Abstract
New isatin-triazole based hybrids have been synthesized and evaluated for their inhibitory activity of TNF-α induced expression of Intercellular Adhesion Molecule-1 (ICAM-1) on the surface of human endothelial cells. Structure-activity relationship (SAR) studies revealed that the presence of the electron-attracting bromo substituent at position-5 of the isatin moiety played an important role in enhancing the anti-inflammatory potential of the synthesized compounds. Z-1-[3-(1H-1,2,4-Triazol-1-yl)propyl]-5-bromo-3-[2-(4-methoxyphenyl)hydrazono]indolin-2-one (19) with an IC50 = 20 μM and 89% ICAM-1 inhibition with MTD at 200 μM was found to be the most potent of all the synthesized derivatives. Introduction of 1,2,4-triazole ring and electron-donating methoxy group on the phenylhydrazone moiety resulted in four-fold increase of the anti-inflammatory activity.
Introduction
Indoline-2,3-diones or indole-1H-2,3-diones, commonly known as isatins, have been extensively studied due to their diverse pharmacological properties and synthetic versatility. Isatins are a well-known class of natural products found in plants of the genus IsatisCitation1, Calanthe discolor LINDLCitation2 and Couroupita guianensis AublCitation3. Various substituted isatins have been isolated from plants, e.g. melosatin alkaloids from Melochia tomentosa, a Caribbean tumorigenic plantCitation4; 6-(3′-methylbuten-2′-yl)isatin from fungi, Streptomyces albusCitation5 and 5-(3′-methylbuten-2′-yl)isatin from Chaetomium globosumCitation6. It is also known that isatins act as endogenous biological regulators, found in the brain, peripheral tissues, and body fluids of humans and animalsCitation7. Isatin was first synthetically obtained as an oxidation product of indigo in the early 19th century by Erdman and LaurentCitation8.
The synthetic interest in the chemistry of isatin and its derivatives stemmed from its easy synthetic accessibility and exhibition of broad spectrum biological effects, including antibacterial, antifungal, anticonvulsant, antiviral, anticancer, antioxidant, anti-inflammatory and antiproliferative activitiesCitation9–16. Isatin derivatives, such as hydrazones, Schiff’s and Mannich bases are of great medicinal value owing to their numerous chemotherapeutic propertiesCitation17–19.
The 1,2,4-triazole moiety is present in a wide variety of therapeutically interesting drugs, such as ribavarinCitation20, triazolamCitation21, fluconazoleCitation22 and voriconazoleCitation23. AnticancerCitation24, antitubercularCitation25, analgesicCitation26, antimicrobialCitation27 and anti-inflammatory activities of 1,2,4-triazole derivatives have also been reportedCitation28. Recently, some 1,2,4-triazole containing isatin derivatives possessing interesting biological activities have been describedCitation29–31.
During an inflammatory cascade, various inflammatory mediators, including cytokines, such as TNF-α, IL-1β and bacterial lipopolysaccharides induce the expression of endothelial cell adhesion molecules, viz. intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin, on the vascular endotheliumCitation32. The increased levels of expression of cell adhesion molecules on the endothelial cells alter the adhesive property of the vasculature, leading to indiscriminate infiltration of the leukocytes across the blood vessels, and thus causing inflammation. A promising approach for the therapeutic intervention of inflammatory disorders is by pharmacological inhibition of the CAM expression of endothelial cellsCitation33. In order to develop safer and potent anti-inflammatory agents/drugs, our laboratories have identified a number of small molecules from natural/synthetic sources that efficiently block nuclear accumulation of NF-κB and abrogate TNF-α-induced expression of E-selectin, VCAM-1 and ICAM-1 on human umbilical vein endothelial cells (HUVEC’s)Citation34–36.
In drug discovery, the development of hybrid molecules through the combination of different pharmacophores leads to compounds with interesting biological profiles. Prompted by the anti-inflammatory activities associated with 1,2,4-triazole and isatin derivatives, we have synthesized a series of novel isatin derivatives by connecting the isatin core moiety with triazole moiety using various alkyl chain linkers. Also, the Schiff’s bases and oximes were prepared using substituted hydrazines and hydroxylamine. Both p-methoxyphenyl hydrazine and pentafluorophenyl hydrazine were used as amines for the condensation step, as we wanted to evaluate the effects of both electron donating and electron withdrawing substituents on the phenyl ring of the amine for the ICAM-1 expression inhibition studies.
The synthesized triazolylisatins were then screened for their inhibition of TNF-α induced expression of ICAM-1 in HUVEC’s. The structure activity relationship (SAR) of the synthesized compounds has also been well established to search for potential lead compounds as anti-inflammatory drugs. Our findings revealed that Z-1-[3-(1H-1,2,4-triazol-1-yl)propyl]-5-bromo-3-[2-(4-methoxyphenyl)hydrazono]indolin-2-one (19) with an IC50 value of 20 μM and 89% inhibition in HUVEC’s is the most potent ICAM-1 expression inhibitor.
Materials and methods
General
Analytical TLCs were performed on Merck silica gel 60 F254 plates. All flash chromatographic separations were performed on 100-200 mesh silica gel. The IR spectra were recorded on a Perkin-Elmer 2000 FT-IR spectrometer (Waltham, MA). The 1H NMR and 13C NMR spectra were recorded on a Bruker AC-300 Avance spectrometer (Billerica, MA) at 300 and 75.5 MHz, respectively, using TMS as internal standard. Chemical shifts are reported on δ scale and coupling constants (J) are in Hz. The HRMS determinations were made in FAB positive mode on a JEOL JMS-AX505W high-resolution mass spectrometer using bis-hydroxyethyldisulfide (HEDS) doped with sodium acetate as matrix in the Laboratory of Dr. Carl-Erik Olsen at the University of Copenhagen (Denmark). Melting points were recorded in a sulfuric acid bath and are uncorrected.
