Abstract
Novel sulfonamide derivatives 6a–i, as new carbonic anhydrase inhibitors which candidate for glaucoma treatment, were synthesized from the reactions of 4-amino-N-(4-sulfamoylphenyl) benzamide 4 and sulfonyl chloride derivatives 5a–i with high yield (71–90%). The structures of these compounds were confirmed by using spectral analysis (FT-IR, 1H NMR, 13C NMR, LC/MS and HRMS). The inhibition effects of 6a–i on the hydratase and esterase activities of human carbonic anhydrase isoenzymes, hCA I and II, which were purified from human erythrocytes with Sepharose®4B-l-tyrosine-p-aminobenzene sulfonamide affinity chromatography, were studied as in vitro, and IC50 and Ki values were determined. The results show that newly synthesized compounds have quite powerful inhibitory properties.
Introduction
Sulfonamide compounds are an important class of drugs for the medicine industry and they possess various types of biological activities such as antibacterialCitation1, anticancerCitation2, anticarbonic anhydrase for glaucoma treatmentCitation3–6, acetylcholinesterase inhibitor agents for Alzheimer’s diseaseCitation7,Citation9, antiobesityCitation10 and high-ceiling diureticCitation11. They are commonly used in human and veterinary medicine for therapeutic and prophylactic purposes to fight many dangerous illnessesCitation12.
Carbonic anhydrase (CA, EC 4.2.1.1) is a metalloenzyme that contains Zn2+ ion in its active site, which catalyzes the interconversion between carbon dioxide and bicarbonate and protonCitation13. Although this reaction is simple, it plays an important role in many physiological and pathological processes such as transport of carbon dioxide and bicarbonate between metabolizing tissues and lungs, electrolyte secretion, pH and carbon dioxide homeostasis, some biosynthetic reactions, calcification and tumorigenicityCitation14. These enzymes were encoded by six different unrelated gene families (α, β, γ, δ, ζ, η) in living organismsCitation15,Citation16. Nowadays, it is known that there are 16 different isozymes of mammalian CAs that belongs to α gene familyCitation17. Among these isozymes CA I, CA II, CA III, CA VII and CA XIII are cytosolic; CA IV, CA IX, CA XII, CA XIV and CA XV are membrane bound; CA VA and CA VB are mitochondrial and CA VI is secreted into saliva and milkCitation15–17. CA I is a major CA isozyme in most of the vertebrates and CA II is also present in human eyeCitation15–18. hCA II is involved in ciliary processes for aqueous humor secretion. Therefore, the inhibition of hCA II is therapeutic for glaucoma, characterized by the elevation of intraocular pressure as a result of excessive secretion of aqueous humorCitation19,Citation20. Some sulfonamide class CA inhibitors (acetazolamide (AAZ), dorzolamide (DZA) and brinzolamide (BRZ)) have been developed for the treatment of glaucoma so farCitation3. But they have some side effects including metallic taste, depression, weight loss, blurred vision, burning of the eye, etc.Citation20 This situation reveals that there is a need for the development of new inhibitory agents.
This paper reports the synthesis, characterization and investigation of inhibitory properties of some new sulfonamide derivatives on hCA I and hCA II (ciliary process isozyme). Inhibition effects of the compounds have been investigated under in vitro conditions and structure–activity relationships of them have been explained.
Methods
Chemistry
The chemicals used in the synthesis of sulfonamide derivatives were provided by Merck and Aldrich Chemical Company and CNBr activated Sepharose®4B for affinity column and electrophoresis reagents were obtained from Sigma Chem. Co. All chemicals and solvents used for the synthesis were spectroscopic reagent grade. Melting points were measured on a Bibby Scientific Stuart Digital, Advanced, SMP30 apparatus. Fourier Transform Infrared (FT-IR) spectra were recorded on a Bruker Optics, ALPHA FT-IR spectrometer. The 1H NMR, and 13C NMR spectra were obtained with a Bruker AV 400 Ultra Shield instrument in DMSO-d6 as solvent with trimethylsilane as the internal reference, at 400 and 100 MHz, respectively. HRMS spectra were detected by an Agilent Technologies 6530 Accurate-Mass Q-TOF LC/MS at the Advanced Technology Research Center of Dumlupinar University (ILTEM).
