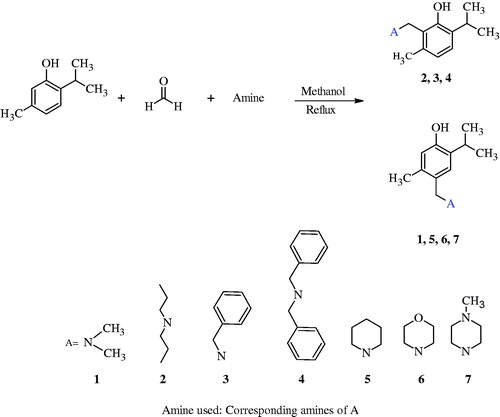
Abstract
Mannich bases of thymol were synthesized. The aminomethylation reaction was realised in the ortho position of the phenol for compounds 2 (dipropylamine), 3 (benzylamine), and 4 (dibenzylamine) while it was from para position for 1 (dimethylamine), 5 (piperidine), 6 (morpholine) and 7 (N-methylpiperazine). The carbonic anhydrase (CA, EC 4.2.1.1) inhibitory effects of the compounds were asssessed against hCA I and hCA II. All compounds moderately inhibited hCA I and hCA II. The cytotoxicity of the compounds against four human oral squamous cell carcinoma cell lines were compared those against three normal oral cells. Tumor specificity values were about 2 or slightly more for the compounds 2, 3, 4, 5 and 6. Compound 2 showed cytostatic activity against OSCC cell lines at 16 to 32-fold lower concentrations as compared with normal cells. This suggests that compound 2 can be considered as cytotoxicity enhancing drug candidate for further investigations.
Introduction
Carbonic anhydrase (CA, EC 4.2.1.1), a zinc-dependent metalloenzyme, catalyzes the reversible hydration of CO2Citation1. This enzyme plays an important role in physiologic and pathologic processes, such as pH and CO2 homeostasis, electrolyte secretion, lipogenesis, ureagenesis and calcificationCitation2. Sixteen different isoforms are known in mammals which are cytosolic (CA I, CA II, CA III, CA VII and CA XIII), membrane-bound (CA IV, CA IX, CA XII, CA XIV and CA XV), mitochondrial (CA VA and CA VB), or secreted (CA VI) proteinsCitation2,Citation3. There are known diuretics, antiepileptic, antiglaucoma, anticancer drugs based on CA inhibitors (CAIs) and they target different human α-CA isoformsCitation2. Recently, it has been showed that these enzymes are also present in protozoa, bacteria and fungiCitation1. Therefore, these enzymes are the target of potential antibacterial, antimalarial and antifunfal agentsCitation4.
Some primary sulfonamides with CA inhibitory action and their derivatives have been used for a long time in the treatment of some diseases because of their diuretic and antiglaucom effectsCitation1,Citation2. Commercially available CAIs, such as acetazolamide, ethoxzolamide and dorzolamide are also known for their potential anticonvulsantCitation2, anticancerCitation3, analgesicCitation1 and antiinfectiveCitation2 activities. But the compounds mentioned above are not selective inhibitors of CAs and can cause some side effectsCitation2.
The CA inhibitory effects of some natural products and their derivatives have been recently investigatedCitation5. It was reported that natural compounds carring phenol or polyphenol moiety had carbonic anhydrase inhibitory activity at micromolar concentrationCitation6. In addition, the compounds having phenol functional group are known to have anticancer, antibacterial, antiviral, antifungal and antioxidant activitiesCitation7–9.
Thymol, 2-isopropyl-5-methylphenol, is a bioactive natural phenolic compound which was isolated from Thymus sulgarisCitation10. Thymol is known to show antioxidantCitation11–13, anticancerCitation14,Citation15, antidepressantCitation16, antibacterialCitation17,Citation18 and antifungalCitation19 activities.
Mannich bases are an important group of compounds in medicinal chemistry and may be synthsized by Mannich reaction. There are several type of Mannich bases, such as carbon Mannich bases and nitrogen Mannich basesCitation20. Phenolic Mannich bases are a group of carbon Mannich basesCitation20. CAs inhibitionCitation9,Citation21,Citation22, cytotoxicCitation8,23–25, anticonvulsantCitation26–28, diureticCitation29, antifungalCitation30 and antioxidantCitation10 activities of several Mannich bases had been reported.