Materials
Materials were obtained from commercial suppliers and were used without further purification unless otherwise noted. Petroleum ether and ethyl acetate were distilled over P2O5 and K2CO3, respectively, prior to use. The compounds 1 and 2, the endothelial cell growth factor (ECGF), M199 medium, l-glutamine, methylthiazolydiphenyl-tetrazolium bromide (MTT), trypsin, o-phenylenediamine and goat anti-mouse IgG–HRP conjugate were procured from Sigma Chemical Co. (St. Louis, MO). The fetal calf serum (FCS) was procured from Biological Industries (Kibbutz Beit Haemek, Israel).
Methods
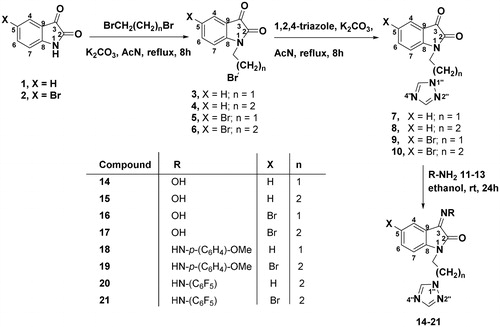
General method for the preparation of compounds 3–6
To a solution of compound 1/2 (2 mmol) and anhydrous potassium carbonate (2 mmol) in acetonitrile (20 mL), 1,2-dibromoethane/1,3-dibromopropane (0.03 mol) was added dropwise and the resultant mixture was refluxed for 5 h. On completion of the reaction, the reaction mixture was filtered and solvent evaporated under reduced pressure. The residue was purified by silica gel column chromatography using ethyl acetate/petroleum ether (3:1, v/v) as an eluent to afford the compounds 3–6 in 60-65% yields.
1-(2-Bromoethyl)indoline-2,3-dione (3)
Orange solid. Yield: 65%; m.p.: 128–130 °C (literature m.p.: 131–132 °C)Citation37.
1-(3-Bromopropyl)indoline-2,3-dione (4)
Orange solid. Yield: 60%; m.p.: 182–184 °C (literature m.p.: 187-188 °C)Citation37. IR (KBr): 3454, 2956, 1738, 1614, 1468 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 2.13–2.16 (m, 2H, C-2′H), 3.60 (t, 2H, J = 3.0 Hz, C-1′H), 3.78 (t, 2H, J = 3.0 Hz, C-3′H), 7.09–7.12 (m, 1H, ArH), 7.17–7.20 (m, 1H, ArH), 7.54-7.55 (m, 1H, ArH), 7.64-7.66 (m, 1H, ArH); 13C NMR (75.5 MHz, DMSO-d6): δ 30.04 (C-3′), 31.83 (C-2′), 58.24 (C-1′), 110.48 (C-7), 117.71 (C-4), 123.13 (C-5), 124.44 (C-6), 138.03 (C-8), 150.50 (C-9), 158.32 (C-2), 183.28 (C-3). HRMS-FAB: m/z [M + Na+] calcd for C11H10BrNO2Na: 289.1102; found: 289.1100.
5-Bromo-1-(2-bromoethyl)indoline-2,3-dione (5)
Orange solid. Yield: 62%; m.p.: 180–182 °C (literature m.p.: 180-184 °C)Citation37. IR (KBr): 3458, 1738, 1602, 1470, 1437 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 3.67 (t, 2H, J = 6.6 Hz, C-1′H), δ 4.10 (t, 2H, J = 6.6 Hz, C-2′H), 7.30 (d, 1H, J = 8.4 Hz, C-6H), 7.66 (s, 1H, C-4H), 7.85 (d, 1H, J = 7.2 Hz, ArH); 13C NMR (75.5 MHz, DMSO-d6): δ 28.95 (C-2′), 41.28 (C-1′), 113.29 (C-7), 115.14 (C-5), 119.17 (C-6), 126.80 (C-4), 139.90 (C-8), 149.12 (C-9), 157.74 (C-2), 181.73 (C-3). HRMS-FAB: m/z [M + Na]+ calcd for C10H7Br2NO2Na: 353.8736; found: 353.8726.
5-Bromo-1-(3-bromopropyl)indoline-2,3-dione (6)
Orange solid. Yield: 60%; m.p.: 155–158 °C (literature m.p.: 155 °C)Citation38. 1H NMR (300 MHz, DMSO-d6): δ 3.75 (t, 2H, J = 6.6 Hz, C-3′H), δ 2.16–2.73 (m, 2H, C-2′H), 7.1 (d, 1H, J = 8.4 Hz, C-6H), 7.68 (s, 1H, C-4H), 7.83 (d, 1H, J = 8.4 Hz, C-7H); 13C NMR (75.5 MHz, DMSO-d6): δ 30.17 (C-3′), 31.70 (C-2′), 40.34 (C-1′), 112.62 (C-7), 114.80 (C-6), 119.52 (C-5), 126.60 (C-4), 139.60 (C-8), 149.38 (C-9), 157.93 (C-2), 182.01 (C-3). HRMS-FAB: m/z [M + Na+] calcd for C10H7Br2NO2Na: 367.8892; found: 367.8889.
General method for the preparation of compounds 7–10
To a solution of compounds 3–6 (2 mmol) and anhydrous potassium carbonate (2 mmol) in acetonitrile (10 mL), 1,2,4-triazole (2 mmol) in acetonitrile (10 mL) was added dropwise and the reaction mixture was stirred and refluxed for 5 h. On completion, the reaction mixture was filtered and solvent evaporated under reduced pressure. The residue thus obtained was purified by silica gel column chromatography using ethyl acetate/petroleum ether (3:1, v/v) as an eluent to afford the compounds 7–10 in 65-75% yields.