General procedure for preparation of 4-nitro-N-(4-sulfamoylphenyl)benzamide compound (3)
4-Aminobenzenesulfonamide (1) (1.739 g, 10.1 mmol), 4-nitro benzoylchloride (2) (1.856 g, 10 mmol), 3 mL dry triethylamine (TEA) and dry 30 mL THF were stirred for 5 h at room temperature. Afterwards the solvent was removed in vacuo and the precipitated crude product was washed with 1000 mL distilled H2O. The product was purified by recrystallization from ethanolCitation4,Citation5.
Procedure for preparation of 4-amino-N-(4-sulfamoylphenyl)benzamide compound (4)
Na2S·9H2O (1 mmol) and sulfur (2 mmol) were dissolved by boiling 20 mL of water. This solution (sodium poly-sulfur) was then added dropwise to a stirred and warm solution of 4-nitro-N-(4-sulfamoylphenyl)benzamide (3) (1 mmol) in ethanol–water. The progress of the reaction was monitored by TLC. Once the reaction was completed, the mixture was cooled to room temperature and solid was filtered off and washed with H2O. The sulfonamide product was purified and recrystallized from the ethanol (90%). The melting point of compound (4) was found to be 313 °CCitation4,Citation5.
General procedure for preparation of sulfonamide derivatives 6a–i
A mixture of a 4-amino-N-(4-sulfamoylphenyl)benzamide (4) 0.5 mmol (0.1455 g) and methanesulfonyl chloride (5a) 0.55 mmol (0.06325 g) in dry 5 mL pyridine were stirred for 4 h at room temperature. The progress of the reaction was monitored by TLC. Once the reaction is completed, the solvent was removed in vacuo and residue was washed with 1000 mL water. The sulfonamide derivatives were obtained as pure products.
4-(Methylsulfonamido)-N-(4-sulfamoylphenyl)benzamide (6a)
As white solid, 132.98 mg, 72%, mp 291 °C (dec.). IR (cm−1): 3377, 3306 and 3280 w (–NH and –NH2), 3039 w (Ar–H), 2939 w (C–H), 1650 s (C=O), 1509 s (C=C); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 10.48 (s, 1H, –NH), 10.27 (s, 1H, –NH), 8.00–7.95 (m, 4H, Ar–H), 7.83 (d, 2H, J = 8.8 Hz, Ar–H), 7.34 (d, 2H, J = 8.8 Hz, Ar–H), 7.30 (s, 2H, SO2NH2), 3.12 (s, 3H, –CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 165.22, 142.19, 141.84, 138.56, 128.93, 129.33, 126.51, 119.78, 117.82, 39.75; HRMS (QTOF-ESI): m/z [M] calcd. for C14H15N3O5S2: 369.0453; found [M − H]−: 368.0377.
4-(Ethylsulfonamido)-N-(4-sulfamoylphenyl)benzamide (6b)
As white solid, 141.87 mg, 74%, mp 311 °C (dec.). IR (cm−1): 3399, 3306 and 3280 w (–NH and –NH2), 3095 w (Ar–H), 2944 w (C–H), 1650 s (C=O), 1507 s (C=C); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 10.38 (s, 1H, –NH), 10.20 (s, 1H, –NH), 7.90–7.86 (m, 4H, Ar–H), 7.73 (d, 2H, J = 9.0 Hz, Ar–H), 7.27 (d, 2H, J = 8.8 Hz, Ar–H), 7.21 (s, 2H, SO2NH2), 3.13 (q, 2H, J = 7.36 Hz, –CH2), 1.14 (t, 3H, J = 7.3 Hz, –CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 165.22, 142.19, 141.90, 138.56, 128.88, 129.34, 126.49, 119.75, 117.65, 45.56, 8.02; HRMS (QTOF-ESI): m/z [M] calcd. for C15H17N3O5S2: 383.0610; found [M − H]−: 382.0535.
