Abstract
The synthesis of a series of benzimidazole-N-benzylpropan-1-amines and adenine-N-benzylpropan-1-amines is described. Subsequent evaluation against two strains of the anaerobic bacterium Clostridium difficile was performed with three amine derivatives displaying MIC values of 16 μg/mL. Molecular docking studies of the described amines determined that the amines interact within two active site pockets of C. difficile methionyl tRNA synthetase with methoxy substituents in the benzyl ring and an adenine biaryl moiety resulting in optimal binding interactions.
Introduction
The Clostridium difficile infection (CDI) rate has shown a dramatic increase over the past decade and this increase is associated with the emergence of hypervirulent strain B1/NAP1/027Citation1. CDI can also now be found more commonly outside healthcare facilities and in previously low-risk groups, such as children, pregnant women, and individuals with irritable bowel syndromeCitation2. Recently, CDI has exceeded MRSA as the most common healthcare facility-associated infectionCitation3. First-line treatment for CDI is usually metronidazole or vancomycin, for patients with severe infection, oral vancomycin is the preferred therapy as the clinical cure rate for vancomycin in patients with severe CDI was significantly better than that for metronidazoleCitation4. Unfortunately, recurrent disease occurs in 20–25% of patients receiving metronidazole and vancomycinCitation5. For this reason, there is a need to exploit other C. difficile targets in the development of new therapies. A promising target is aminoacyl-tRNA synthetase (aaRS).
Research in our lab in Cardiff has focused on methionyl tRNA (MetRS) as an antibacterial target, using our published homology model of C. difficile MetRSCitation6 to aid the design process. Most MetRS inhibitors studied are against Staphylococcus aureus and Escherichia coli. These inhibitors are divided into either adenosine or non-adenosine analogues. Adenosine analogues () are thought to mimic the high-energy intermediate aminoacyl-AMP, thus blocking the aaRS pocketCitation7. Non-adenosine analogues may have vast structural differences compared with the natural substrate aminoacyl-AMP, but they still have good enzyme inhibitory activity. The challenge for MetRS inhibitors, however, is not biochemical activity but whole-cell activity. The most potent examples at the biochemical level, with IC50 values in the low nanomolar range, including oxazolone dipeptides and methionyl adenylate isosteres, have shown good selectivity versus human MetRS but lack antibacterial activity in whole-cell assaysCitation8.
Figure 1. Adenosine analogue E. coli MetRS inhibitor and non-adenosine C. difficile MetRS inhibitor REP3123 and the natural substrate methionyl-AMP.
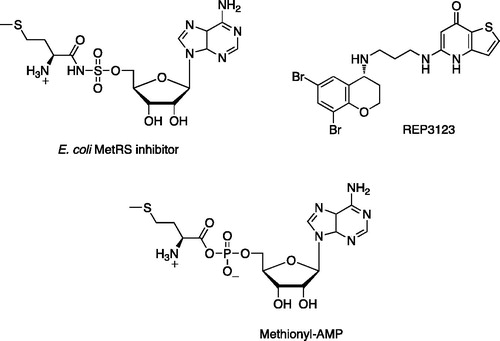
To date, REP3123 (), a novel diaryldiamine, is the only documented selective C. difficile MetRS inhibitor with whole cell activity in a range of clinical C. diffcile isolatesCitation9. REP3123 is reported to have good in vitro activity and selectivity against C. difficile, while sparing other gut floraCitation10.
We report the efficient synthesis (Scheme 1) and whole cell activity against C. difficile () of a series of novel inhibitors incorporating pharmacophore elements of both REP3123 and adenosine analogues, namely benzyl and biaryl moiety (adenine or benzimidazole) and flexible amine linker chain.
Scheme 1. Reagents and conditions: (i) potassium carbonate, 18-crown-6, tert-butyl(3-bromopropyl)carbamate, DMF, 60 °C overnight, (ii) TFA/DCM, (iii) TEA, methanol, different benzaldehydes, overnight, (iv) NaBH4, methanol, overnight [a, R = H; b, R = 4-Cl; c, R = 4-Br; d, R = 4-OMe; e, R = 3,5-diOMe].
![Scheme 1. Reagents and conditions: (i) potassium carbonate, 18-crown-6, tert-butyl(3-bromopropyl)carbamate, DMF, 60 °C overnight, (ii) TFA/DCM, (iii) TEA, methanol, different benzaldehydes, overnight, (iv) NaBH4, methanol, overnight [a, R = H; b, R = 4-Cl; c, R = 4-Br; d, R = 4-OMe; e, R = 3,5-diOMe].](/cms/asset/1f1bad85-02d6-4b1a-94d8-5b5f2e0dea4a/ienz_a_1140754_sch0001.gif)
Table 1. MIC (μg/mL) against C. difficile strain 1813.
Results and discussion
Chemistry
Treatment of benzimidazole (1) or adenine (2) with potassium carbonate followed by the addition of tert-butyl(3-bromopropyl)carbamate resulted in N-alkylation products 3 and 4 in yields of 76 and 68%, respectively. Subsequent deprotection of BOC under acidic conditions gave the trifluoroacetic acid salts 5 and 6 in quantitative and 93% yield, respectively. Imine formation was achieved on base catalyzed reaction of 5 and 6 with a range of benzaldehydes, giving the imine derivatives 7 and 8 in variable yields. NaBH4 reduction of the imines gave the required benzimidazole and adenine N-benzylpropan-1-amines 9 and 10 in good yields (Synthetic methods and analytical data for all described compounds are available in Supplementary material).