1-[2-(1H-1,2,4-Triazol-1-yl)ethyl]-indoline-2,3-dione (7)
Orange solid. Yield: 65%; m.p.: 105–108 °C. IR (KBr): 3116, 1739, 1612, 1510, 1469 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 4.04 (t, 2H, J = 5.4 Hz, C-1′H), 4.45 (t, 2H, J = 5.7 Hz, C-2′H), 6.77 (d, 1H, J = 8.1 Hz, C-7H), 7.07 (t, 1H, J = 7.5 Hz, C-5), 7.50-7.56 (m, 2H, C-4H and C-6H) 7.90 (s, 1H, C-3″), 8.50 (s, 1H, C-5″); 13C NMR (75.5 MHz, DMSO-d6): δ 46.37 (C-1′ and C-2′), 110.0 (C-7), 117.35 (C-4), 123.27 (C-5), 124.52 (C-6), 138.12 (C-8), 144.77 (C-3″), 150.36 (C-9), 151.68 (C-5″), 158.18 (C-3), 183.03 (C-2). HRMS-FAB: m/z [M + Na+] calcd for C12H10N4O2Na: 265.0696; found: 265.0696.
1-[3-(1H-1,2,4-Triazol-1-yl)propyl]-indoline-2,3-dione (8)
Orange solid. Yield: 68%; m.p.: 103–106 °C. IR (KBr): 3446, 3100, 1724, 1605, 1468 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 2.11–2.15 (m, 2H, C-2′H), 3.65-3.70 (m, 2H, C-3′H), 4.24–4.29 (m, 2H, C-1′ H), 7.09–7.15 (m, 2H, C-4H, C-6H), 7.52 (d, 1H, J = 7.2 Hz, C-7H), 7.64 (t, 1H, J = 7.8 Hz, C-5H), 8.44 (s, 1H, C-3″), 8.50 (s, 1H, C-5″); 13C NMR (75.5 MHz, DMSO-d6): δ 27.06 (C-2′), 36.83 (C-1′), 46.13 (C-3′), 110.49 (C-7), 117.69 (C-5), 123.14 (C-6), 124.41 (C-4), 138.01 (C-8), 144.10 (C-3″), 150.38 (C-9), 151.46 (C-5″), 158.29 (C-3), 183.28 (C-2). HRMS-FAB: m/z [M + Na+] calcd for C13H12N4O2Na: 279.2696; found: 279.2694.
1-[2-(1H-1,2,4-Triazol-1-yl)ethyl]-5-bromoindoline-2,3-dione (9)
Orange solid. Yield: 70%; m.p.: 104–106 °C. IR (KBr): 3449, 3117, 1739, 1611, 1440 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 4.06 (t, 2H, J = 5.4 Hz, C-1′H), 4.46 (t, 2H, J = 4.5 Hz, C-2′H), 6.75 (d, 1H, J = 8.2 Hz, C-7H), 7.55–7.70 (m, 1H, C-6H), 7.70 (s, 1H, C-4H), 7.93 (s, 1H, C-3″H), 8.51 (s, 1H, C-5″H); 13C NMR (75.5 MHz, DMSO-d6): δ 46.45 (C-2′ and C-1′), 112.45 (C-7), 119.12 (C-5), 121.44 (C-6), 128.23 (C-4), 138.72 (C-8), 144.11 (C-3″), 148.39 (C-9), 151.47 (C-5″), 160.32 (C-3), 183.07 (C-2). HRMS-FAB: m/z [M + Na+] calcd for C12H9BrN4O2Na: 342.9801; found: 342.9801.
1-[3-(1H-1,2,4-Triazol-1-yl)propyl]-5-bromoindoline-2,3-dione (10)
Orange solid. Yield: 75%; m.p.: 102–105 °C. IR (KBr): 3451, 2928, 1739, 1607, 1509 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 2.10 (brs, 2H, C-2′), 3.65 (brs, 2H, C-3′H), 4.24 (brs, 2H, C-1′H), 7.12 (d, 1H, J = 8.4 Hz, C-7H), 7.52 (d, 1H, J = 7.5 Hz, C-6H), 7.79 (s, 1H, C-4H), 7.92 (s, 1H, C-3″H), 8.47 (s, 1H, C-5″); 13C NMR (75.5 MHz, DMSO-d6): δ 27.08 (C-2′), 36.97 (C-1′), 45.89 (C-3′), 110.52 (C-7), 112.70 (C-6), 119.46 (C-5), 126.16 (C-4), 139.71 (C-8), 144.11 (C-3″), 150.39 (C-9), 151.47 (C-5″), 158.32 (C-3), 182.07 (C-2). HRMS-FAB: m/z [M + H+] calcd for C13H12BrN4O2: 335.0138; found: 335.0129.
General method for the preparation of compounds 14–21
Equimolar quantities of compounds 7–10 (2 mmol) and hydroxylamine (11)/aryl hydrazine 12–13 (2 mmol) were taken in 20 mL of absolute ethanol. The reaction mixture was stirred at room temperature for 24 h, the completion of the reaction was checked by TLC. Upon completion, the solvent was evaporated under reduced pressure and the product was recrystallized from ethanol (99.5%) yielding 14–21 in 68-72% yields.
Z-1-[2-(1H-1, 2, 4-Triazol-1-yl)ethyl]-3-(hydroxyimino)indolin-2-one (14)
Orange solid. Yield: 70%; m.p.: 228–230 °C. IR (KBr): 3437, 1715, 1611, 1518, 1465 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 4.17 (t, 2H, J = 5.7 Hz, C-1′H), 4.54 (t, 2H, J = 5.7 Hz, C-2′H), 6.90 (d, 1H, J = 7.8 Hz, C-4H), 7.12 (t, 1H, J = 7.5 Hz, C-6H), 7.41 (t, 1H, J = 7.5 Hz, C-5H), 7.97 (s, 1H, C-3″H), 8.02 (d, 1H, J = 7.2 Hz, C-7H), 8.53 (s, 1H, C-5″H), 13.49 (d, 1H, J = 3.6 Hz, OH); 13C NMR (75.5 MHz, DMSO-d6): δ 46.42 (C-1′ & C-2′), 108.49 (C-7), 115.14 (C-6), 122.58 (C-5), 126.85 (C-4), 131.83 (C-8), 142.70 (C-3), 143.27 (C-9), 144.48 (C-3″), 151.55 (C-5″), 163.03 (C-2). HRMS-FAB: m/z [M + Na+] calcd for C12H11N5O2Na: 280.0805; found: 280.0815.
