Abstract
The aim of this research is to evaluate the current streptokinase thrombolytic treatment and to identify or improve new techniques that will base new approaches with a higher efficiency in this area of expertise. In order to be as realistic as possible a new method was set up using magnetic vectorized nanoparticles streptokinase and human blood thrombus. The experimental data confirm the maximum 83% thrombus lyses whenever increase streptokinase concentration. It is very probable to happen because of the presence of high concentration of antiplasmin in the blood that neutralizes around half of the thrombolytic potential of the sanguine plasminogen. The experiment shows also that only free serum plasminogen are available for streptokinase action in order to generate plasmin.
Introduction
The biochemical processes that govern the genesis, and especially the dissolution of the thrombus present in the blood stream, have been and continue to represent an important research topic, given the practical implications of this problem, mainly regarding the therapy of the myocardial infarction and/or the cerebrovascular accident.
Currently, there a several options for the thrombolytic treatment, but the most utilized is the administration of streptokinase (STK). Although the results do not always live up to the expectations, the elements concerning the costs of the treatment have imposed and are maintaining this therapy method as one of the most utilized options in the acute myocardial infarction or cerebral microthrombosisCitation1.
One of the elements that set back the streptokinase therapy is that of the high heterogeneity of the response of the patients to this type of treatment. It is due to this fact that the streptokinase therapy, even if it does not have a significant number of adversaries, has come across a high degree of criticism based on clear clinical dataCitation2. In this study, we have tried to systematize the most positive effects and also to clarify as much as possible the limits of this treatment.
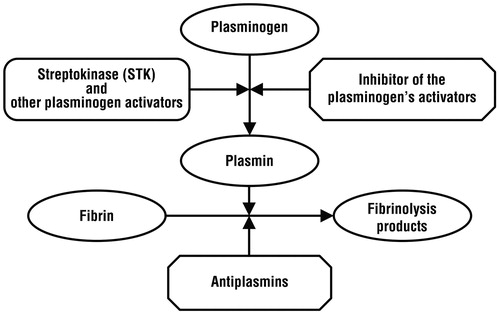
Streptokinase, EC 3.4.99.22, is a bacterial proteinCitation3, formed out of 414 amino acids with a 44 kDa mass, pI 4,7 and a 7.5 max pH activity. This protein has a specific biological activity, meaning that it can engage the plasminogen proteins (Pg) and form the STK-Pg compound that subsequently gains a proteolytic activity on other Pg proteins, thus generating plasmin (Pm) that combined with STK form the compound STK-Pm, a compound that has a high Pg and STK proteolytic activity. Through this succession of reactions, the STK interacts with the plasminogen, having as a final result plasmin and peptide segments derived from STK and PgCitation4,Citation5:
The next phase identifies Pm acting with a proteolytic activity on the fibrin, which represents the frame of the blood clot, determining its degradation and the disappearance of the thrombusCitation6. All these processes are concisely point up in the .
The clinical-extensive experience in the field of using STK in the thrombolytic treatment has shown three categories of aspects that, if understood accordingly, can lead to the increase in the efficiency of the streptokinase thrombolytic treatment:
Even if the producers indicate the active dosage of STK, it has been noted that, at the circulatory system level, there are important variations between the STK concentrations that prove active in every individual, a fact that leads the current physician to elevate the dose of the administrated STK, even with the risk of causing microbleedings;
Currently, there is no possibility of a targeted administration directly into the thrombus or at least in the general area of the body where the thrombus is present, leading to the dissipation of the STK effects into the entire mass of the organism;
Given the nature and important doses utilized for the reasons above-mentioned of STK, the genesis of the antigenic reply of the organism becomes a certainty, involving the anti-STK antibodies that lower upon the point of total elimination of ulterior STK therapies for a period of several weeks.
Methods
Although STK does not act as an authentic enzyme, consuming itself in the process of chemical reactions that lead to the fibrin hydrolysis, most methods of evaluating the biologic activity of STK are based on the principles and reagents used for general proteolytic enzymes. The most utilized are based on synthetic substances hydrolysis that contain derivatives of benzoyl-para-nitroanilide (b-PNA) and also the accurate spectrometric measuring of the development grade of the reactionCitation7. Another category of methods implies the incubation of STK with Pg (from different sources) and measuring the proteolytic activity of the Pm resulted from different substrates like fibrin or caseinCitation8,Citation9.
For evaluating as precisely as possible, reporting to the limitations of the STK therapy, we have imagined and elaborated an experiment concerning blood clots using magnetically vectored STK, a technique that has the advantage of conferring a high degree of handling both in a liquid environment and also in the mass of the thrombus, using an external magnetic field.
Principle of action
The STK action on the thrombus has been evaluated by joining the blood clot with the magnetite nanoparticle (Fe3O4)-marked STK and monitoring the thrombus lysis percentage in different moments in time.
