Abstract
This study reports the synthesis, characterisation and antimicrobial activity of five novel silver N-heterocyclic carbene (Ag–NHC) complexes obtained by N-propylphthalimide and N-methyldioxane substituted benzimidazolium salts with silver oxide. The reactions were performed at room temperature for 24 h in the absence of light. The obtained complexes were identified and characterised by 1H and 13C NMR, FT-IR and elemental analysis techniques. The minimum inhibitory concentration (MIC) of the complexes was determined for E. coli, P. aeruginosa, E. faecalis, S. aureus, C. tropicalis and C. albicans in vitro through agar and broth dilution. The results indicated that these complexes exhibit antimicrobial activity. In particular, complex 3 presented the significant broad spectrum antimicrobial activity.
Introduction
It has been well established that silver cations (Ag+) have antimicrobial activity against a broad range of microorganismsCitation1–4. Ag+ in low concentrations is non-toxic to humans, therefore a compound that releases Ag+ to the environment in a steady rate make an effective antimicrobial agentCitation2–10. The effectiveness of a silver complex as an antimicrobial agent depends on the bioavailability of Ag+ when the compound is solubilised. Many factors can influence this bioavailability of Ag+ including the solubility and ionisation of the compound, the delivery route and the presence of anions in the environment such as chloride, bromide and sulphideCitation1–3,7.
It has also been well established that silver N-heterocyclic carbenes (Ag–NHCs) are a class of organometallic compounds that are stable in the presence of air or moisture; the release of Ag+ to the environment is slow and consistentCitation4,Citation5,Citation7,Citation8. As Ag–NHC complexes can be easily and efficiently synthesised, the characterisation of this class of compounds has generated considerable interest in order to determine their effectiveness as antimicrobial agents. From investigations to date, many groups of researchers have synthesised and determined the biological activity of a broad range of Ag–NHC complexesCitation10–12. Specifically, compounds derived from benzimidazole-based NHC ligands have shown promising antimicrobial activityCitation9,11,13–20 and various studies have extended their research to anticancer activityCitation6,Citation9,Citation13,Citation14.
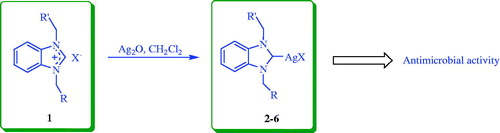
The aim of this study was to synthesise novel benzimidazolium-based Ag–NHC complexes (2–6) in the pursuit of finding biologically active compounds that can be further investigated for their antimicrobial activity. The complexes (2–6) were characterised by IR, elemental analysis, 1H and 13C NMR. The antimicrobial activities of the compounds (2–6) were determined against a panel of non-fastidious bacteria and yeasts. Complexes 3 and 6 were particularly effective against gram negative bacteria and yeast, with compound 3 having the highest antimicrobial activity across the panel of microorganisms in comparison to the other complexes.
Materials and methods
Chemicals and equipment
All reactions were made under argon gas using Standard Schlenk techniques. All primary chemicals were purchased commercially from the following suppliers: Merck (Darmstadt, Germany), Sigma-Aldrich (Aldrich and Fluka, St. Louis, MO), Introgen (Carlo-Erba, Val de Reuil, France and Acros Organics, Geel, Belgium) and ThermoFisher Scientific (Alfa-Aesar, Lancashire, UK). The purchased chemicals include: o-phenylenediamine, formic acid, dimethylformamide (DMF), hexane, silver oxide, 1-(chloromethyl)naphthalene, benzyl chloride, 3-methylbenzyl chloride, 4-methylbenzyl chloride, 2,4,6-trimethylbenzyl chloride, diethyl ether, dichloromethane and ethyl alcohol. Prior to use, the solvents were further purified through conventional methodsCitation21: diethyl ether and hexane by distillation over Na and dichloromethane by distillation over P4O10.
Mueller-Hinton Broth was purchased from HiMedia Laboratories Pvt. Ltd. (Mumbai, India) and RPMI 1640 broth was purchased from Sigma-Aldrich (Chemie GmbH, Taufkirchen, Germany).
