Abstract
Rosmarinic acid (RA) is a natural polyphenol contained in many aromatic plants with promising biological activities. Carbonic anhydrases (CAs, EC 4.2.1.1) are widespread and intensively studied metalloenzymes present in higher vertebrates. Acetylcholinesterase (AChE, E.C. 3.1.1.7) is intimately associated with the normal neurotransmission by catalysing the hydrolysis of acetylcholine to acetate and choline and acts in combination with butyrylcholinesterase (BChE) to remove acetylcholine from the synaptic cleft. Lactoperoxidase (LPO) is an enzyme involved in fighting pathogenic microorganisms, whereas glutathione S-transferases (GSTs) are dimeric proteins present both in prokaryotic and in eukaryotic organisms and involved in cellular detoxification mechanisms. In the present study, the inhibition effects of rosmarinic acid on tumour-associated carbonic anhydrase IX and XII isoenzymes, AChE, BChE, LPO and GST enzymes were evaluated. Rosmarinic acid inhibited these enzymes with Kis in the range between micromolar to picomolar. The best inhibitory effect of rosmarinic acid was observed against both AChE and BChE.
Introduction
For many natural phenolic compounds, such as polyphenols directly provided by human diet, their beneficial properties have been primarily related to antioxidant activities, which are associated with their redox behaviour. Polyphenolic compounds derived from natural products are well known to possess a range of biological activitiesCitation1. They incorporate one or more hydroxyl moieties bonded directly to an aromatic carbon atom with the substitution patterns on the aromatic ring creating a large chemical variety. Therefore, free-radical scavenging by natural polyphenols has been widely studiedCitation2.
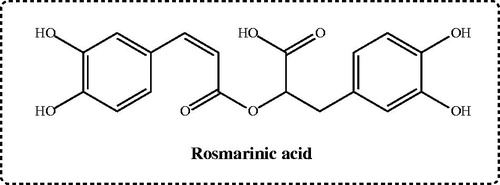
Rosmarinic acid () is an ester of caffeic and 3,4-dihydroxyphenyllactic acids. It is generally found in a wide range of species and plants. It is especially present as a secondary metabolite in medicinal and food plants. Rosmarinic acid was first isolated and characterised in 1958 from rosemary (Rosmarinus officinalis)Citation3. This polyphenol and its derivatives are abundantly found in sage or savoury herbs. Also, these plants are widely used in Mediterranean and Anatolian folk medicine as culinary herbs. It is also used as a fragrant additive in cosmetic applications and other applications. It was reported that rosmarinic acid had several biological activities, namely antioxidant, anti-inflammatory, antimutagenic, antibacterial, antiviral, antitumor, hepatoprotective and cardioprotective properties. Also, rosmarinic acid had health promoting and beneficial effects. It is supposed to act as a preformed constitutively accumulated defence compound in plants. Plant extracts that contain rosmarinic acid are a good potential source of antioxidants for food protection and pharmaceutical applications. Rosmarinic acid is a natural polyphenol carboxylic acid, an ester of caffeic and 3,4-dihydroxyphenyllactic acids and an important phenolic bioactive compoundCitation4.
Figure 1. Chemical structure of rosmarinic acid as glutathione S-transferase, lactoperoxidase, acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase isoenzymes inhibitor.
Carbonic anhydrases (CAs, EC 4.2.1.1) are zinc-containing metalloenzymes that catalyse the reversible hydration of carbon dioxide (CO2) in a two-step reaction, to yield bicarbonate (HCO3−) and protons (H+)Citation5. CO2, HCO3− and H+ are essential molecules or ions in many important physiologic processes in all life kingdoms, throughout the tree of life and for this reason, relatively high amounts of CAs are present in different tissues or cell compartments of most investigated organismsCitation6.