The aims of this study were as follows:
We aimed to synthesize several phenolic mono Mannich bases of thymol by using acyclic and cyclic amines having different chemical structure, properties and pKa values, which may govern the chemical reactions and bioactivities.
To investigate the CA inhibitory activity of the compounds against the human (h) isoforms hCA I and hCA II since it is a known phenol function, which is a group of carbonic anhydrase inhibitors. There is no study reporting the carbonic anhydrase inhibitory activities of mono Mannich bases of natural compound thymol.
To investigate the cytotoxic activities of thymol mono Mannich bases against four human oral squamous cell carcinoma cell lines: Ca9-22 (derived from the gingival tissue), HSC-2, HSC-3 and HSC-4 (derived from the tongue) as compared with three normal oral mesenchymal cells: HGF (human gingival fibroblasts), HPC (human pulp cells) and HPLF (human periodontal ligament fibroblasts). The results to be obtained may be useful for new drug candidate/s development.
Experimental
Materials
1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were taken using a Varian Mercury Plus spectrometer (Varian Inc., Palo Alto, CA). Chemical shifts (δ) were reported in ppm. Melting points were determined using an Electrothermal 9100 (IA9100, Bibby Scientific Limited, Staffordshire, UK) instrument and are uncorrected. Liquid chromatography ion trap-time of flight tandem mass spectrometer (Shimadzu, Kyoto, Japan) equipped with an electrospray ionization (ESI) source, operating in both positive and negative ionization mode. Shimadzu's LCMS Solution software was used for data analysis.
Methods
Synthesis of mono Mannich bases, 1–7
The mixture of thymol (6.6 mmol), formaline (6.6 mmol, 37%) and amine (6.6 mmol) [Dimethylamine (1), dipropylamine (2), benzylamine (3), dibenzylamine (4), piperidine (5), morpholine (6) and N-methylpiperazine (7)] was heated in methanol (15 ml) for 15-26 h (Scheme 1). Reactions were monitored by TLC. When the reaction was stopped, the solvent was reduced half of volume under vacuum. The content was kept at +4°C for 24 h. The solids obtained [for the compounds 5, 6 and 7] were crystallized from suitable solvents [it was dipropylether (5) and acetonitrile (6 and 7)]. On the other hand, the compounds 1, 2, 3 and 4 were purified by column chromatography on silica gel (SiO2) using chloroform as a mobile phase.
5-Methyl-4-[(dimethylamino)methyl]-2-(propan-2-yl)phenol, compound 1
A viscous liquid, yield 15%. 1H NMR (400 MHz, CDCl3) δ: 6.99 (s, 1H), 6.37 (s, 1H), 3.33 (s, 2H), 3.20-3.15 (m, 1H), 2.31–2.20 (m, 9H), 1.23–1.20 (m, 6H).
3-Methyl-2-[(dipropylamino)methyl]-6-(propan-2-yl)phenol, compound 2
A viscous liquid, yield 6%. 1H NMR (400 MHz, CDCl3) δ: 6.99 (d, J = 7.7 Hz, 1H), 6.60 (d, J = 7.7 Hz, 1H), 3.75 (s, 2H), 3.33-2.26 (m, 1H), 2.49-2.45 (m, 4H), 2.21 (s, 3H), 1.62-1.53 (m, 4H), 1.21 (d, J = 7.2 Hz, 6H), 0.90 (t, J = 7.2 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ: 156.1, 133.7, 133.5, 124.5, 120.6, 119.9, 55.7, 54.4, 26.6, 22.9, 19.8, 12.1. HRMS (ESI-MS) Calc. for C17H29NO [M + H]+ 264.2322; found 264.2346.
3-Methyl-2-[(benzylamino)methyl]-6-(propan-2-yl)phenol, compound 3
A viscous liquid, yield 10%. 1H NMR (400 MHz, CDCl3) δ: 7.39-7.28 (m, 5H), 7.01 (d, J = 8.1 Hz, 1H), 6.72 (d, J = 8.1 Hz, 1H), 3.91(s, 2H), 3.89 (s, 2H), 3.28–3.25 (m, 1H), 2.08 (s, 3H), 1.23 (d, J = 6.6 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ: 151.6, 138.6, 133.9, 133.7, 129.3, 128.7, 127.6, 123.8, 121.8, 118.4, 81.6, 56.2, 48.8, 26.5, 22.9, 18.3. HRMS (ESI-MS) Calc. for C18H23NO [M + H]+ 270.1852; found 270.1855.