4-(4-Methoxyphenylsulfonamido)-N-(4-sulfamoylphenyl)benzamide (6c)
As white solid, 200.75 mg, 87%, mp 259 °C (dec.). IR (cm−1): 3370, 3283 and 3260 w (–NH and –NH2), 3105 w (Ar–H), 2976 w (C–H), 1651 s (C=O), 1503 s (C=C); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 10.60 (s, 1H, NH), 10.33 (s, 1H, NH), 7.83 (d, 2H, J = 8.78 Hz, Ar–H), 7.78 (d, 2H, J = 8.8 Hz, Ar–H), 7.73–7.70 (m, 4H, Ar–H), 7.21 (s, 2H, SO2NH2), 7.16 (d, 2H, J = 8.5 Hz, Ar–H), 7.02 (d, 2H, J = 8.8 Hz, Ar–H), 3.72 (s, 3H, –CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 165.18, 162.61, 142.16, 142.32, 138.55, 130.81, 129.17, 128.98, 126.49, 119.68, 118.04, 114.52, 55.65; HRMS (QTOF-ESI): m/z [M] calcd. for C20H19N3O6S2: 461.0715; found [M − H]−: 460.0638.
4-(4-Methylphenylsulfonamido)-N-(4-sulfamoylphenyl)benzamide (6d)
As white solid, 182.65 mg, 82%, mp 289 °C (dec.). IR (cm−1): 3375, 3299 and 3276 w (–NH and –NH2), 3088 w (Ar–H), 1648 s (C=O), 1506 s (C=C); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 10.66 (s, 1H, NH), 10.33 (s, 1H, NH), 7.84–7.65 (m, 8H, Ar–H), 7.30 (d, 2H, J = 8.0 Hz, Ar–H), 7.20 (s, 2H, SO2NH2), 7.16 (d, 2H, J = 8.5 Hz, Ar–H), 2.27 (s, 3H, –CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 165.17, 143.63, 142.14, 141.19, 138.56, 136.38, 129.38, 129.83, 129.18, 126.76, 126.48, 119.67, 118.10, 20.94; HRMS (QTOF-ESI): m/z [M] calcd. for C20H19N3O5S2: 445.0766; found [M − H]−: 444.0689.
4-(Naphthalene-2-sulfonamido)-N-(4-sulfamoylphenyl)benzamide (6e)
As white solid, 204.65 mg, 85%, mp 254 °C (dec.). IR (cm−1): 3356 and 3324, 3262 and 3230 w (–NH and –NH2), 1653 s (C=O), 1506 s (C=C); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 10.96 (s, 1H, –NH), 10.39 (s, 1H, –NH), 8.60 (s, 1H, Ar–H), 8.19 (d, 1H, J = 7.8 Hz, Ar–H), 8.13 (d, 1H, J = 8.8 Hz, Ar–H), 8.02 (d, 1H, J = 7.8 Hz, Ar–H), 7.91–7.79 (m, 7H, Ar–H), 7.73–7.65 (m, 2H, Ar–H), 7.32 (d, 2H, J = 8.8, Ar–H) 7.30 (s, 2H, SO2NH2); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 165.14, 142.13, 141.04, 138.54, 136.17, 134.33, 131. 50, 129.69, 129.39, 129.29, 129.20, 129.16, 128.29, 127.83, 127.80, 126.48, 121.85, 119.66, 118.19; HRMS (QTOF-ESI): m/z [M] calcd. for C23H19N3O5S2: 481.0766; found [M − H]−: 480.0690.
N-(4-sulfamoylphenyl)-4-(2,4,6-trimethylphenylsulfonamido)benzamide (6f)
As white solid, 179.95 mg, 76%, mp 262 °C (dec.). IR (cm−1): 3378 and 3359, 3314 and 3285 w (–NH and –NH2), 3188 w (Ar–H), 2979 w (C–H), 1658 s (C=O), 1508 s (C=C); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 10.78 (s, 1H, NH), 10.37 (s, 1H, NH), 7.91 (d, 2H, J = 8.8 Hz, Ar–H), 7.85 (d, 2H, J = 8.8 Hz, Ar–H), 7.79 (d, 2H, J = 8.8 Hz, Ar–H), 7.29 (s, 2H, SO2NH2), 7.10 (d, 2H, J = 8.8 Hz, Ar–H), 7.05 (s, 2H, Ar–H), 2.63 (s, 6H, 2x–CH3), 2.23 (s, 3H, –CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 165.14, 142.43, 142.19, 141.18, 138.73, 136.51, 133.42, 128.62, 131.92, 129.22, 126.48, 119.60, 116.82, 22.38, 20.94; HRMS (QTOF-ESI): m/z [M] calcd. for C22H23N3O5S2: 473.1079; found [M − H]−: 472.1001.
