Abstract
The effects of isatin Mannich bases incorporating (1-[piperidin-1-yl (P1)/morpholin-4-yl (P2)/N-methylpiperazin-1-yl (P3)]methyl)-1H-indole-2,3-dione) moieties against human (h) carbonic anhydrase (CA, EC 4.2.1.1) isoenzymes hCA I and hCA II, acetylcholinesterase (AChE), and butyrylcholinesterase (BChE) enzymes were evaluated. P1–P3 demonstrated impressive inhibition profiles against AChE and BChE and also inhibited both CAs at nanomolar level. These inhibitory effects were more powerful in all cases than the reference compounds used for all these enzymes. This study suggests that isatin Mannich bases P1–P3 are good candidate compounds especially for the development of new cholinesterase inhibitors since they were 2.2–5.9 times better inhibitors than clinically used drug Tacrine.
Introduction
Alzheimer’s disease (AD) is characterised by a progressive decline of memory and cognition. Based on the world Alzheimer’s reports, there were 36 million people in 2010, predicted to increase to 115 million by 2050Citation1. One of the therapeutic strategies is based on the cholinergic hypothesis targeting cholinesterase enzymes.
Cholinesterases (ChE) are an enzyme family that catalyse the hydrolysis of acetylcholine (ACh) into choline and acetic acid, an essential process for the restoration of the cholinergic neurotransmission. There are two cholinesterase types: acetylcholinesterase (AChE, EC 3.1.1.7) and butyrylcholinesterase (BChE, EC 3.1.1.8). AChE is known to be abundant in the muscle, brain and erythrocyte membrane, whereas BChE has a higher activity in liver, intestine, heart, kidney and lung. They have similar molecular forms and active sites despite being products of different genes on the human chromosomes. Inhibition of hydrolyses of acetylcholine (ACh) and butrylcholine (BCh) by using cholinesterase inhibitors have been considered to increase the level of the ACh and BCh in synapses. Although there are many ongoing research activities for treatment of AD, only some drugs were approved by the Food and Drug Administration (FDA), such as Tacrine, Donepezil and RivastigminCitation2. The 1H-indol motif which is also available in isatin structure was also used to design some new compounds which can be good candidate for the treatment of neurodegenerative diseasesCitation3. In addition, some isatin derivatives were considered to develop new cholinesterase inhibitorsCitation1,Citation4.
Carbonic anhydrases (CAs, EC 4.2.1.1) catalyse a very simple but physiologically essential reaction in all life kingdoms, the hydration of carbon dioxide (CO2) to bicarbonate (HCO3−) and protons (H+), with a high efficiency. Up to now, six distinct genetic CA families (α-, β-, γ-, δ-, ζ- and η-CAs) were discovered. Mammals including humans possess 16 different α-CA isoforms, which are involved in many crucial physiological or pathological processes connected with respiration and transport of CO2/HCO3−, pH and CO2 homeostasis, chemosensing, electrolyte secretion in a variety of tissues and organs, biosynthetic reactions, bone resorption, calcification, tumorigenicity, etc. CA inhibitors (CAIs) have found applications as important therapeutic agents. The most well known clinically established sulphonamide CA inhibitors include acetazolamide, dorzolamide, methazolamide, brinzolamide, dichlorophenamide and ethoxzolamide. Dorzolamide and brinzolamide are topically acting drugs, whereas the others are administered systemically and therefore have many undesirable side effects associated with them due to lack of sufficient selectivity towards CA isozymesCitation5,Citation6.
Isatin (1H-Indole-2,3-dion) and its derivatives are biologically active compounds and have significant importance in medicinal chemistry. Isatin moiety has many biological activities, such as anticonvulsant, MAO-A and MAO-B inhibitory, antipsychotic, sedative, antianxiety, antimicrobial, antiviral, anti-inflammatory, analgesic, antioxidant, anticancer, and cholinesterase inhibitory activitiesCitation1–3,7. On the other hand, Schiff bases of isatin have been reported as selective carbonic anhydrase inhibitorsCitation8.
Mannich bases are an important group of compounds in medicinal chemistry and they are synthesised by Mannich reactionCitation9,Citation10. Mannich bases have wide range of biological activities, such as cytotoxicCitation11–13, antiinflammatoryCitation14 and anticonvulsantCitation15 activities. Several types of phenolic Mannich bases were also reported with cytotoxicCitation13,Citation16 and CA inhibitoryCitation17 activities. The reported mechanism of Mannich bases are thiol alkylationCitation18,Citation19, interaction with some enzymes which are important for antioxidant mechanisms, inhibition of mitochondrial respirationCitation20,Citation21, topoisomerase enzyme inhibitionCitation22,Citation23 and tubulin polimerisationCitation24.