3-Methyl-2-[(dibenzylamino)methyl]-6-(propan-2-yl)phenol, compound 4
A viscous liquid, yield 6%. 1H NMR (400 MHz, CDCl3) δ: 11.40 (brs, 1H, –OH), 7.38–7.27 (m, 10H), 7.00 (d, J = 7.9 Hz, 1H), 6.63 (d, J = 7.9 Hz, 1H), 3.76 (s, 2H), 3.62 (s, 2H), 3.58 (s, 1H), 3.49 (d, J = 8.4 Hz, 2H), 3.35–3.33 (m, 1H), 2.24 (s, 2H), 1.24 (d, J = 6.6 Hz, 6H).
5-Methyl-4-[(piperidin-1-yl)methyl]-2-(propan-2-yl)phenol, compound 5
A white solid, yield 12%, m.p. 148 °C, 145 °CCitation31. 1H NMR (400 MHz, CDCl3) δ: 6.99 (s, 1H), 6.26 (s, 1H), 3.32 (s, 2H), 3.16 (q, J = 6.9 Hz 1H), 2.42 (brs, 4H), 2.18 (s, 3H), 1.60–1.55 (m, 4H), 1.43 (brs, 2H), 1.21 (d, J = 6.9 Hz, 6H).
5-Methyl-4-[(morpholino-4-yl)methyl]-2-(propan-2-yl)phenol, compound 6
A white solid, yield 16%, m.p. 152 °C, 152 °C10. 1H NMR (400 MHz, CDCl3) δ: 7.00 (s, 1H), 6.48 (s, 1H), 3.69 (brs, 4H), 3.38 (s, 2H), 3.16–3.13 (m, 1H), 2.44 (brs, 4H), 2.26 (s, 3H), 1.22 (d, J = 6.9 Hz, 6H).
5-Methyl-4-[(N-methylpiperazin-1-yl)methyl]-2-(propan-2-yl)phenol, compound 7
A white solid, yield 29%, m.p. 159 °C. 1H NMR (400 MHz, CDCl3) δ: 7.00 (s, 1H), 6.45 (s, 1H), 3.38 (s, 2H), 3.21–3.14 (m, 1H), 2.47 (brs, 8H), 2.29 (s, 3H), 2.24 (s, 3H), 1.22 (d, J = 6.9 Hz, 6H).
Biological activity
Carbonic anhydrase enzyme assay
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activityCitation32. Phenol red (at a concentration of 0.2 mM) has been used as an indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalyzed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor, at least six traces of the initial 5-10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled-deionized water and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. The inhibition percentages were obtained by using PRISM 3, as reported earlierCitation33, and represent the mean from at least three different determinations. All CA isoforms were recombinant ones obtained in-house as reported earlierCitation34. The cell pellets were lysed, and hCA II and hCA I were purified through affinity chromatography using pAMBS resin.
Cytotoxicity evaluation
The cytotoxicity of the compounds were assayed towards human oral squamous cell carcinoma (Ca9-22, HSC-2, HSC-3, HSC-4) and human normal oral cells (HGF, HPLF, HPC) as describedCitation35 with some minor modifications. In brief, all cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS). The following concentrations of the compounds in dimethylsulfoxide (DMSO) were added to the medium and incubated at 37°C for 48 h: compounds 1–7 (0.32, 1, 3.2, 10, 31.6, 100, 316, 1000 μM) and Melphalan (3.12, 6.25, 12.5, 25, 50, 100, 200, 400 μM). The media that contained the same concentration of DMSO (0.0078, 0.156, 0.03125, 0.0625, 0.125, 0.25, 0.5 or 1%) were used as controls, since DMSO above 0.25% is cytotoxic. The viable cell numbers were determined by the MTT method. The CC50 values were determined from dose-response curves.