Reagents and materials
All of the substances used have been analytically pure.
STK, Streptase, CSL Behring 250000 I.U./vial.
NP-STK suspension (equivalent of 5.000 I.U./mL) was prepared by mixing magnetite nanoparticle previously obtainedCitation10 with STK in phosphate buffer citrate solution and NP-STK separation with external magnetic field.
Buffer solution pH 7 phosphate, 0.2 M
Clotted blood 2 mL/tube
Saline solution (0.9% NaCl)
Magnet, pipettes, laboratory glassware.
Procedure
About 36–40 mL of blood was harvested from a healthy volunteer and was split into 2 mL fractions in graded tubes with 5–10 mL volume. A 5–15 clotting time is necessary, followed by freezer storage (3 °C–7 °C) until it is utilized as a reaction environment. A strict tab will have been kept on the time (hour and date) of the harvesting/storage and utilization of the blood clot. The serum is not to be separated from the formed clot.
The NP-STK compound is cleansed with saline solution, and then, it is resuspended in buffer solution so that a suspension with 500 I.U./mL activity is obtained. The reagents are placed in a thermostat for 25 min at 37 °C.
About 250 I.U. of NP-STK suspension (0.5 mL) are placed into contact with 1 mL of phosphate buffer solution and 2 mL of clotted blood. NP-STK is vibrated through the clot using an external magnet for 5–10 s. The mixtures are then placed in a thermostat for 5–90 min and then the fraction from the blood clot that has been hydrolyzed by the action of NP-STK is measured and the expressed as a percentage of the initial clot volume. The initial clot volume is calculated as a arithmetic average from three tubes, by washing the clot in the graded tubes, followed by a sieve decantation and an adding of a well-determined volume, using a pipette, of distilled water until a desired gradation on the tube containing the clot is achieved. The volume of the clot is obtained by the difference between the volume indicated on the tube and the volume of distilled water added with the pipette.
Results
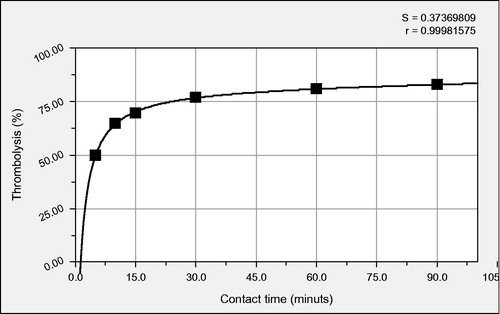
The degree of the blood clot hydrolysis at several time intervals of contact with NP-STK and certain work conditions are shown in .
Table 1. The proteolysis of the integral sanguine thrombus obtained from 2 mL of blood, 1 mL of phosphate buffer, 0,2 mM, pH = 7 and 250 I.U. STK-NP.
Discussion
The experimental results show more than one aspects that influence the effectiveness of the streptokinase thrombolytic treatment, from which the most important are: the high concentrations of blood antiplasminCitation7,Citation11, the lack of an antiplasmin inactivation/elimination treatment before administrating STK and the proteolytic activity of plasmin over STK. Along the therapy act, however, these can be mixed with other processes, as shown in .
Table 2. Limiting factors in thrombolytic therapy.
Thus, the most important process that lowers the thrombolytic activity of STK is represented by the presence of the plasmin inhibitors, mainly alfa2-antiplasmine (API), present in a concentration of approximately 1 mM. The reaction between Pm and API, being extremely quick, neutralizes the plasmin and inhibits the thrombus proteolysis. But this process only occurs in a first stage, until the whole blood quantity of API is consumed. Considering the differences of the concentration between antiplasmin (0.7–1 mM) and plasminogen (1.5–2 mM), we can state that normally, given the best scenario possible, the thrombolytic activity of the STK can only achieve half of the theoretically possible activity in the lack of API. Accordingly, the physician must take into consideration that in many occasions these proportions can be modified by different pathologies like hipoplasminogenesis, sepsis, leukemia, hyperthyroidism, hepatitis, etc. Leading to a highly lowered level of plasmin (subsequently inducing a low thrombolytic activity), regardless of the amount of administrated STK.
Another process that lowers the efficiency of the STK therapy is due to the proteolytic action of plasmin (and other reaction products between Pg and STK) over STK with an appreciable speed (the half-time of STK is approximately 30 min).
These facts explain the allure of the curve that illustrates the advancement of the degree of thrombolysis together with the action time of the STK, presented in , as well as the ascertainment that the magnetically recovered STK in the form of NP-STK no longer produces thrombolysis (, no.7).
Through the nonlinear regression of the degree of the thrombus hydrolysis (y, %) dependent of the contact time (x, minutes), a non-linear relationship has been highlighted, y = (71.95x - 89.95)/(1 + 0.86x – 0.00033x2), that can characterize the kinetics of the blood clot lysis processes under the action of STK.