4-(3,5-Dichloro-2-hydroxyphenylsulfonamido)-N-(4-sulfamoylphenyl)benzamide (6g)
As white solid, 209.13 mg, 81%, mp 266 °C (dec.). IR (cm−1): 3390 br (OH), 3347, 3282 and 3250 w (–NH and –NH2), 3090 w (Ar–H), 1653 s (C=O), 1505 s (C=C); 1H NMR (DMSO–d6, 400 MHz) δ (ppm): 11.27 (s, 1H, OH), 10.85 (s, 1H, NH), 10.41 (s, 1H, NH), 7.91 (d, 2H, J = 9.0 Hz, Ar–H), 7.87–7.85 (m, 3H, Ar–H), 7.80–7.78 (m, 3H, Ar–H), 7.28 (s, 2H, SO2NH2), 7.25 (d, 2H, J = 8.8 Hz, Ar–H); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 165.15, 150.26, 142.16, 140.66, 138.53, 129.25, 128.83, 123.88, 122.86, 134.21, 129.15, 128.41, 126.48, 119.64, 117.83; HRMS (QTOF-ESI): m/z [M] calcd. for C19H15Cl2N3O6S2: 514.9779; found [M − H]−: 513.9723.
4-(Phenylsulfonamido)-N-(4-sulfamoylphenyl)benzamide (6h)
As white solid, 153.17 mg, 71%, mp 279 °C (dec.). IR (cm−1): 3386 and 3274 w (–NH and –NH2), 3090 and 3066 w (Ar–H), 1649 s (C=O), 1505 s (C=C); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 10.84 (s, 1H, NH), 10.43 (s, 1H, NH), 7.93–7.80 (m, 8H, Ar–H), 7.67–7.58 (m, 3H, Ar–H), 7.30 (s, 2H, SO2NH2), 7.27 (d, 2H, J = 8.5 Hz, Ar–H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 165.18, 142.15, 141.06, 139.25, 138.56, 133.21, 129.42, 129.21, 126.70, 126.50, 119.70, 118.25; HRMS (QTOF-ESI): m/z [M] calcd. for C19H17N3O5S2: 431.0610; found [M − H]−: 430.0535.
4-(4-Bromophenylsulfonamido)-N-(4-sulfamoylphenyl)benzamide (6i)
As white solid, 229.67 mg, 90%, mp 287 °C (dec.). IR (cm−1): 3417 and 3387, 3291 and 3273 w (–NH and –NH2), 3089 w (Ar–H), 1649 s (C=O), 1507 s (C=C); H1 NMR (DMSO-d6, 400 MHz) δ (ppm): 10.91 (s, 1H, NH), 10.45 (s, 1H, NH), 7.95–7.89 (m, 4H, Ar–H), 7.84–7.77 (m, 6H, Ar–H), 7.31 (s, 2H, SO2NH2), 7.27 (d, 2H, J = 8.5 Hz, Ar–H); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 165.15, 142.14, 140.72, 138.58, 138.47, 129.74, 127.16, 132.53, 129.29, 128.72, 126.51, 119.73, 118.58; HRMS (QTOF-ESI): m/z [M] calcd. for C19H16BrN3O5S2: 508.9715; found [M − H]−: 507.9619.