Isatin derivatives, which are carrying aminomethyl moiety, i.e. Mannich bases have important biological activities including anticancerCitation25 and anti-HIVCitation26 activities. Mannich bases of isatin were generally used as starting compounds or as intermediate compounds for the synthesis of various chemical designs. There is a study reporting the cytotoxicities of Mannich bases having the isatin motif and their corresponding Schiff base analogues. In this study, Manich bases had higher cytotoxicity than their corresponding Schiff basesCitation27.
In this study, it was aimed to investigate the CA, AChE and BChE inhibitory activities of some newly prepared isatin Mannich bases.
Experimental
Materials
1H NMR (400 MHz) spectra were taken using a Varian Mercury Plus spectrometer (Varian Inc., Palo Alto, CA). Chemical shifts (δ) were reported in ppm. Melting points were determined using an Electrothermal 9100 (IA9100, Bibby Scientific Limited, Staffordshire, UK) instrument and are uncorrected.
Methods
Synthesis of mono Mannich bases, P1–P3
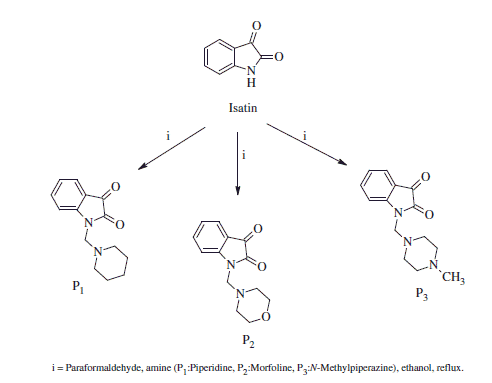
A mixture of secondary amine compound (3.4 mmol) and paraformaldehyde (3.4 mmol) in ethanol (5 mL) was added into the solution of isatin (3.4 mmol) in ethanol (10 mL) (Scheme 1). The reaction mixture was heated for 4 h and then kept at +4 °C for 36 h to form crystals. The crystalline products were separated by filtration and dried at room temperature. Recrystallisation from ethanol provided pure compounds (P1–P3). Chemical structures of the compounds were confirmed by 1H NMR and melting point.
1-(Piperidin-1-yl)methyl)-1H-indole-2,3-dione, P1
Orange coloured solid (65%); m.p. 134–136 °C, 126–128 27. 1H NMR (400 MHz, CDCl3,) δ: 7.59–7.57 (m, 2H, Ar-H), 7.12–7.09 (m, 2H, Ar-H), 4.43 (s, 2H), 2.58–2.55 (m, 4H), 1.59–1.42 (m, 6H).
1-(Morpholino-4-yl)methyl)-1H-indole-2,3-dione, P2
Orange coloured solid (68%); m.p. 199–200 °C, 194–196 Citation27. 1H NMR (400 MHz, CDCl3,) δ: 7.62 (d, J = 7.4 Hz, 1H, Ar-H), 7.59 (d, J = 7.7 Hz, 1H, Ar-H), 7.15 (t, J = 7.4 Hz, 1H, Ar-H), 7.08 (d, J = 7.7 Hz, 1H, Ar-H), 4.44 (s, 2H), 3.70–3.68 (m, 4H), 2.64–2.61 (m, 4H).
1-[(4-Methylpiperazin-1-yl)methyl]-1H-indole-2,3-dione, P3
Orange red coloured solid (72%); m.p. 138–140 °C. 1H NMR (400 MHz, CDCl3,) δ: 7.61–7.56 (m, 2H, Ar-H), 7.12 (t, J = 7.5 Hz, 1H), 7.07 (d, J = 8.1 Hz, 1H), 4.47 (s, 2H), 2.66 (brs, 4H), 2.41 (brs, 4H), 2.26 (s, 3H).
Biological activity
Carbonic anhydrase inhibition assay
The purification of cytosolic CA isoenzymes (CA I and CA II) were previously described with a simple one-step method by a Sepharose-4B-L tyrosine-sulphanilamide affinity chromatoghraphyCitation28. The protein quantity in the column effluents was determined spectrophotometrically at 280 nm. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was applied with a Bio-Rad Mini Gel system Mini-PROTEIN® system, Bio-Rad Laboratories, Inc., China after purification of both CA isoenzymes. Briefly, it was performed in acrylamide for the running (10%) and the stacking gel (3%) contained SDS (0.1%), respectively.
