.3, 128.9, 131.2, 131.4, 133.8, 136.3 and 137.9 (Ar–C and CH2C6H4CH=CH2); 141.2 (NCHN). Anal. calc. for C19H21N2Cl (312.84 g/mol): C, 72.95%; H, 6.77%; N, 8.95%. Found: C, 72.86%; H, 6.81%; N, 8.99%.
1-(4-Vinylbenzyl)-3-(3,4,5-trimethoxybenzyl)benzimidazolium chloride, 1f
Yield: 90%, m.p.: 176–177 °C, IR(CN): 1557.5 cm−1. 1H NMR (300 MHz, CDCl3), δ: 3.80 [s, 3H, CH2C6H2(OCH3)3–3,4,5]; 3.86 [s, 6H, CH2C6H2(OCH3)3–3,4,5]; 5.26 (d, 1H, CH2C6H4CH=CH2, J = 11.1 Hz); 5.96 [s, 2H, CH2C6H2(OCH3)3–3,4,5]; 5.87 (s, 2H, CH2C6H4CH=CH2); 5.72 (d, 1H, CH2C6H4CH=CH2, J = 18.3 Hz); 6.63 (m, 1H, CH2C6H4CH=CH2); 6.87 [s, 2H, CH2C6H2(OCH3)3–3,4,5]; 7.29–7.69 (m, 8H, Ar–H); 12.05 (s, 1H, NCHN). 13C NMR (75 MHz, CDCl3), δ: 56.7 and 60.8 [CH2C6H2(OCH3)3–3,4,5]; 51.4 (CH2C6H4CH=CH2); 51.8 [CH2C6H2(OCH3)3–3,4,5]; 105.9, 113.7, 115.3, 127.1, 128.3, 129.5, 131.2, 131.3, 131.4, 132.1, 135.8, 138.4, 138.5 and 153.9 (Ar–C and CH2C6H4CH=CH2); 143.9 (NCHN). Anal. calc. for C26H27N2O3Cl (450.96 g/mol): C, 69.25%; H, 6.03%; N, 6.21%. Found: C, 69.38%; H, 6.21%; N, 6.19%.
1-(4-Vinylbenzyl)-3-(2,3,4,5,6-pentamethylbenzyl)benzimidazolium chloride, 1 g
Yield: 84%, m.p.: >330 °C, IR(CN): 1557.7 cm−1. 1H NMR (300 MHz, DMSO-d6), δ: 2.24 [s, 6H, CH2C6(CH3)5–3,5]; 2.26 [s, 6H, CH2C6(CH3)5–2,6]; 2.28 [s, 3H, CH2C6(CH3)5–4]; 5.29 (d, 1H, CH2C6H4CH=CH2, J = 10.5 Hz); 5.73 [s, 2H, CH2C6(CH3)5–2,3,4,5,6]; 5.77 (s, 2H, CH2C6H4CH=CH2); 5.85 (d, 1H, CH2C6H4CH=CH2, J = 17.7 Hz); 6.71 (m, 1H, CH2C6H4CH=CH2); 7.41–8.24 (m, 8H, Ar–H); 9.45 (s, 1H, NCHN). 13C NMR (75 MHz, DMSO-d6), δ: 16.9, 17.2 and 17.5 [CH2C6(CH3)5–2,3,4,5,6]; 46.9 [CH2C6(CH3)5–2,3,4,5,6]; 50.0 (CH2C6H4CH=CH2); 114.5, 115.7, 126.2, 127.1, 127.3, 127.4, 128.7, 129.2, 131.5, 132.0, 133.5, 134.4, 136.4, 136.5 and 137.8 (Ar–C and CH2C6H4CH=CH2); 141.8 (NCHN). Anal. calc. for C28H31N2Cl (431.01 g/mol): C, 78.03%; H, 7.25%; N, 6.50%. Found: C, 78.12%; H, 7.40%; N, 6.44%.