Purification of carbonic anhydrase I and II isoenzymes from human erythrocytes
Erythrocytes were purified from human blood. The blood samples were centrifuged at 1500 rpm for 20 min and plasma was removed. Later, red cells were washed with isotonic solution (0.9% NaCl), and the erythrocytes were hemolyzed with 1.5 volumes of ice-cold water. Cell membranes were removed by centrifugation at 4 °C, 20 000 rpm for 30 min. The pH of hemolysate was adjusted to 8.7 with solid TRIS (tris(hydroxymethyl)aminomethane). The hemolysate was applied to affinity column (Sepharose®4B-l-tyrosine-p-aminobenzene sulfonamide) pre-equilibrated with 25.0 mM TRIS–HCl/0.1 M Na2SO4 (pH 8.7). After the extensive washing with a solution of 25.0 mM TRIS–HCl/22.0 mM Na2SO4 (pH 8.7), the hCA I and hCA II isoenzymes were eluted with the solution of 1.0 M NaCl/25.0 mM Na2HPO4 (pH 6.3) and 0.1 M NaCH3COO/0.5 M NaClO4 (pH 5.6), respectivelyCitation21. For quantitative protein determination, the Bradford method was used with bovine serum albumin as a standardCitation22. Also purity control of the isoenzymes was performed with SDS-PAGE after the purificationCitation23.
Determination of hydratase and esterase activities of hCA I and hCA II
The CO2 hydratase activity of the enzyme was determined at 0 °C in a veronal buffer (pH 8.15) with the pH-stat method as the indicator and saturated carbon dioxide solution as the substrate in a final volume of 4.2 mL. The time (in seconds) taken for the solution to change from pH 8.15 to pH 6.50 was measured. The enzyme unit (EU) is the enzyme amount that reduces the non-enzymatic reaction time by 50%. The activity of an enzyme unit was calculated by using the equation ((t0−tc)/tc), where t0 and tc are times for pH change of the non-enzymatic and enzymatic reactions, respectivelyCitation24.
Esterase activity was assayed by following the change in the absorbance at 348 nm of 4-nitrophenylacetate to 4-nitrophenylate ion over a period of 3 min at 25 °C using a spectrophotometer according to the method described in the literatureCitation25. The enzymatic reaction, in a total volume of 3.0 mL, contained 1.4 mL of 0.05 M TRIS–SO4 buffer (pH 7.4), 1.0 mL of 3.0 mM 4-nitrophenylacetate, 0.5 mL H2O and 0.1 mL enzyme solution. A reference measurement was obtained by preparing the same cuvette without enzyme solution.
Determination of IC50 and Ki values of the compounds
To determine the IC50 values of the inhibitors, hydratase and esterase activities of CA isoenzymes were assayed in the presence of various inhibitor concentrations as mentioned above. Regression analysis graphs were drawn by plotting the percent enzyme activity versus inhibitor concentration and IC50 values were calculatedCitation3,Citation6.
To determine Ki values as well as the inhibition type, three different inhibitor concentrations giving 30%, 50% and 70% inhibition were selected. At each of these inhibitor concentrations, enzyme activity was measured in the presence of various substrate concentrations (0.3 mM, 0.4 mM, 0.5 mM, 0.6 mM and 0.7 mM) and the data were linearized with Lineweaver–Burk plot for Vmax and the Ki determination. Enzyme activity was also measured in the presence of the same substrate concentrations but in the absence of any inhibitor to determine the VmaxCitation3,Citation6.
Statistical analysis
All the presented data were confirmed in at least three independent experiments and are expressed as the mean ± standard deviation (SD). Data were analyzed by using a one-way analysis of variance for multiple comparisons (SPSS 13.0, SPSS Inc., Chicago, IL). p < 0.00001 was considered to be statistically significant.
Result and discussion
Chemistry
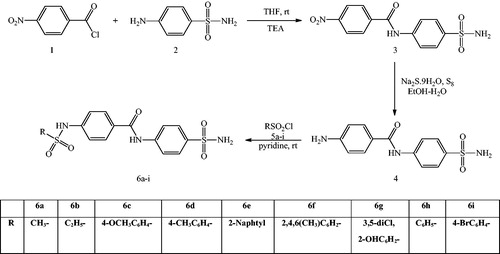
The general synthetic procedure to obtain sulfonamide derivatives in three step reaction is described in Scheme 1.
The 4-nitro-N-(4-sulfamoylphenyl)benzamide (3) was prepared in the presence of TEA in THF at room temperatureCitation3–5. The 4-amino-N-(4-sulfamoylphenyl)benzamide (4) was obtained in polysulfide solution by reduction of the nitro compound (3). Novel sulfonamide derivatives 6a–i were synthesized from the reactions of 4-amino-N-(4-sulfamoylphenyl)benzamide 4 and sulfonyl chloride derivatives 5a–i in pyridine.