These enzymes are present in all organisms, from the very simple to the complex ones. This metalloenzyme superfamily includes six distinct genetic families (α-, β-, γ-, δ-, ζ- and η-CAs) known to date, which constitute an interesting example of convergent evolution at the molecular levelCitation7. They vary in their preference for the catalytic metal ions used within the active site, since Zn2+, Cd2+ or Fe2+ can be used within their active sitesCitation8. The α-CA isoforms differ significantly in their localization and tissue distribution. They are present in vertebrates, protozoa, algae and cytoplasm of green plants and in some bacteria. CA I, II, III, VII and XIII are cytosolic isoforms, CA IV, IX, XII and XIV are membrane-bound, CA VA and VB are mitochondrial, whereas CA VI is secreted. CA IX and XII are known as the membrane tumour-associated CAs, being found in a limited number of normal tissues, such as the gastrointestinal mucosa and body cavity liningCitation9. An important role of CA IX and XII as tumour pH-regulating enzymes, involved in the survival or proliferation of the tumour cells within the hypoxic, acidic niche typical of many solid cancers. CA I, II, IX and XII were studied in this study. CA I and II are present at high concentrations in the cytosol of erythrocytes and the gastrointestinal tract. CA IX is found in tumour cells and absent or is present in very limited amount in normal tissuesCitation10.
Cholinesterases (ChE) are an enzyme family that catalyse the hydrolysis of acetylcholine (ACh) into choline and acetic acid, an essential process for the restoration of the cholinergic neurotransmission. There are two cholinesterase types: acetylcholinesterase (AChE; EC 3.1.1.7) and butyrylcholinesterase (BChE; EC 3.1.1.8)Citation11.
Butyrylcholinesterase is plasma ChE and a non-specific ChE enzyme that hydrolyses many different choline-based esters. In humans, it is made in the liver, found mainly in blood plasma and encoded by the BChE gene. AChE is known to be abundant in the muscle, brain and erythrocyte membrane, whereas BChE has a higher activity in liver, intestine, heart, kidney and lung. AChE and BChE share 65% amino acid sequence homology and have similar molecular forms and active sites despite being products of different genes on the human chromosomesCitation12.
Both cholinesterases participate in cholinergic neurotransmission by hydrolysing ACh in the central and peripheral nervous system. The symptomatic Alzheimer’s disease (AD) treatment involves the use of cholinesterase inhibitors (ChEIs) such as Rivastigimine. ChEIs are the first-line drugs in the symptomatic treatment of AD, as by inhibiting cholinesterase they lead to an increased synaptic level of the neurotransmitterCitation13.
Lactoperoxidase (LPO, E.C. 1.11.1.7) is of growing interest due to its distinctive biological activity, such as biocidal and biostatic ones. The mechanism of the LPO in antimicrobial action has been studied thoroughly regarding the conversion of thiocyanate (SCN-) to hypothiocyanite ion (OSCN-) as antimicrobial product and some other highly reactive and short-lived oxidation products. These oxidations occur in the presence of hydrogen peroxideCitation14.
LPO is found in the salivary glands, in the breast secretory epithelial cells, lacrimal glands and in their secretions, such as saliva, milk and tears. Based on its antibacterial characteristics, currently LPO has extensive applications, including preservation of raw milk during collection or transportation to processing plants in dairy industry, the extending shelf-life of pasteurised milk and the supplementation of salivary peroxidase antimicrobial system in toothpastes and mouth rinses to reduce acid production by oral microorganismsCitation15.
Glutathione S-transferases (GSTs, E.C. 2.5.1.18) catalyse the conjugation of the reduced form of glutathione (GSH) to xenobiotic substrates for the purpose of detoxification. They are multifunctional enzymes for the cellular defence against xenobiotics and provide protection for organism. They are essential and found in all kingdoms of life. GSTs catalyse the conjugation of GSH by a sulfhydryl group (-SH) to electrophilic centres on a wide variety of substrates in order to make the compounds more water solubleCitation12. For example, the detoxification of 1,2-epoxyethylbenzene can be given.
In this study, we investigated the inhibition effect of rosmarinic acid against human carbonic anhydrase IX and XII isoenzymes, AChE, BChE, LPO and GST enzymes.
Experimental section
Biochemical assays
An applied photophysics stopped-flow instrument was used to assay the catalytic/inhibition of four CA isozymes, as reported by KhalifahCitation16. Briefly, phenol Red (20 mM) was used as an indicator, with an absorbance maximum of 557 nm, with HEPES (10 mM, pH 7.4) as a buffer and 0.1 M Na2SO4 or NaClO4 for maintaining constant the ionic strength; these anions are not inhibitory at the used concentration. The CA-catalysed CO2 hydration was followed for a period of 10–100 s.