General preparation of 1-(4-vinylbenzyl) substituted Ag-NHC complexes, 2
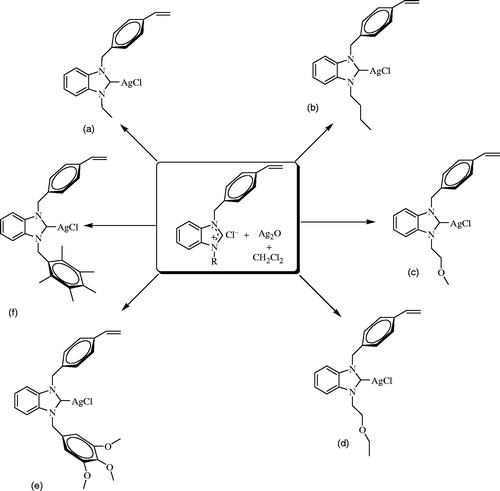
New Ag-NHC complexes were prepared according to literatureCitation1,Citation5,Citation22,Citation23. Silver oxide (1 mmol), benzimidazolium salt (2 mmol) and activated molecular sieves in CH2Cl2 (25 ml) were stirred at 25 °C for 1 day in dark conditions. After the reaction finished, the mixture was filtered through celite. The solvent in the medium was aspirated under reduced pressure. The synthesized compound was crystallized from a dichloromethane/diethyl ether (2:1) mixture at room temperature.
[1-(4-Vinylbenzyl)-3-ethylbenzimidazol-2-ylidene]chlorosilver(I), 2a
Yield: 68%, m.p.: 131–132 °C, IR(CN): 1396.6 cm−1. 1H NMR (300 MHz, DMSO-d6), δ: 1.44 (t, 3H, CH2CH3, J = 7.2 Hz); 4.52 (q, 2H, CH2CH3, J = 7.2 Hz); 5.24 (d, 1H, CH2C6H4CH=CH2, J = 10.8 Hz); 5.71 (s, 2H, CH2C6H4CH=CH2); 5.72 (d, 1H, CH2C6H4CH=CH2, J = 15.3 Hz); 6.68 (m, 1H, CH2C6H4CH=CH2); 7.33–7.47 (m, 8H, Ar–H). 13C NMR (75 MHz, DMSO-d6), δ: 16.4 and 44.4 (CH2CH3); 52.1 (CH2C6H4CH=CH2); 112.5, 112.8, 115.3, 124.5, 126.9, 128.2, 133.4, 133.7, 136.3, 136.4, 136.5 and 137.4 (Ar–C and CH2C6H4CH=CH2); 188.3 (C–Ag). Anal. calc. for C18H18N2AgCl (405.67 g/mol): C, 53.29%; H, 4.47%; N, 6.91%. Found: C, 53.18%; H, 4.59%; N, 6.86%.
[1-(4-Vinylbenzyl)-3-butylbenzimidazol-2-ylidene]chlorosilver(I), 2b
Yield: 64%, m.p.: 132–133 °C, IR(CN): 1395.7 cm−1. 1H NMR (300 MHz, DMSO-d6), δ: 0.89 (t, 3H, CH2CH2CH2CH3, J = 7.2 Hz); 1.31 (hex., 2H, CH2CH2CH2CH3, J = 7.2 Hz); 1.84 (p, 2H, CH2CH2CH2CH3, J = 7.2 Hz); 4.49 (t, 2H, CH2CH2CH2CH3, J = 7.2 Hz); 5.22 (d, 1H, CH2C6H4CH=CH2, J = 10.8 Hz); 5.72 (s, 2H, CH2C6H4CH=CH2); 5.79 (d, 1H, CH2C6H4CH=CH2, J = 18.2 Hz); 6.68 (m, 1H, CH2C6H4CH=CH2); 7.32–7.46 (m, 8H, Ar–H). 13C NMR (75 MHz, DMSO-d6), δ: 14.1, 19.9, 32.4 and 48.9 (CH2CH2CH2CH3); 52.0 (CH2C6H4CH=CH2); 112.6, 112.8, 116.2, 124.5, 126.3, 128.1, 133.7, 133.8, 136.4, 136.5 and 137.4 (Ar–C and CH2C6H4CH=CH2); 188.9 (C–Ag). Anal. calc. for C20H22N2AgCl (433.72 g/mol): C, 55.38%; H, 5.11%; N, 6.46%. Found: C, 55.54%; H, 5.29%; N, 6.40%.