The novel sulfonamide derivatives were obtained under mild conditions, in short times and in high yields.
Spectral data are consistent with the chemical structures of the compounds. When the IR spectrums were examined; the C=O groups stretching vibrations, which belong to the structure of sulfonamide derivatives 6a–i, were observed between 1658 and 1648 cm−1 (Supplemental Figures).
Compound 6g aromatic O–H stretching band was observed at 3390 cm−1. Besides, sulfonamide derivatives 6a–i aromatic C–H stretching bands and aliphatic C–H stretching bands were observed between 3188–3039 cm−1 and 2979–2939 cm−1, respectively. –NH and –NH2 groups stretching vibration bands that belong to these compounds 6a–i were observed in the region between 3399–3317 cm−1 and 3314–3230 cm−1, respectivelyCitation26,Citation27. Stretching vibration of the C=C bonds in the aromatic structures was observed at 1509–1503 cm−1. Symmetric and asymmetric vibration bands of SO2, existing in the structure of the novel sulfonamide compounds were determined to give severe vibrations in the range of 1159–1135 cm−1 (Supplemental Figures).
The 1H NMR spectra of sulfonamide compounds (6a, 6c, 6d, and 6f), which belong to protons of the methyl groups, showed singlet peaks in between 2.27 and 3.72 ppm. The compound 6b ethyl group protons were observed in triplet peak at 1.14 ppm (3H) and quartet peak at 3.12 ppm (2H) (Supplemental Figures).
Aromatic protons of all the sulfonamide derivatives 6a–i showed signals in the region between 7.02 and 8.60 ppm. The compound 6g hydroxyl group proton was observed as a broad peak at 11.27 ppm. SO2NH2 group protons of sulfonamide compounds 6a–i were observed as singlet peaks between 7.20 and 7.31 ppm. The carboxamide group protons (–CONH–) of all compounds 6a–i showed broad peaks between 10.20 and 10.96 ppm (Supplemental Figures).
Carbonic anhydrase inhibition
In this part of the study we aimed to:
investigate the inhibition potentials of synthesized compounds on hCA I and II,
compare the contribution of different functional groups on the inhibition potentials of the compounds.
For this purpose, firstly, the carbonic anhydrase isozymes (hCA I and II) were purified separately from human erythrocyte cells with a single-step method, Sepharose®4B-p-aminobenzene sulfonamide affinity chromatography. Purity control of the isozymes was performed by SDS-PAGE and single bands were observed and then the inhibition potentials of newly synthesized compounds on hydratase and esterase activities of hCA I and II were investigated as in vitroCitation24,Citation25.
Kinetic studies showed that the compounds have inhibitory properties on hydratase and esterase activities of hCA I and II isoforms. The inhibition effects of synthesized sulfonamide derivatives on the hydratase activities of the isozymes demonstrate that these compounds are attached to zinc ion in the active siteCitation16.
As shown in , synthesized derivatives have more potential inhibition effects on hCA II than hCA I. Because hCA II is a target enzyme for glaucoma treatment, more powerful inhibition of this isozyme is clinically important. Also the compounds showed remarkable inhibition effects on the esterase activities of hCA I and hCA II when compared with AAZ, which is intraocular pressure reducing agent.
The compounds have inhibitory effects, with micromolar range, on the hydratase activities of the isozymes. The IC50 values were in the range of 0.518–1.654 μM for hCA I and 0.138–1.060 μM for hCA II. Compound 6c has the most powerful inhibition effect on the hydratase activity of hCA I (IC50 value 0.518 μM), whereas 6d is the strongest inhibitor for the hydratase activity of hCA II (IC50 value 0.138 μM). Also 6i has the weakest inhibition effect on the hydratase activities of the isoforms, hCA I and II (IC50 values 1.654 μM and 1.060 μM, respectively). Synthesized compounds are the more potent inhibitors for hydratase activity of hCA II than the other isoform’s hydratase activity. According to hydratase IC50 values, the inhibition potentials were in the order of 6c > 6d > 6e > 3 > 4>6f > 6a > 6g > 6b > 6h > 6i for hCA I, and 6d > 6a > 6c > 3 > 6e > 4 > 6f > 6b > 6g > 6h > 6i for hCA II.