The melting points (m.p.) of compounds were performed on an Electrothermal 9200 melting point apparatus. Elemental analyses of 2–6 were conducted on a LECO CHNS-932 Elemental Analyser. FT-IR analyses were done on a Mattson 1000 spectrophotometer. The NMR spectra of the Ag–NHC complexes were performed on a Bruker AC300P FT spectrometer. Chemical shifts (δ) were given in ppm relative to tetramethylsilane.
Synthesis and characterisation of Ag–NHC complexes (2–6)
The benzimidazolium salts were prepared as follows: KOH was added to benzimidazole solution in ethyl alcohol and alkyl halide was added to this solution after 1 h. The solution was refluxed for 6 h then it was allowed to cool to room temperature. The precipitated KCl was removed by filtering. The obtained N-alkylbenzimidazole was purified by crystallization. Subsequently, alkyl halide was added to the DMF solution of synthesised N-alkylbenzimidazole and it was stirred for 24 h at 80 °C. Benzimidazolium salts were synthesised and purified by crystallisation in a mixture of ethyl alcohol–diethylether (2:1)Citation17,22–24.
The Ag(I)-NHC complexes (2–6) were synthesised from N-propylphthalimide and N-methyldioxane substituted benzimidazolium salts (1) (2 mmol) and Ag2O (1 mmol) in 20 mL dry dichloromethane (Scheme 1). All reactions were conducted in a Schlenk tube that was wrapped with aluminium foil for 24 h at room temperature. After the completion of the reactions, the dichloromethane was removed by vacuum to obtain the crude products. The products were washed twice with diethyl ether, filtered and recrystallised from dichloromethane–diethylether (2:1) or chloroform–diethylether (2:1) mixtures at room temperature. The structures of the compounds were confirmed by elemental analysis, FT-IR, 1H and 13C NMR.
Chloro [1-(N-propylphthalimide)-3-benzylbenzimidazol-2-ylidene] silver(I), 2
Compound 2 was synthesised as described above from 1-(N-propylphthalimide)-3-benzylbenzimidazolium chloride (2 mmol) and Ag2O (1 mmol). The reaction provided complex 2 (0.45 g, 72%) as a solid. M.p.: 212–213 °C. IR ν(CN) = 1431.2 cm−1. Anal. calcd. for C25H21AgClN3O2 (538.77 g/mol) was determined as C, 55.73%; H, 3.93%; N, 7.80%. Found C, 55.61%; H, 4.08%; N, 7.75%.
1H NMR (300 MHz, DMSO-d6), δ; 2.40 (p, 2H, CH2CH2CH2N, J = 7.2 Hz); 3.84 and 4.61 (t, 4H, CH2CH2CH2N, J = 7.2 Hz); 5.66 (s, 2H, CH2C6H5); 7.26–7.87 (m, 13H, Ar–H). 13C NMR (75 MHz, DMSO-d6), δ; 29.3 (CH2CH2CH2N); 35.4 and 45.5 (CH2CH2CH2N); 53.4 (CH2C6H5); 111.3, 112.3, 123.5, 124.3, 127.2, 128.5, 129.1, 131.9, 133.8, 134.1 and 134.9 (Ar–C); 168.2 (C=O).
Chloro [1-(N-propylphthalimide)-3-naphthalen-1-yl-methylbenzimidazol-2-ylidene] silver (I), 3
Compound 3 was synthesised as described above from 1-(N-propylphthalimide)-3-naphthalen-1-yl-methylbenzimidazolium chloride (2 mmol) and Ag2O (1 mmol). The reaction produced complex 3 (0.51 g, 83%) as a solid. M.p.: 195–196 °C. IR ν(CN) = 1430.5 cm−1. Anal. calcd. for C29H23AgClN3O2 (588.83 g/mol): C, 59.15%; H, 3.94%; N, 7.14%. Found C, 59.03%; H, 4.08%; N, 7.15%.
1H NMR (300.13 MHz, DMSO-d6), δ; 2.10 (p, 2H, CH2CH2CH2N, J = 7.2 Hz); 3.56 and 4.46 (t, 4H, CH2CH2CH2N, J = 7.2 Hz); 6.10 (s, 2H, CH2C10H7); 6.89–8.08 (m, 15H, Ar-H). 13C NMR (75 MHz, DMSO-d6), δ; 29.4 (CH2CH2CH2N); 35.5 and 47.5 (CH2CH2CH2N); 50.4 (CH2C10H7); 112.8, 123.3, 124.7, 124.9, 125.1, 125.9, 126.7, 127.2, 128.9, 129.2, 130.7, 132.2, 133.6, 133.8, 134.2, 134.6 and 134.9 (Ar–C); 168.5 (C = O).