[1-(4-Vinylbenzyl)-3-(2-methoxyethyl)benzimidazol-2-ylidene]chlorosilver(I), 2c
Yield: 71%, m.p.: 134–135 °C, IR(CN): 1394.5 cm−1. 1H NMR (300 MHz, DMSO-d6), δ: 3.42 (s, 3H, CH2CH2OCH3); 3.43 (t, 2H, CH2CH2OCH3, J = 4.8 Hz); 4.64 (t, 2H, CH2CH2OCH3, J = 4.8 Hz); 5.23 (d, 1H, CH2C6H4CH=CH2, J = 11.1 Hz); 5.69 (s, 2H, CH2C6H4CH=CH2); 5.76 (d, 1H, CH2C6H4CH=CH2, J = 17.7 Hz); 6.67 (m, 1H, CH2C6H4CH=CH2); 7.14–7.81 (m, 8H, Ar–H). 13C NMR (75 MHz, DMSO-d6), δ: 49.0, 52.0 and 71.2 (CH2CH2OCH3); 58.8 (CH2C6H4CH=CH2); 112.6, 112.9, 115.4, 124.6, 126.9, 128.1, 133.5, 134.3, 136.3, 136.4 and 137.4 (Ar–C and CH2C6H4CH=CH2); 191.4 (C–Ag). Anal. calc. for C19H20N2OAgCl (435.7 g/mol): C, 52.38%; H, 4.63%; N, 6.43%. Found: C, 52.27%; H, 4.55%; N, 6.47%.
[1-(4-Vinylbenzyl)-3–(2-ethoxyethyl)benzimidazol-2-ylidene]chlorosilver(I), 2d
Yield: 63%, m.p.: 148–149 °C, IR(CN): 1455.0 cm−1. 1H NMR (300 MHz, DMSO-d6), δ: 0.99 (t, 3H, CH2CH2OCH2CH3, J = 6.9 Hz); 3.39 (q, 2H, CH2CH2OCH2CH3, J = 6.9 Hz); 3.79 (t, 2H, CH2CH2OCH2CH3, J = 4.2 Hz); 4.66 (t, 2H, CH2CH2OCH2CH3, J = 4.2 Hz); 5.24 (d, 1H, CH2C6H4CH=CH2, J = 10.8 Hz); 5.73 (s, 2H, CH2C6H4CH=CH2); 5.79 (d, 1H, CH2C6H4CH=CH2, J = 16.8 Hz); 6.68 (m, 1H, CH2C6H4CH=CH2); 7.33–7.85 (m, 8H, Ar–H). 13C NMR (75 MHz, DMSO-d6), δ: 15.4, 49.2, 52.0 and 66.1 (CH2CH2OCH2CH3); 68.9 (CH2C6H4CH=CH2); 112.7, 112.9, 115.3, 124.4, 124.5, 126.9, 127.0, 128.1, 133.5, 133.8, 134.3, 136.4, 136.5 and 137.4 (Ar–C and CH2C6H4CH=CH2); 189.3 (C–Ag). Anal. calc. for C20H22N2OAgCl (449.72 g/mol): C, 53.41%; H, 4.93%; N, 6.23%. Found: C, 53.59%; H, 4.81%; N, 6.29%.