Table 1. The effects of synthesized compounds on hCA I and II isozymes under in vitro conditions.
When the inhibition effects of the compounds on the esterase activities of the isozymes, hCA I and II were examined, a similar situation to the inhibition of the hydratase activity could be observed clearly. But the compounds showed more effective inhibition, nearly in nanomolar range, on the esterase activities of the isozymes. The IC50 values were in the range of 0.049–0.276 μM for hCA I and 0.027–0.237 μM for hCA II. Unlike the inhibition of the hydratase activity, newly synthesized compounds 6a–i have more powerful inhibition effects on the esterase activities of hCA I and hCA II than the starting compounds 3 and 4. 6f, containing 2,4,6–trimethylphenyl, has the strongest inhibition effect on the esterase activities of hCA I and II (IC50 values 0.049 and 0.027 μM, respectively). Also 6h and 6d have nearly the same inhibition potentials on the isozymes (see ). These compounds contain phenyl and tolyl moieties, respectively. Esterase IC50 values of 6d, 6f and 6h are close to each other. Furthermore, their substituents are similar to each other as structurally. Therefore it can be thought that these compounds make similar interactions with the active site. However, contrary to our expectations, the increase of the polarity in the substituents, reduced the inhibitory potentials of the compounds. This situation is clearly observed on 6c, 6g and 6i. Also the compounds containing non-aromatic substituents, 6a and 6b, have weaker inhibition potentials on the esterase activities of hCA I and hCA II. The reason why compounds 6d, 6f and 6h, which have apolar substituents, are potentially better inhibitors is that these compounds better interact with the hydrophobic pocket of the active site. A similar compound that has been studied previously supports this claim and PDB code of this compound (3N3J) shows how this compound interacts with this enzymeCitation28.
The esterase Ki values are in agreement with the esterase IC50 values. Therefore, the aforementioned structure–activity relationships for esterase activity can also be said for Ki values. These values were in the range of 0.020–0.149 μM for hCA I, and 0.011–0.141 μM for hCA II. hCA I and hCA II isozymes have more affinity against 6d, 6f and 6h derivatives that have apolar aromatic substituents (Ki values 0.025 ± 0.008 μM, 0.020 ± 0.003 μM and 0.022 ± 0.002 μM for hCA I, and 0.015 ± 0.006 μM, 0.011 ± 0.001 μM and 0.013 ± 0.001 μM for hCA II, respectively). But the affinity of the isozymes against inhibitors decreased in the presence of non-aromatic substituents (6a and 6b) and polar aromatic substituents (6c, 6g and 6i). According to Ki values, inhibition potentials were in the order of 6f > 6h > 6d > 6e > 6i > 6c ≈ 6a > 6b > 6g ≈ 4 > 3 for hCA I and II isoforms.
Conclusion
Today, some carbonic anhydrase inhibitors for the treatment of glaucoma are present on the market, but such drugs have many side effects. Strong carbonic anhydrase inhibitors can be developed to reduce dosage, and as a result of this, reduction of side effects can be achieved. In the present study, the synthesized compounds have remarkable inhibition effects on hCA I and II. Consequently, these potential inhibitors may be sufficient to reduce the dosage. So we can say that the synthesized inhibitors can be candidates for in vivo studies in the treatment of glaucoma.
IENZ_1134524_SupplementaryMaterial.pdf
Download PDF (3.1 MB)Declaration of interest
This research was financed by Dumlupınar University Research Fund (Grant No. 2014-20).