Results and discussion
The compounds investigated here were synthesized by the conventional heating method. The mixture of thymol, formalin solution and amine was refluxed in methanol; the reagents had the same mole ratios. Although the same experimental procedure was applied for the synthesis of phenolic mono Mannich bases of thymol, the aminomethylation reaction took place at the ortho position of phenol in the case of the reactions for compounds 2, 3 and 4, in which secondary or primary aliphatic amines were used. On the other hand, aminomethylation reaction took place at para position of phenol for the reactions of the compounds 1, 5, 6 and 7. Secondary cyclic amines were used for the reactions of 5, 6 and 7. Compounds obtained with the secondary cyclic amines were solid apart from the others. Unique pattern of reaction was observed when the dimethyl amine (for compound 1) was used as an amine component. Although compound 1 is aliphatic and non-cyclic amine like dipropylamine (2), benzylamine (3) and dibenzylamine (4), it gave different reaction from compounds 2 to 4. Aminomethylation reaction took place at para position of phenol function in a similar way with secondary cyclic amines. Actually, to occupy the place of aminomethylation, i.e. Mannich reaction at ortho or para positions of phenol is an expected scientific fact, since hydroxyl group is a first-class substituent and direct substituent which will come to ortho or para position by itself. What are the substituents on nitrogen and how the substituents are located on it seems to be of great importance to direct aminomethylation process of thymol to ortho or para position of phenol function. Aminomethylation took place at para position of phenol function when the substituents on nitrogen were two methyls or bigger than this in size and these were acceptable on the condition that the amine used was in cyclic form in which free rotation was prevented as in the case of piperidine, morpholine and N-methylpiperazine. On the other hand, aminomethylation took place at ortho position of phenol function when the two substituents on nitrogen had longer chain than methyl (dipropylamine) and the hydrogens of methyl substituents located on nitrogen were replaced by phenyl rings (dibenzylamine). Ortho aminomethylation also occurred when the substituent was benzylamine. In this case, there is one methyl substituent on nitrogen and one of its hydrogens was replaced by phenyl (benzylamine). The common point for the ortho substitution of phenol function was that substituents present on nitrogen have the ability of free rotation.
1H NMR spectra of the ortho substituted thymols gave two doublets for the hydrogens located at 4 and 5 position of phenol function, while 1H NMR spectra of the para substituted thymol derivatives gave two singlets for the hydrogens located at 3 and 6 position of phenol function as reported in the “Experimental” section. The compounds 2 and 3 were reported for the first time. Chemical structure of the compounds were confirmed by 1H NMR and/or melting points for the literature registered compounds (1, 4, 5, 6 and 7). 1H NMR, 13C NMR and HRMS for the compounds 2 and 3 reported here for the first time.
The CA inhibition (% inhibition at 0.1 μM concentration of inhibitor) of the Mannich bases prepared here is shown in . This percentage was in the range of 18–26% for hCA I and of 28–33% for hCA II. Contrary to other phenols investigated earlierCitation5,Citation6,Citation9, the Mannich bases reported here show poor inhibitory activity against these two CA isoforms. This is probably due to the fact that in the ortho position to the OH moiety there is one or two rather bulky functionalities, which may interfere with the binding of the compound to the enzyme active site.
Table 1. hCA I and hCA II inhibition percentage of the Mannich bases 1–7.
The cytotoxic activity of the compounds were compared between four human oral squamous cell carcinoma (OSCC) cell lines (Ca9-22, HSC-2, HSC-3, HSC-4) and three human normal oral cells (HGF, HPLF, HPC). The results are presented in . Tumor-specificity (TS) value reflects the selectivity of the compounds against cancer tissues rather than normal ones. In this study, two types of TS values were calculated. First, TS was calculated by dividing the mean CC50 value of each compound against three human oral normal cells (Column D) by the mean CC50 value against four human OSCC cell lines (Column B). Second, TS was also calculated by dividing the value CC50 of each compound against HGF cells (Column C) by the CC50 value against Ca9-22 cell lines (Column A), both cells being originated from the same tissue (gingiva). Tumor-specificity values are presented at . All compounds showed lower TS values than reference drug melphalan by these two types of criteria. According to TS values obtained by first calculation method, the order of the potency of TS values of the compounds was as follow: The compound number (TS value): 5 (2.2) > 6 (2.0) > 3 (1.9) > 2 (1.8). The calculation of TS value for 4 was difficult due to much lower cytotoxicity of this compound to both malignant and non-malignant cells. When the second calculation was considered, compound 4 showed TS value greater than 2.1 due to the scale over of CC50 value, while the compound 5 (TS=2.6) had higher TS value than the compounds 2 and 3, which showed comparable tumor-specificity (TS = 2.2). On the other hand, compound 2 showed some selectivity against human oral squamous cell lines, especially at lower concentration. The growth of all four OSCC cell lines declined at 7.8–15.6 μg/ml, whereas the growth of all three normal cells declined at 16–32-fold higher concentration (250 μg/ml) ( and ). The TS values may be significantly affected by the type of growth inhibition, either cytotoxic or cytostatic.