For the determination of kinetic parameters and inhibition constants, the saturated CO2 concentrations ranged from 1.7 to 17 mM. For rosmarinic acid, at least six traces of the initial 5–10% of the reaction were used for determining the initial velocity. The uncatalysed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (10 mM) were prepared in distilled-deionised water, and dilutions up to 0.01 μM were performed with distilled-deionised water. Rosmarinic acid and enzyme solutions were preincubated together for 15 min at room temperature prior to the assay to allow for the formation of the EI complex. The inhibition constant of rosmarinic acid was obtained by non-linear least-squares methods using PRISM 3, as reported earlier, and represents the mean from at least three different determinations. Both CA isozymes (CA IX and XII) were prepared in recombinant form as reported earlierCitation17.
The inhibitory effect of rosmarinic acid on AChE/BChE activities were measured according to spectrophotometric method of Ellman et al.Citation18 as described previouslyCitation12. Acetylthiocholine iodide (AChI) or butyrylthiocholine iodide (BChI) was used as substrates of the reaction. 5,5′-Dithio-bis(2-nitro-benzoic)acid (DTNB) was used for the measurement of the AChE/BChE activities. The hydrolysis of both substrates was monitored spectrophotometrically by formation of the yellow 5-thio-2-nitrobenzoate anion as the result of the reaction of DTNB with thiocholine, released by enzymatic hydrolysis of AChI/BChI, with absorption maximum at a wavelength of 412 nmCitation19.
CNBr-activated-sepharose 4B was used for the purification of LPO from bovine milkCitation20. LPO purity was checked by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)Citation21. Protein concentration was determined according to the Bradford method as reported previouslyCitation22. The stacking and running gels contained 3% (w/v) and 10% (w/v) acrylamide, respectively, and SDS (0.1%, w/v), according to a previously published proceduresCitation23.
LPO activities were determined according to the procedure of Shindler and BardsleyCitation24. One unit of enzyme is defined as the amount of enzyme catalysing the 100 oxidation of l μmol of ABTS min−1 at 298 K (Molar absorption coefficient, 32.400 M −1 cm−1). This activity method is based on the oxidation of ABTS as a chromogenic substrate by H2O2, resulting in a product that absorbs at 412 nm.
GST activity was measured as according to the previous studyCitation12. The kinetic constants of GST-catalysed reaction were determined using GSH (20 mM) or CDNB (25 mM), in phosphate buffer (pH 7.2) at room temperature. The enzyme solution replaced by phosphate buffer was used as the control.
In order to determine the effect of rosmarinic acid on enzymes, different rosmarinic acid concentrations were added into the reaction medium. enzymes activities was measured, and an experiment in the absence of rosmarinic acid was used as controlCitation25. The IC50 values were obtained from activity (%) versus rosmarinic acid concentration plots. To determine the Ki constant in the media with rosmarinic acid as inhibitor, different substrate concentrations were used. Rosmarinic acid solution was added into the reaction medium, resulting in three different fixed rosmarinic acid concentrations as inhibitor. Lineweaver–Burk graphsCitation26 were used to determine Vmax and other kinetic parameters. The Ki was calculated from these graphsCitation27.
Results and discussion
Phenolic compounds are secondary plants metabolites that play an important role in the pigmentation, growth and reproduction of plants as well as plant resistance to pathogens. Also, they are biologically active substances and possess biological activities including antioxidant, antimutagenic, anticancer, anticarcinogenic, antiviral, anti-inflammatory and antibacterial activitiesCitation28.
Phenolic compounds are slightly acidic and have weak tendencies to lose the proton (H+) ion from the hydroxyl group (--OH), resulting in the highly water-soluble phenolate anion. Phenols effectively inhibit CA isoenzymes. The inhibition profile of various isozymes with this class of agent is variable, with inhibition constants ranging from the millimolar to the submicromolar range for many simple phenolsCitation29. Also, they inhibit the CA isozymes because of the presence of different functional groups in their scaffold, mainly the phenolic --OH and acetate (--COOH) groups, which may bind to the Zn2+ ion or the water coordinated to the zinc ion from the CA-active site. CA isoenzymes are interesting targets for the design of pharmacological agents that are useful in the treatment or prevention of a variety of disorders, such as glaucoma, epilepsy, as diuretics or antitumor agents and diagnostic tools. The design of CAIs as therapeutic agents is related to the large number of isoforms in humans, their rather diffuse localisation in many tissues or organs. CAIs have lately emerged that CAIs could have potential as anticancer, anti-obesity and anti-infective drugsCitation29.