References
- Kaya M, Demir E, Bekci H. Synthesis, characterization and antimicrobial activity of novel xanthene sulfonamide and carboxamide derivatives. J Enzyme Inhib Med Chem 2013;28:885–93
- Monti SM, Supuran CT, De Simone G. Anticancer carbonic anhydrase inhibitors: a patent review (2008–2013). Expert Opin Ther Patents 2013;23:737–49
- Kaya M, Basar E, Cakir E, et al. Synthesis and characterization of novel dioxoacridine sulfonamide derivatives as new carbonic anhydrase inhibitors. J Enzym Inhib Med Chem 2012;27:509–14
- Esirden İ, Ulus R, Aday B, et al. Synthesis of novel acridine bis-sulfonamides with effective inhibitory activity against the carbonic anhydrase isoforms I, II, IX and XII. Bioorg Med Chem 2015;2:6573–80
- Ulus R, Yeşildağ İ, Tanç M, et al. Synthesis of novel acridine and bis acridine sulfonamides with effective inhibitory activity against the cytosolic carbonic anhydrase isoforms II and VII. Bioorg Med Chem 2013;21:5799–805
- Yeşildağ İ, Ulus R, Basar E, et al. Facile, highly efficient, and clean one-pot synthesis of acridine sulfonamide derivatives at room temperature and their inhibition of human carbonic anhydrase isoenzymes. Monatsh Chem 2014;145:1027–34
- Göksu S, Naderi A, Akbaba Y, et al. Carbonic anhydrase inhibitory properties of novel benzylsulfamides using molecular modeling and experimental studies. Bioorg Chem 2014;56:75–82
- Bag S, Tulsan R, Sood A, et al. Sulfonamides as multifunctional agents for Alzheimer's disease. Bioorg Med Chem Lett 2015;25:626–30
- Akincioglu, A, Gulcin, H, Durdagi, IS, et al. Discovery of potent carbonic anhydrase and acetylcholine esterase inhibitors: novel sulfamoylcarbamates and sulfamides derived from acetophenones. Bioorg Med Chem 2015;23:3592–602
- Arechederra RL, Waheed A, Sly WS, et al. Effect of sulfonamides as carbonic anhydrase VA and VB inhibitors on mitochondrial metabolic energy conversion. Bioorg Med Chem 2013;21:1544–8
- Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin Ther Patents 2013;23:681–91
- Pator-Navarro N, Gallego-Iglesias E, Maquieira Á, Puchades R. Development of a group-specific immunoassay for sulfonamides. Application to bee honey analysis. Talanta 2007;71:923–33
- Carta F, Di Cesare Manneli L, Pinard M, et al. A class of sulfonamide carbonic anhydrase inhibitors with neuropathic pain modulating effects. Bioorg Med Chem 2015;23:1828–40
- Żołnowska B, Sławiński J, Pogorzelska A, et al. Carbonic anhydrase inhibitors. Synthesis, and molecular structure of novel series N-substituted N′-(2-arylmethylthio-4-chloro-5-methylbenzenesulfonyl)guanidines and their inhibition of human cytosolic isozymes I and II and the transmembrane tumor-associated isozymes IX and XII. Eur J Med Chem 2014;71:135–47
- Sarikaya B, Ceruso M, Carta F, Supuran CT. Inhibition of carbonic anhydrase isoforms I, II, IX and XII with novel Schiff bases: identification of selective inhibitors for the tumor-associated isoforms over the cytosolic ones. Bioorg Med Chem 2014;22:5883–90
- Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68
- Chegaev K, Lazzarato L, Tamboli Y, et al. Furazan and furoxan sulfonamides are strong α-carbonic anhydrase inhibitors and potential antiglaucoma agents. Bioorg Med Chem 2014;22:3913–21
- Sugrue MF. Pharmacological and ocular hypotensive properties of topical carbonic anhydrase inhibitors. Prog Retin Eye Res 2000;19:87–112
- Zaib S, Saeed A, Stolte K, et al. New aminobenzenesulfonamide–thiourea conjugates: synthesis and carbonic anhydrase inhibition and docking studies. Eur J Med Chem 2014;78:140–50
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Rickli EE, Ghazanfar SAS, Gibbons BH, Edsall JT. Carbonic anhydrases from human erythrocytes. Preparation and properties of two enzymes. J Biol Chem 1964;239:1065–78
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948;176:147–54
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–9
- Kaya M, Yıldırır Y, Türker L. Synthesis and laser activity of halo-acridinedione derivatives. J Heterocyclic Chem 2009;46:294–7
- Ulus R, Yeşildağ İ, Elmastaş M, Kaya M. Rapid synthesis of novel 1,8-dioxoacridine carboxylic acid derivatives by microwave irradiation and their free radical scavenging activity. Med Chem Res 2015;24:3752–9
- Pacchiano F, Aggarwal M, Avvaru BS, et al. Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem Commun (Camb.) 2010;46:8371–3