Figure 1. Viable cell number percentange of human OSCC and normal cells treated with increasing concentrations with compound 2.
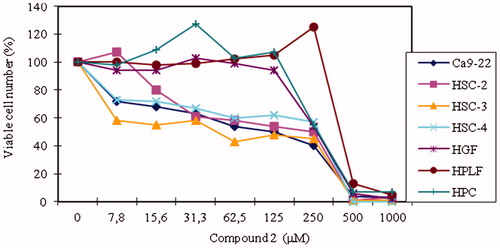
Table 2. Cytotoxic activities of the Mannich bases 1–7.
Table 3. Cytotoxic activities of the compound 2 against human OSCC and normal oral cells.
Conclusion
The chemical structure and tumor-specific cytotoxicity of compounds 2 and 3 are reported in this study for the first time. At the same experimental condition ortho and para aminomethylation occurred depending on the nature of amine used. The compounds were slightly more selective against hCA II rather than hCA I. Based on the cytostatic property of compound 2, it is important to consider the possibility that this compound may enhance the cytotoxicity of popular chemotherapeutic agents.
Acknowledgements
Prof. Gul and Prof. Supuran thank ERASMUS office of Ataturk University, Turkey for their financial support to Yamali C.
Declaration of interest
The authors report no conflict of interest and are responsible for the contents and writing of the paper.
References
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–50
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–89
- Innocenti A, Hall RA, Schlicker C, et al. Carbonic anhydrase inhibitors. Inhibition of the beta-class enzymes from the fungal pathogens Candida albicans and Cryptococcus neoformans with aliphatic and aromatic carboxylates. Bioorg Med Chem 2009;17:2654–7
- Bayram E, Senturk M, Kufrevioglu OI, Supuran CT. In vitro inhibition of salicylic acid derivatives on human cytosolic carbonic anhydrase isozymes I and II. Bioorg Med Chem 2008;16:9101–5
- Innocenti A, Beyza Ozturk Sarikaya S, Gulcin I, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg Med Chem 2010;18:2159–64
- Gorlach A, Kietzmann T. Superoxide and derived reactive oxygen species in the regulation of hypoxia-inducible factors. Methods Enzymol 2007;435:421–46
- Gul HI, Cizmecioglu M, Zencir S, et al. Cytotoxic activity of 4′-hydroxychalcone derivatives against Jurkat cells and their effects on mammalian DNA topoisomerase I. J Enzyme Inhib Med Chem 2009;24:804–7
- Bilginer S, Unluer E, Gul HI, et al. Carbonic anhydrase inhibitors. Phenols incorporating 2- or 3-pyridyl-ethenylcarbonyl and tertiary amine moieties strongly inhibit Saccharomyces cerevisiae beta-carbonic anhydrase. J Enzyme Inhib Med Chem 2014;29:495–9
- Ai-Yu S, Mei-Han H, Li-Fang L, Try-Shy W. Thymol analogues with antioxidant and L-type calcium current inhibitory activity. Drug Dev Res 2005;64:195–202
- Aeschbach R, Loliger J, Scott BC, et al. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem Toxicol 1994;32:31–6
- Aboelwafa HR, Yousef HN. The ameliorative effect of thymol against hydrocortisone-induced hepatic oxidative stress injury in adult male rats. Biochem Cell Biol 2015;93:282–9
- Huang MH, Liao LF, Kuo SH, et al. Effects of 4-piperidinomethyl-2-isopropyl-5-methylphenol on oxidative stress and calcium current. J Pharm Pharmacol 2005;57:1191–7
- Deb DD, Parimala G, Saravana Devi S, Chakraborty T. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem Biol Interact 2011;193:97–106
- Karkabounas S, Kostoula OK, Daskalou T, et al. Anticarcinogenic and antiplatelet effects of carvacrol. Exp Oncol 2006;28:121–5