Phenolic and polyphenolic compounds have a lot of biological activities in fact been evaluated as CAIs by our group earlier, being shown that many such derivatives are potent inhibitors of several physiologically relevant isoforms, such as CA IX and XIICitation4,6,12,17,29. They may constitute interesting lead molecules for identifying novel CAIs. Here, we report the inhibition effect of rosmarinic acid on two catalytically active isoforms, CA IX and XII, as well as against LPO, GST, AChE and BChE. An applied photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activityCitation16. Rosmarinic acid has two diphenolic moieties in its scaffolds. We discovered nanomolar inhibition against some of these metabolic enzymes. The inhibition data of Rosmarinic acid are summarized in , and the following comments can be drawn from these data:
Until now, sixteen isoforms of CA have been discovered in α-CA family; among them the dimeric transmembrane glycoproteins CA IX and XII are also human-associated CA isoforms having extracellular-active site and are found in a broad spectrum of hypoxic tumour types. CA IX and XII are overexpressed in many such tumours in response to the hypoxia inducible factor pathway and research on the involvement of these isozymes in cancer has progressed significantly in recent yearsCitation30. CA IX showed moderate inhibition activity with rosmarinic acid, with an inhibition constant of 4.63 μM.
It has been known that CA IX and XII are associated with tumour progression, metastasis, and response to therapyCitation31. An important role of CA IX and XII as major tumour prosurvival pH-regulating enzymes was suggested in genetic study. CA XII was also poorly inhibited by rosmarinic acid, with a Ki value of 4.71 μM. Whereas, AZA, a positive standard for CA inhibition, showed an effective inhibitory activity with a Ki value 6.0 mMCitation17. Additionally, because the CA XII is more spread in some healthy tissues than CA IX, it is less interesting target than CA IX in the cancer therapyCitation31.
ACh behaves as an excitatory neurotransmitter for voluntary muscles in the somatic nervous system and as a preganglionic and a postganglionic transmitter in the parasympathetic nervous system of vertebrates and invertebrates. On the other hand, AChE is intimately associated with the normal neurotransmission by catalysing the hydrolysis of ACh to acetate and choline and acts in combination with BChE to remove ACh from the synaptic cleftCitation6,Citation32. Selective inhibition of AChE may cause accumulation of ACh in the synaptic cleft, leading to overstimulation and the disruption of nerve impulses and ultimately causing symptoms such as ataxia, central respiratory paralysis, seizures, coma and death. Inhibition of AChE activity was determined on commercially available purified AChE from the electric organ of E. electricus L. based on the method of Ellman methodCitation18. This assay has been used most widely for the detection of AChE and BChE inhibitors and for the quantification of a ChE inhibitory activity. AChE inhibition has generally been used to determine the neurotoxic properties of chemicals or drugs capable of interfering with normal neurotransmission of the sympathetic and parasympathetic nervous system of living organisms. Most AChE inhibitors (ChEIs) interact with the catalytic site of the AChE located at the bottom of the gorge, where the hydrolysis of ACh takes placeCitation19. Currently, the most prescribed ChEIs are donepezil, galantamine and rivastigmine. These drugs are used to treat patients with mild-to-moderate AD. BChE has a specific role in cholinergic neurotransmission and it has been associated with ADCitation21. Individual ChEIs differ from each other with respect to their pharmacologic properties. Primary target of donepezil and galantamine is AChE; however, rivastigmine shows equal affinity for both AChE and BChE enzymes. It was also shown that the main AChE inhibitory effect was primarily associated with aromatic compounds and, to a lesser degree, with aliphatic compounds. AChE was very effectively inhibited by rosmarinic acid, with Ki value of 42.52 pM (). On the other hand, rosmarinic acid inhibited BChE with Ki value of 121.60 pM. The Ki values of rosmarinic acid for AChE and BChE were calculated from Lineweaver–Burk plotsCitation26. On the other hand, donepezil hydrochloride, which is used for the treatment of mild-to-moderate AD and various other memory impairments, had been shown to lower AChE inhibition activity (IC50: 55.0 nM)Citation12.