Z-1-[3-(1H-1,2,4-Triazol-1-yl)propyl]-3-(hydroxyimino)indolin-2-one (15)
Orange solid. Yield: 68%; m.p.: 239–241 °C. IR (KBr): 3449, 1681, 1611, 1581, 1540 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 2.14-2.19 (m, 2H, C-2′H), 3.75 (t, 2H, J = 6.6 Hz, C-1′H), 4.30 (t, 2H, J = 6.9 Hz, C-3′H), 7.07–7.13 (m, 2H, C-5H and C-7H) 7.43 (t, 1H, J = 7.8 Hz, C- 6H), 7.98 (d, 1H, J = 7.5 Hz, C-4H), 8.33 (s, 1H, C-3″H), δ 8.96 (s, 1H, C-5″H), 13.51 (br s, 1H, OH); 13C NMR (75.5 MHz, DMSO-d6): δ 27.05 (C-2′), 36.42 (C-3′), 46.93 (C-1′), 109.02 (C-7), 115.33 (C-6), 122.66 (C-5), 126.89 (C-4), 131.98 (C-8), 142.70 (C-3) 143.39 (C-9 and C-3″), 149.11 (C-5″), 163.19 (C-2). HRMS-FAB: m/z [M + Na+] calcd for C13H13N5O2Na: 294.9896; found: 294.9894.
Z-1-[2-(1H-1,2,4-Triazol-1-yl)ethyl]-5-bromo-3-(hydroxyimino)indolin-2-one (16)
Orange solid. Yield: 72%; m.p.: 251–252 °C. IR (KBr): 3433, 3042, 1722, 1607, 1437 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 4.10 (t, 2H, J = 5.4 Hz, C-2′H), 4.50 (t, 2H, J = 5.1 Hz, C-1′H), 6.85 (t, 1H, J = 7.2 Hz, C-7H), 7.50-7.52 (m, 1H, C-6H), 8.05 (s, 1H, C-4H), 8.08 (s, 1H, C-3″H) and 8.73 (s, 1H, C-5″H), 13.84 (brs, 1H, OH); 13C NMR (75.5 MHz, DMSO-d6): δ 46.73 (C-2′ and C-1′), 110.67 (C-7), 114.02 (C-6), 116.71 (C-4), 128.77 (C-5), 138.02 (C-8), 141.77 (C-3), 142.29 (C-3″), 144.16 (C-9), 150.31 (C-5″), 162.60 (C-2). HRMS-FAB: m/z [M + H+] calcd for C12H10BrN5O2: 336.0091; found: 336.0085.
Z-1-[3-(1H-1,2,4-Triazol-1-yl)propyl]-5-bromo-3-(hydroxyimino)indolin-2-one (17)
Orange solid. Yield: 70%; m.p.: 234–236 °C. IR (KBr): 3487, 2969, 1698, 1604, 1436 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 2.10–2.13 (m, 2H, C-2′H), 3.71 (t, 2H, J = 6.9 Hz, C-2′H), 4.26 (t, 2H, J = 6.9 Hz, C-3′H), 7.05 (d, 1H, J = 5.1 Hz, ArH), 7.58 (dd, 1H, J = 2.1 Hz each ArH), 8.05 (d, 1H, J = 2.1 Hz, ArH), 8.28 (s, 1H, C-3″H), δ 8.90 (s, 1H, C-5″H), 13.80 (brs, 1H, OH); 13C NMR (75.5 MHz, DMSO-d6): δ 26.92 (C-2′), 36.61 (C-1′), 46.91 (C-3′), 111.15 (C-7), 114.06 (C-6), 116.94 (C-5), 128.84 (C-4), 134.17 (C-8), 141.91 (C-3), 142.56 (C-3″), 143.33 (C-9), 148.99 (C-5″), 162.74 (C-2). HRMS-FAB: m/z [M + Na+] calcd for C13H12BrN5O2Na: 372.0067; found: 372.0055.
Z-1-[2-(1H-1,2,4-Triazol-1-yl)ethyl]-3-[2-(4-methoxyphenyl)hydrazono]indolin-2-one (18)
Orange solid. Yield: 70%; m.p.: 154–157 °C. IR (KBr): 3448, 2928, 1671, 1612, 1558, 1514, 1467 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 3.75 (s, 3H, –OCH3), 4.19 (brs, 2H, C-1′H), δ 4.52 (brs, 2H, C-2′H), 6.86 (d, 2H, J = 7.8 Hz, C-2′″H and C-6′″H), 6.96 (d, 2H, J = 8.7 Hz, C-3′″H and C-5′″H), 7.06–7.09 (m, 1H, ArH), 7.20–7.39 (m, 2H, ArH), 7.53–7.55 (m, 1H, ArH), 7.90 (s, 1H, C-3″H), 8.43 (s, 1H, C-5″H), 12.59 (s, 1H, -NH); 13C NMR (75.5 MHz, DMSO-d6): δ 46.60 (C-1′ and C-2′), 55.30 (–OCH3), 114.77 (C-3′″ and C-5′″), 115.58 (C-2′″ and C-6′″), 117.99 (C-7), 120.51 (C-6), 122.21 (C-5), 125.10 (C-4), 127.67 (C-3), 135.98 (C-1′″), 139.50 (C-9), 144.42 (C-3″), 151.54 (C-5″), 155.68 (C-4′″), 161.09 (C-2). HRMS-FAB: m/z [M + H+] calcd for C19H18N6O2: 363.1564; found: 363.1564.