Bromo [1,3-bis(N-propylphthalimide)benzimidazol-2-ylidene] silver (I), 4
Compound 4 was synthesised as described above from 1-(N-propylphthalimide)-3-benzylbenzimidazolium chloride (2 mmol) and Ag2O (1 mmol). The reaction produced complex 4 (0.51 g, 86%) as a solid. M.p.: 246–247 °C. IR ν(CN) = 1434.8 cm−1. Anal. calcd. for C29H24AgBrN3O2 (634.29 g/mol): C, 54.91%; H, 3.81%; N, 6.62%. Found C, 54.71%; H, 4.00%; N, 6.82%.
1H NMR (300 MHz, DMSO-d6), δ; 2.51 (p, 4H, CH2CH2CH2N, J = 7.5 Hz); 3.64 and 4.42 (t, 8H, CH2CH2CH2N, J = 7.5 Hz); 7.47–7.85 (m, 12H, Ar–H). 13C NMR (75 MHz, DMSO-d6), δ; 29.3 (CH2CH2CH2N); 35.1 and 45.2 (CH2CH2CH2N); 112.6, 123.1, 124.6, 131.8, 133.4 and 134.4 (Ar–C); 168.2 (C = O).
Chloro [1-(2-methyl-1,4-benzodioxane)-3-benzylbenzimidazol-2-ylidene] silver(I), 5
Compound 5 was synthesised as described above from 1-(2-methyl-1,4-benzodioxane)-3-benzylbenzimidazolium chloride (2 mmol) and Ag2O (1 mmol). The reaction produced complex 5 (0.51 g, 80%) as a solid. M.p.: 166–167 °C. IR ν(CN) = 1454.1 cm−1. Anal. calcd. for C23H20AgClN2O2 (499.74 g/mol): C, 55.28%; H, 4.03%; N, 5.61%. Found C, 55.14%; H, 4.21%; N, 5.56%.
1H NMR (300 MHz, DMSO-d6), δ; 4.06 and 4.22 (dd, 2H, OCH2CH(CH2)O, J = 9.9 Hz); 4.88 (m, 1H, OCH2CHO); 4.71 and 4.79 (dd, 2H, OCH2CH(CH2)O, J = 9.9 Hz); 5.74 (s, 2H, CH2C6H5); 6.68–7.90 (m, 13H, Ar–H). 13C NMR (75 MHz, DMSO-d6), δ; 49.0 (OCH2CH(CH2)O); 55.6 (OCH2CH(CH2)O); 65.1 (OCH2CH(CH2)O); 71.8 (CH2C6H5); 112.8, 112.9, 117.6, 117.7, 121.9, 122.0, 122.2, 124.6, 124.7, 127.8, 128.5, 129.2, 133.6, 134.4, 136.7, 142.3 and 143.2 (Ar–C).
Chloro [1-(2-methyl-1,4-benzodioxane)-3-(4-methylbenzyl)benzimidazol-2-ylidene]silver(I), 6
Compound 6 was synthesised as described above from 1-(2-methyl-1,4-benzodioxane)-3-(4-methylbenzyl)benzimidazolium chloride (2 mmol) and Ag2O (1 mmol). The reaction produced complex 6 (0.49 g, 77%) as a solid. M.p.: 172–173 °C. IR ν(CN) = 1443.2 cm−1. Anal. calcd. for C24H22AgClN2O2 (513.76 g/mol): C, 56.11%; H, 4.32%; N, 5.45%. Found C, 56.03%; H, 4.52%; N, 5.46%.
1H NMR (300 MHz, DMSO), δ; 4.07 and 4.20 (dd, 2H, OCH2CH(CH2)O, J = 9.9 Hz); 4.93 (m, 1H, OCH2CHO); 4.75 and 4.81 (dd, 2H, OCH2CH(CH2)O, J = 10.8 Hz); 2.30 (s, 3H, CH2C6H4(CH3)–4); 5.67 (s, 2H, CH2C6H4(CH3)-4); 6.69–7.87 (m, 12H, Ar–H). 13C NMR (75 MHz, DMSO), δ; 21.1 (CH2C6H4(CH3)-4); 49.0 (OCH2CH(CH2)O); 52.2 (OCH2CH(CH2)O); 65.3 (OCH2CH(CH2)O); 71.9 (CH2C6H4(CH3)-4); 112.8, 112.9, 117.6, 117.7, 121.9, 122.2, 124.5, 127.8, 129.7, 133.6, 133.7, 134.4, 137.8, 142.3 and 143.2 (Ar–C); 191.4 (C–Ag).