Activities of CA isoenzymes were determined according to a method by Verporte et al.Citation29 The increase in absorbance of reaction medium was spectrophotometrically recorded at 348 nm. Also, the quantity of protein was determined at 595 nm according to the Bradford methodCitation30. Bovine serum albumin was used as standard protein. For determination of inhibition effect of P1–P3 on both hCA isoenzymes, an activity (%)-[P1–P3] graph was drawn. The IC50 values were obtained from activity (%) versus compounds plotsCitation31. For calculation of Ki values, three different concentrations were used. The Lineweaver–Burk curves were drawn and calculations were realisedCitation32.
Cholinesterase inhibition assay
The inhibitory effects of freshly synthesized isatin Mannich bases (P1–P3) on AChE and BChE activities were measured by slightly modifying the spectrophotometric Ellman methodCitation33. Acetylthiocholine iodide (AChI) and Butyrylcholine iodide (BChI) were used as a substrate of the reaction. 5,5′-Dithio-bis(2-nitro-benzoic acid) (DTNB) was used for the determination of the AChE/BChE activities. Briefly, 100 mL of Tris/HCl buffer (1 M, pH 8.0) and 10 mL of sample solution dissolved in ultra pure water at different concentrations and 50 mL AChE/BChE solution were mixed, incubated for 10 min at 25 °C. Then 50 mL of DTNB (0.5 mM) was added. The reaction was then started by the addition of 50 mL of AChI/BChI (10 mM). The hydrolysis of these substrates was observed spectrophotometrically by the formation of yellow 5-thio-2-nitrobenzoate anion as the result of the reaction of DTNB with thiocholine, determined by the enzymatic hydrolysis of AChI/BChI, at a wavelength of 412 nmCitation34. For the determination of inhibition effect of P1–P3 on AChE and BChE, an activity (%)-[P1–P3] graph was drawn. The IC50 values were obtained from activity (%) versus compounds plots. For calculation of Ki values, three different concentrations were used. The Lineweaver–Burk curves were drawn and calculations were realisedCitation32.
Results and discussion
The inhibition data of the isatin Mannich bases P1–P3 are shown in . Our results indicate that P1–P3 had active inhibition profiles against slow cytosolic isoform hCA I, and cytosolic dominant rapid isoenzyme hCA II. The cytosolic hCA I isoenzyme was effectively inhibited by P1–P3 with inhibition constants in the low nanomolar range as 8.57 ± 1.65 − 9.43 ± 3.57 nM. On the other hand, acetazolamide (AZA) which is used as clinical drug had given a Ki value of 10.16 ± 2.46 nM.
Table 1. Inhibitory effects of isatin Mannich bases P1–P3 human carbonic anhydrase isoenzymes I and II (hCA I and II), acetylcholinesterase (AChE) and butyrylcholinesterase (BChE).
The data showed that P1–P3 were more effective than AZA in terms of hCA II inhibitory properties. The compounds P1–P3 showed similar inhibition profile on cytosolic hCA II isoenzyme, with Ki values in the range of 5.02 ± 1.07–8.08 ± 1.98 nM. The reference drug AZA showed a Ki value of 8.29 ± 1.38 nM. It means that the compounds were more powerful inhibitors against hCA II than reference compound.
AChE was effectively inhibited by P1–P3 with Ki values in the range 0.75 ± 0.30–1.01 ± 0.24 nM. Similarly, these compounds inhibited BChE with Ki values in the range 0.42 ± 0.21–1.11 ± 0.43 nM. On the other hand, Tacrine (TAC), which is used for the treatment of AD had been shown to lower AChE and BChE inhibition profiles, Ki values are in the range 2.69 ± 0.56 and 2.49 ± 0.23 nM, respectively. When the results were carefully considered it can be easily noticed that Mannich bases P1 with piperidine (3.4 times), P2 with morpholine (3.6 times), P3 with N-methylpiperazine (2.7 times) were 2.7–3.6 times more potent than reference drug Tacrine towards AchE. On the other hand, P1 (2.7 times), P2 (2.2 times) and P3 (5.9 times) were 2.2–5.9 times more effective than the clinically used reference drug Tacrine towards BChE.