Z-1-[2-(1H-1,2,4-Triazol-1-yl)propyl]-3-[2-(4-methoxyphenyl)hydrazono]indolin-2-one (19)
Orange solid. Yield: 69%; m.p.: 195–198 °C. IR (KBr): 2926, 1664, 1592, 1557, 1466 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 2.14–2.18 (m, 2H, C-2′H), 3.75 (s, 3H, –OCH3), 3.82 (brs, 2H, C-1′H), 4.24 (brs, 2H, C-3′H), 6.95 (d, 2H, J = 9.0 Hz, C-2′″H and C-6′″H), 7.08 (d, 2H, J = 8.1 Hz, C-3′″H and C-5′″H), 7.38–7.44 (m, 2H, C-6 and C-7H), 7.67 (s, 1H, C-4H), 7.97 (s, 1H, C-3″H), 8.51 (s, 1H, C-5″H), 12.69 (s, 1H, –NH); 13C NMR (75.5 MHz, DMSO-d6): δ 27.58 (C-2′), 36.37 (C-1′), 46.21 (C-3′), 55.32 (–OCH3), 111.04 (C-7), 114.24 (C-6), 114.73 (C-3′″ and C-5′″), 115.55 (C-2′″ and C-6′″), 120.33 (C-5), 122.99 (C-4), 123.91 (C-8), 129.68 (C-3), 135.79 (C-1′″), 138.47 (C-9), 144.15 (C-3″), 151.43 (C-5″), 155.65 (C-4′″), 160.80 (C-2). HRMS-FAB: m/z [M + Na+] calcd for C20H19BrN6O2Na: 477.0645; found: 477.0644.
Z-1-[3-(1H-1,2,4-Triazol-1-yl)propyl]-3-[2-(perfluorophenyl)hydrazono]indolin-2-one (20)
Orange solid. Yield: 68%; m.p.: 132–133 °C. IR (KBr): 3436, 2924, 1668, 1609, 1526, 1500, 1467 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 2.17–2.22 (m, 2H, C-2′H), δ 3.81 (t, 2H, J = 6.6 Hz, C-1′H), δ 4.28 (t, 2H, J = 6.9 Hz, C-3′H), δ 7.11–7.19 (m, 2H, ArH), 7.37–7.40 (m, 1H, ArH), 7.48-7.51 (m, 1H, ArH), 7.97 (s, 1H, C-3″H), 8.50 (s, 1H, C-5″H) 12.44 (s, 1H, -NH); 13C NMR (75.5 MHz, DMSO-d6): 27.42 (C-2′), 36.47 (C-1′), 46.19 (C-3′), 109.69 (C-7), 119.18 (C-6), 119.59 (C-5), 122.84 (C-4), 129.92 (C-3), 141.14 (C-3′″ and C-5′″), 144.12 (C-2′″ and C-6′″), 151.44 (C-3″ and C-5″), 161.19 (C-2). HRMS-FAB: m/z [M + H+] calcd for C19H13F5N6O: 437.1144; found: 437.1143.
Z-1-[3-(1H-1,2,4-Triazol-1-yl)propyl]-5-bromo-3-[2-(perfluorophenyl)hydrazono]indolin-2-one (21)
Orange solid. Yield: 68%; m.p.: 178–180 °C. IR (KBr): 3449, 1680, 1577, 1524, 1444 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 2.14-2.18 (m, 2H, C-2′H), δ 3.78 (brs, 2H, C-1′H), δ 4.26 (brs, 2H, C-3′H), δ 7.14 (d, 1H, J = 8.1 Hz, ArH), 7.47–7.55 (m, 2H, ArH), 7.95 (s, 1H, C-3″H), 8.47 (s, 1H, C-5″H), 12.42 (brs, 1H,-NH); 13C NMR (75.5 MHz, DMSO-d6): 27.31 (C-2′), 36.59 (C-1′), 46.12 (C-3′), 111.71 (C-7), 114.70 (C-6), 121.25 (C-5), 121.67 (C-4), 130.22 (C-1′″), 131.93 (C-9), 140.09 (C-3), 141.09 (C-4′″), 144.10 (C-2′″, C-3′″, C-5′″ and C-6′″), 151.42 (C-3″ and C-5″), 160.79 (C-2). HRMS-FAB: m/z [M + H+] calcd for C19H12BrF5N6O: 515.0249; found: 515.0227.
Cell culture experiments
Primary endothelial cells were isolated from human umbilical cord using mild trypsinization as described earlierCitation36.
Cell viability assay
The cytotoxicity of these compounds was determined by colorimetric MTT assay as described earlierCitation35. All experiments were performed at least three times in triplicate wells.
Cell-ELISA for measurement of ICAM-1
Cell-ELISA was used for measuring the expression of ICAM-1 on the surface of endothelial cellsCitation36. The results are expressed in terms of IC50 values (the concentration of the inhibitor that gives 50% inhibition of the ICAM-1 expression) for each derivative. The ICAM-1 assay was validated by using the reference compound “piperine”, which exhibits an IC50 value of 100 μM for ICAM-1 expression inhibition on HUVECs.
Results and discussion
Chemistry
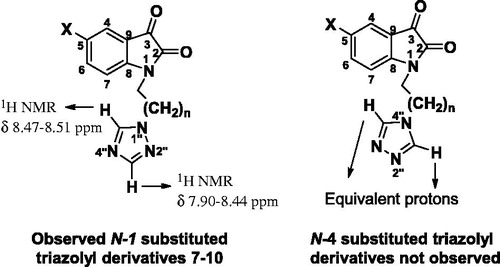
In order to get the desired triazolylisatin derivatives, we first carried out the synthesis of 5-bromoisatin (2) and N-substituted derivatives 3–6 of isatin (1) using 1,2-dibromoethane/1,3-dibromopropane (Scheme 1). Subsequently, the triazolylisatins 7–10 were synthesized by treating N-bromoalkylisatins 3–6 with 1,2,4-triazole and anhydrous potassium carbonate using acetonitrile as solvent in 65–75% yields (Scheme 1). The N-alkylation of 1,2,4-triazole could afford a mixture of 1-substituted and 4-substituted products. The NMR spectra of compounds 7–21 showed protons H-3″ between δ 7.90 and 8.44 ppm, and the H-5″ between δ 8.43 and 8.96 ppm instead of a single signal for two equivalent protons, thus confirming the formation of 1-substituted triazolyl product (, see Supplementary material).