[1-(4-Vinylbenzyl)-3-(3,4,5-trimethoxybenzyl)benzimidazol-2-ylidene]chlorosilver(I), 2e
Yield: 59%, m.p.: 225–226 °C, IR(CN): 1392.1 cm−1. 1H NMR (300 MHz, DMSO-d6), δ: 3.61 [s, 3H, CH2C6H2(OCH3)3–4]; 3.79 [s, 6H, CH2C6H2(OCH3)3–3,5]; 5.24 (d, 1H, CH2C6H4CH=CH2, J = 11.1 Hz); 5.65 [s, 2H, CH2C6H2(OCH3)3–3,4,5]; 5.75 (s, 2H, CH2C6H4CH=CH2); 5.79 (d, 1H, CH2C6H4CH=CH2, J = 18.3 Hz); 6.72 (m, 1H, CH2C6H4CH=CH2); 6.79 [s, 2H, CH2C6H2(OCH3)3–3,4,5]; 7.34–7.89 (m, 8H, Ar–H). 13C NMR (75 MHz, DMSO-d6), δ: 56.3 and 60.5 [CH2C6H2(OCH3)3–3,4,5]; 52.1 (CH2C6H4CH=CH2); 52.4 [CH2C6H2(OCH3)3–3,4,5]; 105.9, 112.9, 115.3, 124.6, 126.9, 127.0, 128.1, 132.0, 133.7, 133.8, 133.9, 136.3, 136.4, 137.4, 137.5 and 153.5 (Ar–C and –CH=CH2); 189.3 (C-Ag). Anal. calc. for C26H26N2O3AgCl (557.82 g/mol): C, 55.98%; H, 4.70%; N, 5.02%. Found: C, 55.87%; H, 4.81%; N, 5.05%.
[1-(4-Vinylbenzyl)-3-(2,3,4,5,6-pentamethylbenzyl)benzimidazol-2-ylidene]chlorosilver(I), 2f
Yield: 55%, m.p.: 289–290 °C, IR(CN): 1395.0 cm−1. 1H NMR (400 MHz, DMSO-d6), δ: 2.15–2.49 [m, 15H, NCH2C6(CH3)5–2,3,4,5,6]; 3.35 [s, 2H, NCH2C6(CH3)–2,3,4,5,6]; 5.59 (s, 2H, CH2C6H4CH=CH2); 5.22–6.73 (m, 3H, CH2C6H4CH=CH2); 7.23–8.23 (m, 8H, Ar–H). Anal. calc. for C28H30N2AgCl (537.87 g/mol): C, 62.52%; H, 5.62%; N, 5.21%. Found: C, 62.64%; H, 5.75%; N, 5.17%.
Evaluation of antibacterial and antifungal activity
The following 11 microorganisms, consisting of 10 bacteria and 1 yeast, were studied: Aeromonas hydrophila (ATCC 7965), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (FMC 5), Proteus mirabilis (BC 3624), Pseudomonas aeruginosa (ATCC 27853), Salmonella typhimurium (NRRLE 4463), Yersinia enterocolitica (ATCC 1501), Bacillus cereus (FMC 19), Listeria monocytogenes (1/2B), Staphylococcus aureus (ATCC 29213) and Candida albicans (ATCC 1223).
The antimicrobial activities of 13 compounds (1a–g; 2a–f) were obtained in vitro using the agar-well diffusion methodCitation19. The stock solutions (10 mg/ml−1) of the compounds were prepared by dissolving them in dimethyl sulfoxide (DMSO). All samples were sterilized through a 0.2-µm membrane filter. Y. enterocolitica and C. albicans were grown in nutrient broths and malt extract at room temperature (25 °C) for 18 h, respectively. The other bacteria were grown in nutrient broth at 35 °C for 18 h and then suspensions were adjusted to 0.5 McFarland standard turbidity. Two hundred and fifty microliters (250 µl) of each microorganism was added to a flask containing 25 ml of sterile Mueller Hinton agar or malt extract agar at 45 °C and poured into Petri dishes (9 cm diameter). Then, the agars were allowed to solidify at 4 °C for 1 h. Holes were made in the agar using sterile cork borers (Ø = 6 mm). The solutions of compounds (50 µl) were applied to the holes and DMSO was used as a control. C. albicans and Y. enterocolitica were incubated at room temperature for 14–24 h in the inverted position. The other microorganisms were incubated at 35 °C for 18–24 h. At the end of the period, the inhibition zones which formed on the medium were measured in millimeters (mm). Tetracycline (10 mg/ml) (Sigma T3258–56) and natamycin (30 mg/ml) (Delvocid DMS) standard antibiotics were used as positive controls.