Milk contains a variety of constituents that protect the neonate and the milk itself from a host of deleterious microorganisms. One such constituent is LPO, which plays an important role in protecting the lactating mammary gland and the intestinal tract of new-borns against pathogenic microorganisms. Our investigation showed that rosmarinic acid moderately inhibited LPO with a Ki value of 0.51 μM.
Recently, many studies demonstrated that GST plays important functions in cellular defence against chemical toxicity. It was reported a link between the lack of GST enzyme activity and the susceptibility to develop different types of cancer including oral, gastric and bladder cancersCitation12. GST was potently inhibited by rosmarinic acid, with a Ki value of 48.74 nM (). It was reported that the inhibitor of GST bearing suitable linkers could concomitantly bind to two active sites of GST and usually possess Ki values at nanomolar levels and excellent enzyme selectivityCitation12.
Table 1. Inhibition constants (Ki) of rosmarinic acid against CA IX and XII isoenzymes, AChE, BChE, LPO and GST enzymes.
Conclusion
Rosmarinic acid is a caffeic acid ester of salvianic acid A (3,4-dihydroxyphenyllactic acid). To effect of rosmarinic acid against CA IX and XII isoenzymes, AChE, BChE, LPO and GST was evaluated in this study. Rosmarinic acid showed low micromolar inhibition against both CA isoenzymes (CA IX and XII) and LPO, nanomolar inhibition against GST and picomolar inhibition against ACE and BChE. The results showed that rosmarinic acid moderately inhibited both CA isoenzymes, but effectively inhibited by GST, ACE and BChE. The catalytic inhibition mechanisms of CA isoenzymes for the physiologic reaction is well-understood processes: most types of classical inhibitors bind to the metal centre, but the discovery of new classes of CAIs possessing different inhibition mechanisms compared to the classical inhibitors. Also, the other metabolic enzymes (AChE, BChE, LPO and GST) have diverse inhibition profiles. These data may explain the beneficial health effects of Rosmarinic acid and may lead to enzyme researchers and drug design campaigns.
Declaration of interest
The authors have declared no conflict of interest.
This work was financed in part by two EU projects of the 7th FP, Metoxia and Dynano. Also, İlhami Gülçin and Saleh H. Alwasel would like to extend his sincere appreciation to the Research Chairs Programme at King Saud University for funding this research.
References
- Şerbetçi TH, Gülçin İ. Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.). Int J Food Propert 2010;13:657–71.
- Gülçin İ, Beydemir Ş, Şat İG, Küfrevioğlu Öİ. Evaluation of antioxidant activity of cornelian cherry (Cornus mas L.). Acta Alimen Hung 2005;34:193–202.
- Martinez-Tome M, Jimenez AM, Ruggieri S, et al. Antioxidant properties of Mediterranean spices compared with common food additives. J Food Protect 2001;64:1412–19.
- Topal M, Gülçin İ. Rosmarinic acid: a potent carbonic anhydrase isoenzymes inhibitor. Turk J Chem 2014;38:894–902.
- Yıldırım A, Atmaca U, Keskin A, et al. N-acylsulfonamides strongly inhibit human carbonic anhydrase isoenzymes I and II. Bioorg Med Chem 2015;23:2598–605.
- Scozzafava A, Kalın P, Supuran CT, et al. The impact of hydroquinone on acetylcholine esterase and certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J Enzyme Inhib Med Chem 2015;30:941–6.
- Capasso C, Supuran CT. Sulfa and trimethoprim-like drugs-antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J Enzyme Inhib Med Chem 2014;29:379–87.
- Akbaba Y, Akıncıoğlu A, Göçer H, et al. Carbonic anhydrase inhibitory properties of novel sulfonamide derivatives of aminoindanes and aminotetralins. J Enzyme Inhib Med Chem 2014;29:35–42.
- Ceruso M, Bragagni M, AlOthman Z, et al. New series of sulfonamides containing amino acid moiety act as effective and selective inhibitors of tumor-associated carbonic anhydrase XII. J Enzyme Inhib Med Chem 2015;30:430–4.