Conclusion
The effects of isatin Mannich bases P1–P3 against hCA I/hCA II isoenzymes and AChE/BChE enzymes were evaluated. P1–P3 demonstrated very impressive inhibition profile on cholinesterases enzymes (AChE and BChE) and they also inhibited both CA isoenzymes (hCA I and hCA II) at the nanomolar level. These inhibitory effects were more powerful in all cases than the references used for CA experiment and cholinesterases experiments.
This pilot study suggests that isatin Mannich bases P1–P3 are good candidate compounds especially for the development of new cholinesterase inhibitors. Since they had 2.2–5.9 times powerful inhibition potency than clinical used drug, Tacrine. They are also useful candidates for the development of new CAIs, since they showed slightly more potent inhibition than the clinically used drug, Acetazolamide. It should be, however, mentioned that these compounds do not possess a chemical structure normally associated with CA inhibitionCitation35–42 and as thus probably possess an unknown mechanism of inhibition which should be investigated in more detailCitation43.
Declaration of interest
The authors report no conflict of interest and are responsible for the contents and writing of the paper.
This study was supported by the Research Foundation of Agri Ibrahim Cecen University, Turkey, Project No: FEF.14.009.
References
- Kia Y, Osman H, Kumar RS, et al. Ionic liquid mediated synthesis of mono- and bis-spirooxindole-hexahydropyrrolidines as cholinesterase inhibitors and their molecular docking studies. Bioorg Med Chem 2014;22:1318–28.
- Ismail MM, Kamel MM, Mohamed LW, Faggal SI. Synthesis of new indole derivatives structurally related to donepezil and their biological evaluation as acetylcholinesterase inhibitors. Molecules 2012;17:4811–23.
- Raj V. Review on CNS activity of isatin derivatives. Inter J Curr Pharm Res 2012;4:1–9.
- Almansour AI, Kumar RS, Arumugam N, et al. A facile ionic liquid promoted synthesis, cholinesterase inhibitory activity and molecular modeling study of novel highly functionalized spiropyrrolidines. Molecules 2015;20:2296–309.
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–89.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–50.
- Bhrigu B, Pathak D, Siddiqui N, et al. Search for biological active isatins: a short review. Inter J Pharm Sci Drug Res 2010;2:229–35.
- Sharma A, Tiwari M, Supuran CT. Novel coumarins and benzocoumarins acting as isoform-selective inhibitors against the tumor-associated carbonic anhydrase IX. J Enzyme Inhib Med Chem 2014;29:292–6.
- Dimmock JR, Shyam K, Logan BM, et al. Syntheses and evaluation of some Mannich bases derived from acetophenones against P388 lymphocytic leukemia and toxicological assessment of 3-dimethyl-amino-2-dimethylaminomethyl-1-(4-methoxyphenyl)-1-propanone dihydrochloride in rats. and of Mannich derived acetophenones P388 leukemia toxicological of dimethylaminomethyl1-(-methoxyphenyl1- dihydrochloride rats. J Pharm Sci 1984;73:471–7.
- Roman G. Mannich bases in medicinal chemistry and drug design. Eur J Med Chem 2015;89:743–816.
- Gul HI, Gul M, Erciyas E. Toxicity of some bis Mannich bases and corresponding piperidinols in the brine shrimp (Artemia salina) bioassay. J Appl Toxicol 2003;23:53–7.
- Gul HI, Yerdelen KO, Gul M, et al. Synthesis of 4′-hydroxy-3′-piperidinomethylchalcone derivatives and their cytotoxicity against PC-3 cell lines. Arch Pharm (Weinheim) 2007;340:195–201.
- Tugrak M, Yamali C, Sakagami H, Gul HI. Synthesis of mono Mannich bases of 2-(4-hydroxybenzylidene)-2,3-dihydroinden-1-one and evaluation of their cytotoxicities. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. DOI:10.3109/14756366.2015.1070263.
- Gul HI, Suleyman H, Gul M. Evaluation of the antiinflammatory activity of N,N-bis(3-dimethylamino-1-phenyl-propylidene)hydrazine dihydrochloride. Pharm Bio 2009;47:968–72.
- Gul HI, Calis U, Ozturk Z, et al. Evaluation of anticonvulsant activities of bis(3-aryl-3-oxo-propyl)ethylamine hydrochlorides and 4-aryl-3-arylcarbonyl-1-ethyl-4-piperidinol hydrochlorides. Arzneimittelforschung 2007;57:133–6.