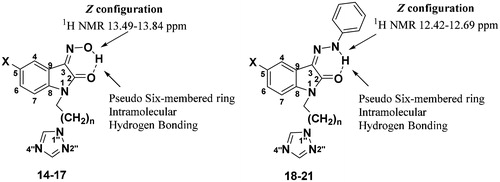
Finally, treatment of compounds 7–10 with hydroxylamine (11) and substituted phenylhydrazines (12–13) in ethanol afforded the compounds 14–21 in 68–72% yields (Scheme 1). The 3-substituted indolin-2-one may exist as either the Z- or E-isomer and there are several factors that can determine the configurationCitation39. In our case, the 1H NMR spectra of the synthesized compounds 14–21 indicated the formation of a single stereoisomer. The downfield shift of the proton peak of the OH group in compounds 14–17 between δ 13.49 and13.84 ppm, and the NH proton peak in compounds 18–21 appearing between δ 12.42 and 12.69 ppm indicates an intramolecularly hydrogen-bonded proton (see Supplementary material). The intramolecular hydrogen bonding between NH of the hydrazone moiety and the carbonyl group of the indolinone unit leading to the formation of the pseudo six-membered ring indicated the formation of Z-hydrazones ().
Similarly, isatin oximes also exist as Z isomers due to hydrogen bonding between hydroxyl proton and carbonyl group. The FTIR spectra also confirmed the Z-configured structure. The frequencies of ν(NH) and ν(C=O) vibrations were found to be lower than usual values due to the intramolecular hydrogen bonding (see Supplementary material). The analysis of the 13C NMR spectra of compounds 14–21 also showed C-2 carbonyl chemical shifts between δ 160.79 and 163.19 ppm, thus confirming the Z stereochemical configuration (see Supplementary material). The structures of all the synthesized compounds were unambiguously established using various spectroscopic techniques, viz. 1H NMR, 13C NMR, IR, HRMS, etc (see Supplementary material).
Anti-inflammatory activity evaluation of compounds 3–10 and 14–21
Compounds 3–10 and 14–21 were screened for their anti-inflammatory activities with respect to the TNF-α induced expression of ICAM-1 inhibition in human endothelial cells. The results summarized in revealed that the synthesized compounds were potent in inhibiting the ICAM-1 expression. Interestingly, Z-1-[3-(1H-1,2,4-triazol-1-yl)propyl]-5-bromo-3-[2-(4-methoxyphenyl)hydrazono]indolin-2-one (19) showed maximum inhibition of ICAM-1 expression – 89% with an IC50 value of 20 μM at a maximal tolerable dose of 200 μM and was found to be the most active compound among the series of 16 synthesized triazolyisatins investigated for anti-inflammatory activities in the present study.
Table 1. ICAM-1 inhibitory activity of compounds 3–10 and 14–21.
Also, Z-1-[3-(1H-1,2,4-triazol-1-yl)propyl]-5-bromo-3(hydroxyimino)indolin-2-one (17) showed 77% inhibition at maximal tolerable dose of 150 μM with an IC50 value of 30 μM (, Scheme 1).
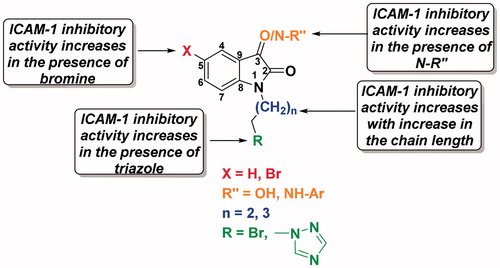
Structure activity relationship
We examined the effect of following structural modifications in isatin scaffold on their ICAM-1 expression inhibitory activity as shown in .
Effect of the bromine at the C-5 position in the benzenoid ring of isatin
As evident from the data given in , introduction of bromine at the C-5 position of the isatin derivatives significantly increases the ICAM-1 expression inhibitory activity ( and ). The IC50 value of 1-[2-(1H-1,2,4-triazol-1-yl)ethyl]-indoline-2,3-dione (7) was found to be 140 μM as compared to its more potent bromo derivative 9 with an IC50 value of 55 μM. Similarly, oxime derivative 14 exhibited an IC50 value of 90 μM in comparison to bromo-substituted oxime 16 having an IC50 value of 50 μM. This is most likely due to the increased cell permeability and hydrophobicity of bromo substituted derivatives. Our results strengthen the earlier findings that electron withdrawing groups at C-5 position of isatin increases the ICAM-1 inhibitory activityCitation14. However, it was also observed that in case of compound 21 bearing bromo group at C-5 and pentafluorophenyl group at C-3, there was not much increase in the inhibitory activity as compared to compound 20 bearing hydrogen at C-5 position of isatin. It can therefore be inferred that increase in activity due to the presence of bromine substituent in the triazolyl-isatin is being masked by the deactivating effect of electron withdrawing pentaflurophenyl group. Thus, simultaneous presence of electron-attracting groups in the isatin scaffold as well as in the phenyl ring at C-3 position could result in an inactive ICAM-1 inhibitor.
Effect of value of ‘n’ in the linker
On comparing the IC50 value of compound 3 with that of 4 (≥150 and 135 μM, respectively), 7 with 8 (140 and 100 μM, respectively) and 14 with 15 (90 and 48 μM, respectively), which differ only in the value of ‘n’, we observed that increasing the chain length of the linker increases the inhibition of TNF-α induced expression of ICAM-I on endothelial cells ( and ).