Another element highlighted by the experimental data is the fact that, under the experiment conditions, even at high concentrations of STK, a complete proteolysis of the thrombus has not been achieved, leading us to the conclusion that plasmin, created as a follow-up of STK administration is not enough to completely hydrolyze the thrombus, which, in real therapy conditions, can migrate into the blood stream, leading to potential fatal consequences. For this reason, the thrombolytic therapy should hold into consideration prolonged administration of STK at least over 2–3 days, so that, as the sanguine Pg reserves are rebuilt, it can maintain a certain level of plasmin and to finally undergo the total thrombus hydrolysis, reported to the entire circulatory system.
Conclusions
We can notice that in the first 5–10 min of the STK action, a thrombus hydrolysis of over 50% is obtained.
In the utilized concentrations, the lack of total thrombus hydrolysis is present even at 60 min of contact time. From the experimental data, in the case of a longer than 60- min contact time, the blood clot hydrolysis stops advancing.
The relationship between the contact time of the NP-STK with the thrombus and the degree of cloth hydrolysis is not linear and is based on a rational connection that can set the base for a useful model for the increase of the STK-based therapy.
The NP-STK recovered using the external magnet after a 90-min contact time no longer presents capacity to act on a new blood clot, confirming once more that the STK has an autolytic activity through the reaction products with plasminogen.
NP-STK manifests no activity on the blood clot cleansed with saline solution 0.9%. This pleads for the hypothesis that basically, plasminogen that can be utilized as a STK substrate is only found freely in the serum and not in the structure of the thrombus. Given the results of other studies that have pinpointed the fact that 14–32% of the blood plasminogen is absorbed by the fibrin filaments through alfa2-API, Citation12, our results plead for the idea that this plasminogen can either generate plasmin that is immediately neutralized by API without being able to manifest its hydrolytic action over fibrin or, by unknown reasons, is not able to react to NP-STK. To clarify this aspect, we have set the goal to begin an extended study in the near future.
As noticed in the case of the blood clot stored in the freezer for 72 h since coagulation, the NP-STK activity is greatly decreased (about five times) by the “age” of the blood clot. This result can fully explained if we accept the fact that while storing the blood clot, its plasminogen levels decrease by degrading, a phenomenon consented by other research studies as well, that indicate a value of 2 days of the plasminogen half-time.
Based on the experimental data, we can appreciate that in order to increase the efficiency of the thrombolytic STK therapy, two directions can be followed: on the one hand, administering an initial massive STK dose so that the natural levels created by the presence of a concentration of antiplasmin in the blood that neutralizes half of the thrombolytic potential of the sanguine plasminogen are passed, and on the other hand, opting for a prolonged administration (3 days) of STK in order to fight the effects of incomplete hydrolysis, fragmentation and migration of the thrombus in the circulatory system.
Declaration of interest
The authors report no declarations of interest.
References
- Collen D, Lijnen HR. Molecular basis of fibrinolysis, as relevant for thrombolytic therapy. Thromb Haemost 1995;74:167–71
- Verstaete M, Vermylen J, Donati MB. The effect of streptokinase infusion on chronic arterial occlusions and stenoses. Ann Int Med 1971;74:377–82
- Lizano S, Kenneth HJ. Structural diversity of streptokinase and activation of human plasminogen. Infect Immun 2005;73:4451–3
- Wakeham N, Terzyan S, Zhai P, et al. Effects of deletion of streptokinase residues 48–59 on plasminogen activation. Protein Eng 2002;15:753–61
- Tharp AC, Laha M, Panizzi P, et al. Plasminogen substrate recognition by the streptokinase-plasminogen catalytic complex is facilitated by Arg253, Lys256, and Lys257 in the streptokinase domain and Kringle 5 of the substrate. J Biol Chem 2009;284:19511–21
- Banerjee A, Chisti Y, Banerjee UC. Streptokinase-a clinically useful thrombolytic agent. Biotech Adv 2004;22:287–307
- Saito H, Goldsmith GH, Moroi M, Aoki N. Inhibitory spectrum of alpha 2-plasmin inhibitor. Proc Natl Acad Sci USA 1979;76:2013–17
- Reed GL, YAN KA, Lin Liu H, et al. A catalytic switch and the conversion of streptokinase to a fibrin-targeted plasminogen activator. Proc Natl Acad Sci USA 1999;96:8879–83
- Rimon A, Shamash Y, Shapiro B. The plasmin inhibitor of human plasma. J Biol Chem 1966;241:5102–7
- Massart R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans Magn 1981;17:1247–8
- Derechin M. The assay of human plasminogen with casein as substrate. Biochem J 1961;78:443–8
- Cederholm-Williams SA. Concentration of plasminogen and antiplasmin in plasma and serum. J Clin Pathol 1981;34:979–81