- Çetinkaya Y, Göçer H, Göksu S, Gülçin İ. Synthesis and carbonic anhydrase isoenzymes inhibitory effects of novel benzylamine derivatives. J Enzyme Inhib Med Chem 2014;29:168–74.
- Topal M, Gocer H, Topal F, et al. Antioxidant, antiradical and anticholinergic properties of cynarin purified from the illyrian thistle (Onopordum illyricum L.). J Enzyme Inhib Med Chem. [Epub ahead of print]. doi: https://doi.org/http://dx.doi:10.3109/14756366.2015.1018244.
- Gülçin İ, Scozzafava A, Supuran CT, et al. The effect of caffeic acid phenethyl ester (CAPE) metabolic enzymes including acetylcholinesterase, butyrylcholinesterase, glutathione s-transferase, lactoperoxidase and carbonic anhydrase ısoenzymes I, II, IX and XII. J Enzyme Inhib Med Chem. [Epub ahead of print]. doi: http://dx.doi.org/10.3109/14756366.2015.1094470.
- Polat Köse L, Gülçin İ, Gören AC, et al. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind Crops Prod 2015;74:712–21.
- Şişecioğlu M, Çankaya M, Gülçin İ, Özdemir M. Interactions of melatonin and serotonin to lactoperoxidase enzyme. J Enzyme Inhib Med Chem 2010;25:779–83.
- Atasaver A, Özdemir H, Gülçin İ, Küfrevioğlu Öİ. One-step purification of lactoperoxidase from bovine milk by affinity chromatography. Food Chem 2013;136:864–70.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- Scozzafava A, Passaponti M, Supuran CT, Gülçin İ. Carbonic anhydrase inhibitors: Guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J Enzyme Inhib Med Chem 2015;30:586–91.
- Ellman GL, Courtney KD, Andres V, Featherston RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
- Akıncıoğlu A, Akıncıoğlu H, Gülçin I, et al. Discovery of potent carbonic anhydrase and acetylcholine esterase inhibitors: novel sulfamoylcarbamates and sulfamides derived from acetophenones. Bioorg Med Chem 2015;23:3592–602.
- Nar M, Çetinkaya Y, Gülçin İ, Menzek A. (3,4-Dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives as carbonic anhydrase isoenzymes inhibitors. J Enzyme Inhib Med Chem 2013;28:402–6.
- Göçer H, Akıncıoğlu A, Göksu S, et al. Carbonic anhydrase and acetylcholinesterase inhibitory effects of carbamates and sulfamoylcarbamates. J Enzyme Inhib Med Chem 2015;30:316–20.
- Ozturk Sarikaya SB, Sisecioglu M, Cankaya M, et al. Inhibition profile of a series of phenolic acids on bovine lactoperoxidase enzyme. J Enzyme Inhib Med Chem 2015;30:479–83.
- Gülçin İ, Küfrevioğlu Öİ, Oktay M. Purification and characterization of polyphenol oxidase from nettle (Urtica dioica L.) and inhibitory effects of some chemicals on enzyme activity. J Enzyme Inhib Med Chem 2005;20:297–302.
- Shindler JS, Bardsley WG. Steady-state kinetics of lactoperoxidase with ABTS as chromogen. Biochem Biophys Res Commun 1975;67:1307–12.
- Öztaşkın N, Çetinkaya Y, Taslimi P, et al. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg Chem 2015;60:49–57.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–66.
- Çoban TA, Beydemir Ş, Gülçin İ, Ekinci D. The effect of ethanol on erythrocyte carbonic anhydrase isoenzymes activity: an in vitro and in vivo study. J Enzyme Inhib Med Chem 2008;23:266–70.
- Sehitoglu MH, Han H, Kalin P, et al. Pistachio (Pistacia vera L.) Gum: a potent inhibitor of reactive oxygen species. J Enzyme Inhib Med Chem 2015;30:264–9.
- Gocer H, Topal F, Topal M, et al. Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin. J Enzyme Inhib Med Chem. [Epub ahead of print]. doi: http://dx.doi.org/10.3109/14756366.2015.1036051.
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77.
- Akıncıoğlu A, Topal M, Gülçin İ, Göksu S. Novel sulfamides and sulfonamides incorporating tetralin scaffold as carbonic anhydrase and acetylcholine esterase inhibitors. Arch Pharm 2014;347:68–76.