In vitro antimicrobial activity testing by agar and broth dilution
The minimum inhibitory concentrations (MICs) for each of the Ag–NHC complexes were determined on a variety of non-fastidious bacteria and yeast strains. The Clinical and Laboratory Standards Institute methods were followed: the agar dilution protocol was conducted on bacterial strainsCitation25 and the broth dilution protocol was conducted on yeast strainsCitation26.
The following strains were used in this study: gram negative bacteria – Escherichia coli ATCC 25922TM and Pseudomonas aeruginosa ATCC 27853TM; gram positive bacteria – Enterococcus faecalis ATCC 29212TM and Staphylococcus aureus ATCC 29213TM; yeasts – Candida tropicalis and Candida albicans. All bacterial strains were sourced from the American Type Culture Collection (Rockville, MD) and all yeast strains were kindly donated from the Department of Microbiology, Faculty of Medicine, Ege University (Izmir, Turkey).
All bacteria were subcultured on Mueller–Hinton Agar and all yeasts were subcultured in RPMI 1640. For antimicrobial activity tests, media was supplemented with Ag–NHC complexes and antimicrobial controls were prepared in serial dilution as described in the CLSI protocols of the agarCitation25 and brothCitation26 dilution methods.
Stocks of the compounds 2–6 were prepared in dimethyl sulfoxide (DMSO) and two-fold serial dilutions of the stocks were prepared in sterile distilled water. The Ag–NHC complexes were prepared as an eight-dilution series from 800 μg/mL to 6.25 μg/mL. Ciprofloxacin, ampicillin and fluconazole were used as positive control drugs to compare against 2–6. In preparation for inoculating antimicrobial supplemented media, the cultures were prepared as a suspension to approximately 106 CFU/mL. The turbidity of the suspension was standardised against a McFarlane Number 0.5 turbidity standard. Antimicrobial supplemented media was inoculated from the cell suspensions with sterile 10 μL inoculating loops. After inoculation, cultures were incubated at 35 °C in static, aerobic conditions, for 16–20 h for bacterial cultures and 48 h for yeast cultures.
Results and discussion
Synthesis and characterisation
The Ag–NHC complexes can remain stable in the presence of air and moisture at room temperature, without evidence of decomposition. Product yields were obtained between 72 and 86%.
Generally, there is a typical proton signal of NCHN in the range at δ 9–11 for benzimidazolium salts in the 1H NMR spectra. However, we did not observed this single signal peak for 2–6. This result verified the synthesis of the Ag–NHC complexes. The pentet proton of N CH2CH2CH2C6H5 resonated at δ 2.40, 2.10 and 2.51 for 2–4, respectively. The triplet proton of N CH2CH2CH2C6H5 resonated at δ 3.84 and 4.61 (for 2), 3.56 and 4.46 (for 3), 3.64 and 4.42 (for 4). The aromatic protons of silver compounds were seen at δ between 6.68 and 8.08 ppm in the 1H NMR. The carbene carbon chemical shift value belonging to compound 6 was observed at δ 191.4 ppm in the 13C NMR spectra. This single signal carbene peak was not observed for other synthesised new compounds (2–5). Carbonyl carbon was resonated at low area for example at δ 168.2, 168.5 and 168.2 for 2–4, respectively. The IR(CN) band was obtained at δ 1431.2, 1430.5, 1434.8, 1454.1 and 1443.2 for 2–6, respectively.
Antimicrobial activity
Protocols from the Clinical and Laboratory Standards Institute were conducted to determine the MICs of 2–6 for E. coli, P. aeruginosa, S. aureus, E. faecalis, C. albicans and C. tropicalis. The MIC was determined as the lowest concentration at which the compound prevented visible growth for each organism.