Results and discussion
Synthesis of N-heterocyclic carbene precursors and their silver complexes
The 1-(4-vinylbenzyl)-3-alkylbenzimidazolium salts (1a–g) as NHC-precursors, which are stable against light, air and humidity both in the hard form and in the solution state, were obtained by quaternization of 1-(4-vinylbenzyl)benzimidazole with various alkyl halides in DMF (Scheme 1). These salts were soluble in polar solvents such as dimethylsulfoxide, ethyl alcohol and dimethyl formamide but were not soluble in common slightly polar or nonpolar solvents like diethyl ether and hexane. The structures of 1a–g were verified using spectroscopic and elemental analysis techniques. The 1H NMR spectra of the benzimidazolium salts, which were collected in CDCl3 and DMSO-d6 over the scan range δ 0–12 ppm, further confirmed the designated structures; the resonances for NCHN were seen as a sharp singlet at δ 10.46, 10.35, 10.05, 11.41, 10.05, 12.05 and 9.45 ppm for 1a–g, respectively.
13C NMR chemical shifts were consistent with the suggested structures. The imino carbon resonances were observed as a typical singlet at 142.8, 143.0, 141.2, 143.9 and 141.8 ppm for benzimidazolium salts (1a, 1b, 1e–g), respectively. The NCHN proton and carbon signals of benzimidazolium salts were obtained in similar field with our previous studiesCitation1,Citation21,Citation24. The FT-IR data clearly indicated the presence of ν(CN) at 1561.0, 1556.2, 1557.9, 1562.7, 1554.8, 1557.5 and 1557.7 cm−1 for the benzimidazolium salts (1a–g), respectively.
The complexes (2a–f) were synthesized by treatment of benzimidazolium salts with Ag2O in CH2Cl2 (Scheme 2). To form of the Ag-NHC complexes, the reaction mixture was mixed for 24 h at 25 °C in dark conditions. In the 1H NMR spectra, the lack of a downfield NCHN signal shows the successful formation of Ag-NHC complexes. The signal of benzylic proton (N–CH2–Ar) belonging to Ag-NHC complexes was observed as a sharp singlet signal at around δ 5 ppm. These resonance values are very similar with resonance values of corresponding salts. The structures of the supposed Ag-NHC compounds were also verified with 13C NMR, because the carbene carbon resonance is shifted downfield, such as at 188.3, 188.9, 191.4 189.3 and 189.3 ppm for new complexes 2a–g. This signal shift is in accordance with literature reportsCitation1,Citation25,Citation26. Carbene carbon signal shifted downfield only. Other carbon signals of Ag-NHC complexes observed were similar to benzimidazolium salts. The FT-IR data clearly indicated the presence of ν(CN) at 1396.6, 1395.7, 1394.5, 1455.0, 1392.1 and 1395.0 cm−1 for the Ag-NHC complexes (2a–f), respectively. Unfortunately, an appropriate single crystal from these synthesized complexes for X-ray diffraction was not obtained. However, in a previous study, we had obtained the single crystals of similar silver complexes in order to prove the coordination bond between the silver and carbon atomsCitation1,Citation27.
Evaluation of the antimicrobial activity
The antimicrobial activity of the synthesized compounds was determined against three Gram (+) bacterial strains (B. subtilis, L. monocytogenes, S. aureus), seven Gram (−) bacterial strains (E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa, S. typhimurium, Y. enterocolitica) and one yeast (C. albicans) by agar diffusion assay. The results are given in . No inhibition zone was seen in the control (DMSO). All of the compounds demonstrated potent antimicrobial activity against the microorganisms tested in this study. Generally, the silver complexes 2a–f were more effective than benzimidazolium salts 1a–g. Compound 1g showed the most activity among the synthesized salts, which may be attributed to the presence of the aromatic group. The alkyl group which contains an electron-donating substituent may have contributed to the antibacterial activity of 1a–e compounds. The antibacterial activity of the silver complexes was almost similar to that of the standard drug, tetracycline. In the case of silver complexes, their antimicrobial activities were similar to each other. However, it may be said that 2d was slightly less effective than the other silver complexes. Also, it was seen that Y. enterocolitica was more resistant to the salts among the Gram (−) bacterial strains. Silver complexes (12–15 mm, inhibition zones) were more potent antifungals than the salts (7–10 mm, inhibition zones). The 1b salt was more effective against P. mirabilis and L. monocytogenes than against other organisms. The 2a and 2d complexes showed the highest activity against P. aeruginosa. Compared to all synthesized compounds, the 2f complex was most effective against the Gram-positive bacteria (S. aureus) and yeast (C. albicans). Many studies have shown that benzimidazole derivatives are effective against different strains of microorganisms. 5,6-Dimethylbenzimidazole is a constituent of naturally occurring vitamin B12. For this reason, benzimidazole derivatives have attracted the attention of researchers. Although vitamin B12 is able to induce the growth of bacteria, benzimidazole and some of its derivatives inhibit bacterial growth. Owing to the structural similarity to purine, the antibacterial ability of benzimidazole is explained by its competition with purines resulting in inhibition of the synthesis of bacterial nucleic acids and proteinsCitation15.