- Yerdelen KO, Gul HI, Sakagami H, Umemura N. Synthesis and biological evaluation of 1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one and its aminomethyl derivatives. J Enzyme Inhib Med Chem 2015;30:383–8.
- Yamali C, Tugrak M, Halise Inci G, et al. The inhibitory effects of phenolic Mannich bases on carbonic anhydrase I and II isoenzymes. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. DOI:10.3109/14756366.2015.1126715.
- Gul M, Gul HI, Hanninen O. Effects of Mannich bases on cellular glutathione and related enzymes of Jurkat cells in culture conditions. Toxicol In Vitro 2002;16:107–12.
- Gul M, Atalay M, Gul HI, et al. The effects of some Mannich bases on heat shock proteins HSC70 and GRP75, and thioredoxin and glutaredoxin levels in Jurkat cells. Toxicol In Vitro 2005;19:573–80.
- Dimmock JR, Hamon NW, De Gooijer CA, et al. Effect of some 4-alkyl-5-dimethylamino-1-phenyl-1-penten-3-one hydrochlorides and related compounds on respiration in mouse liver mitochondria. Pharmazie 1989;44:560–2.
- Kucukoglu K, Gul HI, Cetin-Atalay R, et al. Synthesis of new N, N'-bis[1-aryl-3-(piperidine-1-yl)propylidene]hydrazine dihydrochlorides and evaluation of their cytotoxicity against human hepatoma and breast cancer cells. J Enzyme Inhib Med Chem 2014;29:420–6.
- Canturk P, Kucukoglu K, Topcu Z, et al. Effect of some bis Mannich bases and corresponding piperidinols on DNA topoisomerase I. Arzneimittelforschung 2008;58:686–91.
- Mete E, Gul HI, Canturk P, et al. Biological activity of 1-aryl-3-phenethylamino-1-propanone hydrochlorides and 3-aroyl-4-aryl-1-phenethyl-4-piperidinols on PC-3 cells and DNA topoisomerase I enzyme. Z Naturforsch C J Biosci Naturforsch 2010;65:647–52.
- Das U, Gul HI, Alcorn J, et al. Cytotoxic 5-aryl-1-(4-nitrophenyl)-3-oxo-1,4-pentadienes mounted on alicyclic scaffolds. Eur J Med Chem 2006;41:577–85.
- George RF, Ismail NS, Stawinski J, Girgis AS. Design, synthesis and QSAR studies of dispiroindole derivatives as new antiproliferative agents. Eur J Med Chem 2013;68:339–51.
- Mishra R. Synthesis of enone ester by conventional method for their possible use in anti-HIV chemotherapy. J Chem Pharm Res 2014;6:753–6.
- Solomon VR, Hu C, Lee H. Hybrid pharmacophore design and synthesis of isatin-benzothiazole analogs for their anti-breast cancer activity. Bioorg Med Chem 2009;17:7585–92.
- Akbaba Y, Akincioglu A, Gocer H, et al. Carbonic anhydrase inhibitory properties of novel sulfonamide derivatives of aminoindanes and aminotetralins. J Enzyme Inhib Med Chem 2014;29:35–42.
- Verporte JA, Mehta S Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem Bio 1967;242:4221–9.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.
- Senturk M, Gulcin I, Beydemir S, et al. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–9.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–66.
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
- Oztaskin N, Cetinkaya Y, Taslimi P, et al. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg Chem 2015;60:49–57.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77.
- Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72.
- Winum JY, Supuran CT. Recent advances in the discovery of zinc-binding motifs for the development of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2015;30:321–4.
- Touisni N, Maresca A, McDonald PC, et al. Glycosyl coumarin carbonic anhydrase IX and XII inhibitors strongly attenuate the growth of primary breast tumors. J Med Chem 2011;54:8271–7.
- Del Prete S, De Luca V, Scozzafava A, et al. Biochemical properties of a new alpha-carbonic anhydrase from the human pathogenic bacterium, Vibrio cholerae. J Enzyme Inhib Med Chem 2014;29:23–7.
- Del Prete S, De Luca V, Vullo D, et al. Biochemical characterization of the γ-carbonic anhydrase from the oral pathogen Porphyromonas gingivalis, PgiCA – anhydrase the pathogen gingivalisPgiCA. J Enzyme Inhib Med Chem 2014;29:532–7.
- Capasso C, Supuran CT. Sulfa and trimethoprim-like drugs – antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J Enzyme Inhib Med Chem 2014;29:379–87.
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. DOI:10.3109/14756366.2015.1122001.