- Deng XY, Li HY, Chen JJ, et al. Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav Brain Res 2015;291:12–19
- Wei Z, Zhou E, Guo C, et al. Thymol inhibits Staphylococcus aureus internalization into bovine mammary epithelial cells by inhibiting NF-kappaB activation. Microb Pathog 2014;71:15–19
- Didry N, Dubreuil L, Pinkas M. Activity of thymol, carvacrol, cinnamaldehyde and eugenol on oral bacteria. Pharm Acta Helv 1994;69:25–8
- Mahmoud AL. Antifungal action and antiaflatoxigenic properties of some essential oil constituents. Lett Appl Microbiol 1994;19:110–13
- Roman G. Mannich bases in medicinal chemistry and drug design. Eur J Med Chem 2015;89:743–816
- Sieger GM, Barringer WC, Krueger JE. Mannich derivatives of medicinals. 2. Derivatives of some carbonic anhydrase inhibitors. J Med Chem 1971;14:458–60
- Buyukkidan N, Buyukkidan B, Bulbul M, et al. Synthesis and characterization of phenolic Mannich bases and effects of these compounds on human carbonic anhydrase isozymes I and II. J Enzyme Inhib Med Chem 2013;28:337–42
- Gul HI, Yerdelen KO, Das U, et al. Synthesis and cytotoxicity of novel 3-aryl-1-(3′-dibenzylaminomethyl-4′-hydroxyphenyl)-propenones and related compounds. Chem Pharm Bull 2008;56:1675–81
- Tugrak M, Yamali C, Sakagami H, Gul HI. Synthesis of mono Mannich bases of 2-(4-hydroxybenzylidene)-2,3-dihydroinden-1-one and evaluation of their cytotoxicities. J Enzyme Inhib Med Chem 2015. [Epub ahead of print]. doi:10.3109/14756366.2015.1070263
- Yerdelen KO, Gul HI, Sakagami H, Umemura N. Synthesis and biological evaluation of 1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one and its aminomethyl derivatives. J Enzyme Inhib Med Chem 2015;30:383–8
- Gul HI, Calis U, Vepsalainen J. Synthesis and evaluation of anticonvulsant activities of some bis Mannich bases and corresponding piperidinols. Arzneimittelforschung 2002;52:863–9
- Gul HI, Calis U, Vepsalainen J. Synthesis of some mono-Mannich bases and corresponding azine derivatives and evaluation of their anticonvulsant activity. Arzneimittelforschung 2004;54:359–64
- Gul HI, Calis U, Ozturk Z, et al. Evaluation of anticonvulsant activities of bis(3-aryl-3-oxo-propyl) ethylamine hydrochlorides and 4-aryl-3-arylcarbonyl-1-ethyl-4-piperidinol hydrochlorides. Arzneimittelforschung 2007;57:133–6
- Koechel DA, Rankin GO. Diuretic activity of Mannich base derivatives of ethacrynic acid and certain ethacrynic acid analogues. J Med Chem 1978;21:764–9
- Mete E, Ozelgul C, Kazaz C, et al. Synthesis and antifungal activity of 1-aryl-3-phenethylamino-1-propanone hydrochlorides and 3-aroyl-4-aryl-1-phenethyl-4-piperidinols. Arch Pharm 2010;343:291–300
- Moehrle H, Miller C. Mannich bases. Part 14. Aminomethylation of different substituted phenols by secondary amines. Pharmazie 1978;33:500–5
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73
- Maresca A, Temperini C, Pochet L, et al. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem 2010;53:335–44
- D'Ambrosio K, Smaine FZ, Carta F, et al Development of potent carbonic anhydrase inhibitors incorporating both sulfonamide and sulfamide groups. J Med Chem 2012;55:6776–83
- Motohashi N, Wakabayashi H, Kurihara T, et al. Biological activity of barbados cherry (acerola fruits, fruit of Malpighia emarginata DC) extracts and fractions. Phytother Res 2004;18:212–23