Table 1. Antimicrobial activities of compounds 1a–g, 2a–f and reference drug against the tested microorganisms.
Conclusion
In this study, seven benzimidazolium salts (1a–g) and six Ag-NHC complexes (2a–f) were synthesized and their structures were characterized by NMR, IR and elemental analysis. They were screened for their in vitro antimicrobial activities. These compounds displayed good antimicrobial potential. The biological activity results indicated that the silver complexes were found to have more potent antimicrobial properties than their salts. Thus, these newly synthesized compounds may be used as antimicrobial agents in the food and pharmaceutical industries. It is believed that these compounds will form the basis for the synthesis of pharmacologically important drugs.
Supplementary material available online
Declaration of interest
This work was financially supported by Inönü University Research Fund (I.U.B.A.P. 2012/02).
References
- Gök Y, Akkoç S, Albayrak S, et al. Phenyl-substituted carbene precursors and their silver complexes: synthesis, characterization and antimicrobial activities. Appl Organometal Chem 2014;28:244–51
- Ha SK, Shobha D, Moon E, et al. Anti-neuroinflammatory activity of 1,5-benzodiazepine derivatives. Bioorg Med Chem Lett 2010;20:3969–71
- Haris RC, Straley JM. Cationic polymethine dyes for acrylic fibers. U.S. patent 1,537,757; 1968. [Chem Abstr 1970;73:00054w]
- Shaharyar M, Mazumder A, Salahuddin, et al. Synthesis, characterization and pharmacological screening of novel benzimidazole derivatives. Arab J Chem 2011. [Epub ahead of print]. doi: 10.1016/j.arabjc.2011.04.013
- Gök Y, Akkoç S, Çelikal ÖÖ, et al. Nfunctionalized benzimidazol-2-ylidene silver complexes: synthesis, characterization, and antimicrobial studies. Turk J Chem 2013;37:1007–13
- Hattori K, Yamaguchi K, Yamaguchi J, Itami K. Pd- and Cu-catalyzed C–H arylation of indazoles. Tetrahedron 2012;68:7605–12
- Zulikha HZ, Haque RA, Budagumpi S, Abdul Majid AMS. Topology control in nitrile-functionalized silver(I)-N-heterocyclic carbene complexes: synthesis, molecular structures, and in vitro anticancer studies. Inorg Chim Acta 2014;411:40–7
- El-Emam AA, Al-Deeb AO, Al-Omar M, Lehmann J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg Med Chem 2004;12:5107–13
- Ersmark K, Nervall M, Hamelink E, et al. Synthesis of malarial plasmepsin inhibitors and prediction of binding modes by molecular dynamics simulations. J Med Chem 2005;48:6090–106
- Macaev F, Rusu G, Pogrebnoi S, et al. Synthesis of novel 5-aryl-2-thio-1,3,4-oxadiazoles and the study of their structure–anti-mycobacterial activities. Bioorg Med Chem 2005;13:4842–50
- Mullican MD, Wilson MW, Conner DT, et al. Design of 5-(3,5-di-tert-butyl-4-hydroxyphenyl)-l,3,4-thiadiazoles, -1,3,4-oxadiazoles, and -1,2,4-triazoles as orally-active, nonulcerogenic antiinflammatory agents. J Med Chem 1993;36:1090–9
- Shingalapur RV, Hosamani KM, Keri RS, Hugar MH. Derivatives of benzimidazole pharmacophore: synthesis, anticonvulsant, antidiabetic and DNA cleavage studies. Eur J Med Chem 2010;45:1753–9