Effect of modification at C-3 position in the isatin ring
It was observed that derivatization at C-3 position in the isatin ring influences the ICAM-1 inhibitory activity. According to the data shown in , as a general trend, arylhydrazone and oxime derivatives 14–21 of triazolylisatin were found to be more potent when compared to parent triazolyl derivatives 7–10. The results show that compound 19 with electron-donating methoxy group on phenyl ring at C-3 position is the most promising among the tested compounds. We compared the parent isatin derivatives 7 and 10 having IC50 values of 140 and 52 μM, respectively, with the corresponding C-3 substituted compounds. Isatin-3-hydrazones 18 and 19 bearing electron donating methoxy group had IC50 values of 53 and 20 μM, respectively, and showed improved activity when compared to isatin-3-oxime derivatives 14 and 17 with IC50 values of 90 and 30 μM, respectively. It is worth mentioning that compounds 20 and 21 with electron withdrawing pentafluoro phenyl group showed poor activity (IC50 value = 80 μM) as compared to other derivatives. Thus, it can be inferred that electron donating group at the C-3 position in the isatin ring leads to a potent ICAM-1 expression inhibitor.
Effect of triazole ring
It has been observed that the introduction of aromatic ring bearing electron withdrawing groups through carbon chain linker at N-1 position of isatin increases its pharmacological activityCitation40–42. 1,2,4-Triazole ring is a basic aromatic heterocycle, which has been incorporated in the isatin scaffold at various positions to enhance its pharmacological property by synergetic effectCitation29–31. In the current study, the 1,2,4-traizole ring is tethered to the isatin by carbon chain linker. It was observed by comparing the IC50 values of compounds 3 versus 7, 4 versus 8, 5 versus 9, and 6 versus 10 that the ICAM-1 expression inhibitory activity increases with the addition of triazole ring ( and ). Thus, the IC50 value of compound 10 was 52 μM as opposed to that of 6 that does not have the triazole ring and has the IC50 value of 70 μM. This corroborates the fact that hybrid molecules incorporating two or more biologically active moieties in a single structure are comparatively more active than the individual components.
Conclusions
In the current study, 12 novel and four known triazolylisatin hybrids have been synthesized and their anti-inflammatory activities have been investigated with respect to their inhibition of TNF-α induced expression of ICAM-1 in HUVEC’s. The synthesized compounds exhibited potent ICAM-1 expression inhibition and our findings/evaluations led to the conclusion that compound 19 potently inhibited the ICAM-1 expression by 89% at an IC50 value of 20 μM with a maximal tolerable dose of 200 μM in HUVEC’s. On comparing the ICAM-1 expression inhibition results, structure-activity relationship has also been established with respect to the substituents present on the core isatin moiety of the synthesized compounds, which impose the requirement of the desired functional group/substituents in the targeted compounds. All these results yield valuable information for further optimization of structure-based drug design towards ICAM-1 expression inhibitors.
Supplementary material available online The underlying research materials for this article can be accessed as http://journalauthors.tandf.co.uk/preparation/multimedia.asp for further explanation of supplemental data and underlying research materials.
IENZ_1151015_Supporting_Information.pdf
Download PDF (976.1 KB)Declaration of interest
The authors acknowledge the financial support from the University of Delhi, University Grants Commission (UGC, New Delhi) and the Department of Biotechnology (DBT, New Delhi). B.G. thanks the Council of Scientific & Industrial Research (CSIR, Govt. Of India) for the financial support (Project: BSC0116).
References
- Guo Y, Chen F. TLC-UV-spectrophotometric and TLC-scanning determination of isatin in leaf of Isatis. Zhongcaoyao 1986;17:8–11
- Yoshikawa M, Murakami T, Kishi A, et al. O-bisdesmoside, calanthoside, the precursor glycoside of tryptanthrin, indirubin, and isatin, with increasing skin blood flow promoting effects, from two Calanthe species (Orchidaceae). Chem Pharm Bull 1998;46:886–8
- Bergman J, Lindström JO, Tilstam U. The structures and properties of some indolic constituents in Courouptia guainensis Aubl. Tetrahedron 1985;41:2879–81
- Kapadia GJ, Shukla YN. Melosatin D. A new isatin alkaloid from Melochia tomentosa roots. Planta Med 1993;59:568–9
- Grafe U, Radics L. Isolation and structure elucidation of 6-(3′-methylbuten-2′-yl) isatin, an unusual metabolite from Streptomyces albus. J Antibiotics 1986;39:162–3
- Breinholt J, Demuth H, Heide M, et al. Prenisatin (5-(3-methyl-2-butenyl)indole-2,3-dione): an antifungal isatin derivative from Chaetomium globosum. Acta Chem Scand 1996;50:443–5
- Medvedev AE, Clow A, Sandler M, Glover V. Isatin – a link between natriuretic peptides and monoamines. Biochem Pharmacol 1996;52:385–91
- da Silva JFM, Garden SJ, Pinto AC. The chemistry of isatins: a review from 1975 to 1999. J Brazil Chem Soc 2001;12:273–324
- Pandeya, SN, Smitha S, Jyoti M, Sridhar SK. Biological activities of isatin and its derivatives. Acta Pharm 2005;55:27–46
- Pakravan P, Kashanian S, Khodaei MM, Harding FJ. Biochemical and pharmacological characterization of isatin and its derivatives: from structure to activity. Pharmacol Rep 2013;65:313–35