Microbiology
A stock solution of each amine was prepared in 5% DMSO at a concentration of 256 μg/mL. These were then diluted with nutrient broth to final concentrations of 128 μg/mL, 64 μg/mL, 32 μg/mL, 16 μg/mL, 8 μg/mL, 4 μg/mL, 2 μg/mL, 1 μg/mL, or 0.5 μg/mL and inoculated with a bacterial suspension of either C. difficile strain 1813 or strain R20291. Growth was assessed after incubation at 37 °C for 48 h in an anaerobic cabinet. Metronidazole as a control showed an MIC of <0.5 μg/mL for both C. difficile strain 1813 and R20291.
Three amines 9e, 10a, and 10e displayed MIC values of 16 μg/mL against C. difficile strain 1813 () and one of the imines 8d showed activity with an MIC value of 16 μg/mL. None of the imines or amines showed inhibitory activity against strain R20291 (Microbiological assay methods are available in Supplementary material).
Molecular modeling
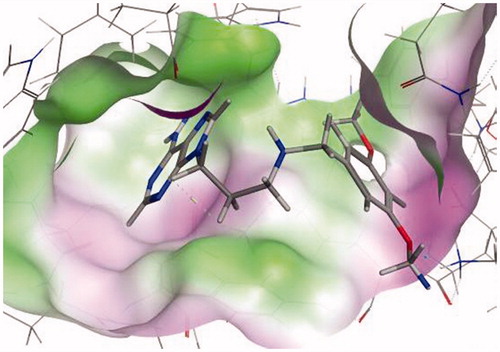
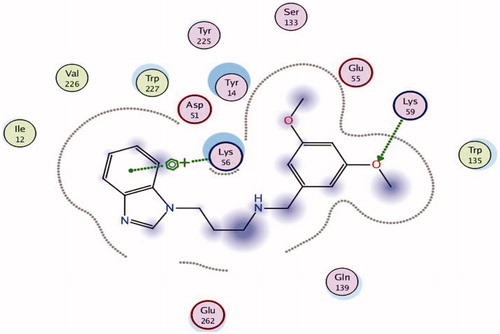
Docking interactions of the imines and amines were explored using molecular modeling software. REP3123 had previously been shown to fit in two pockets within the C. difficile MetRS active site. Pocket 1 (the methionine-binding pocket) has Ile12, Asp51, Ala230, and Trp227 as the main residues, while Glu55, Ser133, and Tyr225 form an adjacent pocket (pocket 2) with Lys56 bridging the two pocketsCitation6,Citation11.
All the amines and imines had reasonable docking results based on both visual inspection of the poses and the docking score and occupied the two pockets previously observed for REP3123 (). The adenine derivatives were found to give better interactions in terms of hydrogen bonding via the NH2 and the 4-methoxy and 3,5-dimethoxy derivatives were able to better fill the two pockets with additional hydrogen bonding interactions between a methoxy group and Lys59 (). Ile12 was found to be the main key interaction in the methionine pocket, while Glu55, Lys59, and Ala230 were found to be the main interactions in the adjacent pocket with Lys56 and Glu262 bridging these two pockets.
Conclusions
This study provides some insight into the functionality required for improved binding interactions within the two pockets of the MetRS active site, although there is still scope for further extension within the pockets. The whole cell activity observed for the imine 8d and amines 9e, 10a, and 10e is promising for further development; however, the different inhibitory activity observed with the two C. difficile strains highlights the challenges of developing a MetRS inhibitor that can inhibit a range of C. difficile clinical isolates.
Supplementary material available online
IENZ_1140754_Supplementary_File.pdf
Download PDF (563.5 KB)Acknowledgements
The authors acknowledge the EPSRC Mass Spectrometry Centre, Swansea, UK, for mass spectroscopy data.
Declaration of interest
The authors report that they have no conflicts of interests to declare.
References
- Lanis JM, Heinlen LD, James JA, et al. Clostridium difficile 027/BI/NAP1 encodes a hypertoxic and antigenically variable form of TcdB. PLoS Pathog 2013;9:e1003523.
- Ananthakrishnan AN. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol 2011;8:17–26.
- Miller BA, Chen LF, Sexton DJ, et al. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol 2011;32:387–90.
- Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007;45:302–7.
- Bauer MP, Kuijper EJ, van Dissel JT. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI). Clin Microbiol Infect 2009;15:1067–79.
- Al-Moubarek E, Simons C. A homology model for Clostridium difficile methionyl tRNA synthetase: active site analysis and docking interactions. J Mol Model 2011;17:1679–93.
- Lee J, Kim SE, Lee JY, et al. N-Alkoxysulfamide, N-hydroxysulfamide, and sulfamate analogues of methionyl and isoleucyl adenylates as inhibitors of methionyl-tRNA and isoleucyl-tRNA synthetases. Bioorg Med Chem Lett 2013;13:1087–92.
- Green LS, Bullard JM, Ribble W, et al. Inhibition of methionyl-tRNA synthetase by REP8839 and effects of resistance mutations on enzyme activity. Antimicrob Agents Chemother 2009;53:86–94.
- Citron DM, Warren YA, Tyrrell KL, et al. Comparative in vitro activities of LFF571 against Clostridium difficile and 630 other intestinal strains of aerobic and anaerobic bacteria. J Antimicrob Chemother 2009;63:972–6.
- Critchley IA, Green LS, Young CL, et al. Spectrum of activity and mode of action of REP3123, a new antibiotic to treat Clostridium difficile infections. J Antimicrob Chemother 2009;63:954–63.
- Evans R, Green L, Sun X, et al. Co-crystal structure of REP3123 bound to Clostridium difficile methionyl tRNA synthetase. ICAAC 2007, Abstracts of the 47th Interscience Conferenceon Antimicrobial Agents and Chemotherapy, Proceedings of the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL. 17–20 September 2007; Washington, DC, USA: American Society for Microbiology. Poster no. F1-2114.