- Kumar M, Narasimhan B, Kumar P, et al. 4(1-Aryl-5-chloro-2-oxo-1,2-dihydro-indol-3-ylideneamino)-N-substituted benzene sulfonamides: synthesis, antimicrobial, anticancer evaluation and QSAR studies. Arab J Chem 2014;7:436–47
- Kumar M, Ramasamy K, Mani V, et al. Synthesis, antimicrobial, anticancer, antiviral evaluation and QSAR studies of 4-(1-aryl-2-oxo-1,2-dihydro-indol-3-ylideneamino)-N-substituted benzene sulfonamides. Arab J Chem 2014;7:396–408
- Özkay Y, Tunalı Y, Karaca H, Işıkdağ İ. Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazone moiety. Eur J Med Chem 2010;45:3293–8
- Tunçbilek M, Kiper T, Altanlar N. Synthesis and in vitro antimicrobial activity of some novel substituted benzimidazole derivatives having potent activity against MRSA. Eur J Med Chem 2009;44:1024–33
- Ansari KF, Lal C. Synthesis, physicochemical properties and antimicrobial activity of some new benzimidazole derivatives. Eur J Med Chem 2009;44:4028–33
- Padalkar VS, Gupta VD, Phatangare KR, et al. Synthesis of novel dipodal-benzimidazole, benzoxazole and benzothiazole from cyanuricchloride: structural, photophysical and antimicrobial studies. J Saudi Chem Soc 2014;18:262–8
- Seenaiah D, Reddy PR, Reddy GM, et al. Synthesis, antimicrobial and cytotoxic activities of pyrimidinyl benzoxazole, benzothiazole and benzimidazole. Eur J Med Chem 2014;77:1–7
- Güven ÖÖ, Erdoğan T, Göker H, Yıldız S. Synthesis and antimicrobial activity of some novel phenyl and benzimidazole substituted benzyl ethers. Bioorg Med Chem Lett 2007;17:2233–6
- Akkoç S, Gök Y. Synthesis and characterization of 1-phenyl-3-alkylbenzimidazol-2-ylidene salts and their catalytic activities in the Heck and Suzuki cross-coupling reactions. J Coord Chem 2013;66:1396–404
- Gök Y, Akkoç S, Özeroğlu Çelikal Ö, et al. In Vitro antimicrobial studies of naphthalen-1-ylmethyl substituted silver N-heterocyclic carbene complexes. Arab J Chem 2015. [Epub ahead of print]. doi: 10.1016/j.arabjc.2015.04.019
- Lin IJB, Vasam CS. Preparation and application of N-heterocyclic carbene complexes of Ag(I). Coord Chem Rev 2007;251:642–70
- Aktaş A, Akkoç S, Gök Y. Palladium catalyzed Mizoroki–Heck and Suzuki–Miyaura reactions using napthalenomethyl-substituted imidazolidin-2-ilidene ligands in aqueous media. J Coord Chem 2013;66:2901–9
- Iqbal MA, Haque RA, Nasri SF, et al. Potential of silver against human colon cancer: synthesis, characterization and crystal structures of xylyl (ortho, meta, para) linked bis-benzimidazolium salts and Ag(I)-NHC complexes: in vitro anticancer studies. Chem Central J 2013;7:27
- Iqbal MA, Umar MI, Haque RA, et al. Macrophage and colon tumor cells as targets for a binuclear silver(I) N-heterocyclic carbene complex, an anti-inflammatory and apoptosis mediator. J Inorg Biochem 2015;146:1–13
- Akkurt M, Akkoç S, Gök Y, et al. Chlorido[1-phenyl-3-(2,3,5,6-tetra-methyl-benz-yl)benzimidazol-2-yl-idene]silver (I). Acta Crystallogr 2012;E68:m590–1