- Jarrahpour A, Khalili D, Clercq ED, et al. Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-Schiff bases of isatin and their derivatives. Molecules 2007;12:1720–30
- Ragavendran JV, Sriram D, Patel SK, et al. Design and synthesis of anticonvulsants from a combined phthalimide-GABA-anilide and hydrazone pharmacophore. Eur J Med Chem 2007;42:146–51
- Vine KL, Matesic L, Locke JM, et al. Cytotoxic and anticancer activities of isatin and its derivatives: a comprehensive review from 2000-2008. Anticancer Agents Med Chem 2009;9:397–414
- Malhotra S, Balwani S, Dhawan A, et al. Synthesis and biological activity evaluation of N-protected isatin derivatives as inhibitors of ICAM-1 expression on human endothelial cells. Med Chem Comm 2011;2:743–51
- Kandile NG, Mohamed MI, Ismaeel HM. Antiproliferative effects of metal complexes of new isatin hydrazones against HCT116, MCF7 and HELA tumour cell lines. J Enzym Inhib Med Chem 2012;27:330–8
- Andreani A, Burnelli S, Granaiola M, et al. New isatin derivatives with antioxidant activity. Eur J Med Chem 2010;45:1374–8
- Sridhar SK, Saravanan M, Ramesh A. Synthesis and antibacterial screening of hydrazones, Schiff and Mannich bases of isatin derivatives. Eur J Med Chem 2001;36:615–25
- Pandeya SN, Sriram D, Nath G, De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4(3H)-one. Pharm Acta Helv 1999;74:11–17
- Sridhar SK, Pandeya SN, Stables JP, Ramesh A. Anticonvulsant activity of hydrazones, Schiff and Mannich bases of isatin derivatives. Eur J Pharm Sci 2002;16:129–32
- Kumarapperuma SC, Sun Y, Jeselnik M, et al. Structural effects on the phosphorylation of 3-substituted 1-beta-D-ribofuranosyl-1,2,4-triazoles by human adenosine kinase. Bioorg Med Chem Lett 2007;17:3203–7
- Mandrioli R, Mercolini L, Raggi MA. Benzodiazepine metabolism: an analytical perspective. Curr Drug Metab 2008;9:827–44
- Sabatelli F, Patel R, Mann PA, et al. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob Agents Chemother 2006;50:2009–15
- Zhou CH, Wang Y. Recent researches in triazole compounds as medicinal drugs. Curr Med Chem 2012;19:239–80
- Shivarama HB, Veerendra B, Shivananda MK, Poojary B. Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur J Med Chem 2003;38:759–67
- Küçükgüzel I, Küçükgüzel SG, Rollas S, Kiraz M. Some 3-thioxo/alkylthio-1,2,4-triazoles with a substituted thiourea moiety as possible antimycobacterials. Bioorg Med Chem Lett 2001;11:1703–7
- Kumar H, Javed SA, Khan SA, Amir M. 1,3,4-Oxadiazole/thiadiazole and 1,2,4-triazole derivatives of biphenyl-4-yloxy acetic acid: synthesis and preliminary evaluation of biological properties. Eur J Med Chem 2008;43:2688–98
- Mudasir, RB, Abdul R. Substituted 1,2,4-triazoles and thiazolidinonce from fatty acids: spectral characterization and antimicrobial activity. Indian J Chem 2009;48B:97–102
- Palaska E, Sahin G, Kelicen P, et al. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and1,2,4-triazole-3-thiones. Farmaco 2002;57:101–7
- Murthy YLN, Govindh B, Diwakar BS, et al. Synthesis and bioevaluation of Schiff and Mannich bases of isatin derivatives with 4-amino-5-benzyl-2,4-dihydro-3H-1,2,4-triazole-3-thione. Med Chem Res 2012;21:3104–10
- Patila SA, Manjunathab M, Kulkarnic AD, Badamid PS. Synthesis, characterization, fluorescence and biological studies of Mn(II), Fe(III) and Zn(II) complexes of Schiff bases derived from isatin and 3-substituted-4-amino-5-mercapto-1,2,4-triazoles. Complex Metals 2014;1:128–37
- Bekircan O, Bektas H. Synthesis of Schiff and Mannich bases of isatin derivatives with 4-amino-4,5-dihydro-1H-1,2,4-triazole-5-ones. Molecules 2008;10:2126–35
- Ghosh S, May MJ, Kopp EB. NF-kB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998;16:225–60
- Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. In: Paul LC, Issekutz TB, eds. Adhesion molecules in health and disease. New York: Marcel Dekker; 1997:1–54
- Malhotra S, Balwani S, Dhawan A, et al. Design, synthesis and biological activity evaluation of regioisomeric spiro-(indoline-isoxazolidines) in the inhibition of TNF-α-induced ICAM-1 expression on human endothelial cells. Med Chem Comm 2012;3:1536–47
- Pandey MK, Balwani S, Sharma PK, et al. Design, synthesis and anti-inflammatory evaluation of PEGylated 4-methyl & 4, 8-dimethylcoumarins. Eur J Pharm Sci 2010;39:134–40
- Kumar S, Arya P, Mukherjee C, et al. Novel aromatic ester from Piper longum and its analogs inhibit expression of cell adhesion molecules on endothelial cells. Biochemistry 2005;44:15944–52
- Bauer DJ, Sadler PW. 1-Substituted isatin-thiosemicarbazones, their preparation and pharmaceutical preparations containing them. British Patent 975357, Chem Abstr 1964;62:6462
- Vandendriessche A, Thomas J, Van Oosterwijck C, et al. Convergent synthesis of dendrimers based on 1,3,3-trisubstituted 2-oxindoles. Eur Polym J 2009;45:3196–209
- Jakusov K, Gaplovský M, Donovalova J, et al. Effect of reactants concentration on the ratio and yield of E, Z isomers of isatin-3-(4-phenyl)semicarbazone and N-methylisatin-3-(4-phenyl)semicarbazone. Chem Pap 2013;67:117–26
- Vine KL, Locke JM, Ranson M, et al. An investigation into the cytotoxicity and mode of action of some novel N-alkyl-substituted isatins. J Med Chem 2007;50:5109–17
- Matesic L, Locke JM, Bremner JB, et al. N-phenethyl and N-naphthylmethyl isatins and analogues as in vitro cytotoxic agents. Bioorg Med Chem 2008;16:3118–24
- Nguyen JT, Wells JA. Direct activation of the apoptosis machinery as a mechanism to target cancer cells. Proc Natl Acad Sci USA 2003;100:7533–8