The results of the MIC values determined for complexes 2–6 are provided in . The complexes showed varied antimicrobial activity against tested microorganisms. In comparison to the antibiotic controls, all complexes had a higher MIC value: however complexes 3 and 6 were more effective than the other complexes. Complex 3, in particular, exhibited the most antimicrobial activity across the whole panel compared to the other complexes. Compound 3 was more effective against the yeast strains C. albicans and C. tropicalis than against the bacterial strains. The antimicrobial activity of complexes 2 and 4 was the least effective, having similar activities across all microorganisms. Complex 5 again exhibited same activities across all microorganisms, although it presented a moderate MIC value. Complexes 2 and 5 both contain a benzyl substituent, although 5 contains a benzodioxane group and had lower MIC value than 2, which contains a propyl phthalimide group. This suggests that the benzodioxane has an effect on the antimicrobial activity of 5.
Table 1. MIC values of the Ag–NHC complexes (2–6) against a panel of non-fastidious microorganisms.
Complex 3 includes a naphthalene-1-ylmethyl group and its performance is consistent with a previous work which indicated that Ag–NHC with naphthalene-1-ylmethyl substituents has higher antimicrobial activity, especially antifungal activityCitation16. Complex 6 includes a 4-methylbenzyl group and its performance is markedly improved compared to a previous workCitation27, in which the authors demonstrated that their complex which included a 4-methylbenzyl group had lower antimicrobial activity.
Conclusion
This study describes the synthesis of five novel benzimidazolium based Ag–NHC complexes (2–6) which were characterised by IR and elemental analysis, 1H and 13C NMR. The complexes were evaluated for biological activity against a panel of non-fastidious bacteria and yeasts. Complexes 3 and 6 were particularly effective against gram negative bacteria and yeast, with complex 3 having the highest antimicrobial activity across the panel of microorganisms in comparison to the other complexes.
Declaration of interest
The authors report no declarations of interest.
We would like to thank the Inonu University Research Fund (BAP 2011/25) for financial support.
References
- Maillard J-Y, Hartemann P. Silver as an antimicrobial: facts and gaps in knowledge. Crit Rev Microbiol 2012;39:373–83
- Kascatan-Nebioglu A, Panzner MJ, Tessier CA, et al N-Heterocyclic carbene–silver complexes: a new class of antibiotics. Coord Chem Rev 2007;251:884–95
- Asekunowo PO, Haque RA. Counterion-induced modulation in biochemical properties of nitrile functionalized silver(I)-N-heterocyclic carbene complexes. J Coord Chem 2014;67:3649–63
- Hindi KM, Panzner MJ, Tessier CA, et al The medicinal applications of imidazolium carbene-metal complexes. Chem Rev 2009;109:3859–84
- Haque RA, Salman AW, Budagumpi S, et al Silver(I)-N-heterocyclic carbene complexes of bis-imidazol-2-ylidenes having different aromatic-spacers: synthesis, crystal structure, and in vitro antimicrobial and anticancer studies. App Organomet Chem 2013;27:465–73
- Gautier A, Cisnetti F. Advances in metal–carbene complexes as potent anti-cancer agents. Metallomics 2012;4:23–32
- Garrison JC, Youngs WJ. Ag(I) N-heterocyclic carbene complexes: synthesis, structure, and application. Chem Rev 2005;105:3978–4008
- Budagumpi S, Haque RA, Endud S, et al Biologically relevant silver(I)-N-heterocyclic carbene complexes: synthesis, structure, intramolecular interactions, and applications. Eur J Inorg Chem 2013;2013:4367–88
- Haque RA, Choo SY, Budagumpi S, et al Silver(I) complexes of mono- and bidentate N-heterocyclic carbene ligands: synthesis, crystal structures, and in vitro antibacterial and anticancer studies. Eur J Med Chem 2015;90:82–92
- Liu W, Gust R. Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs. Chem Soc Rev 2013;42:755–73
- Deblock MC, Panzner MJ, Tessier CA, et al Chapter 4 biologically active N-heterocyclic carbene–metal complexes. In: N-heterocyclic carbenes: from laboratory curiosities to efficient synthetic tools. Royal Soc Chem; 2011:119–33
- Teyssot M-L, Jarrousse A-S, Manin M, et al Metal–NHC complexes: a survey of anti-cancer properties. Dalton Trans 2009;35:6894–902
- Haque RA, Choo SY, Budagumpi S, et al Synthesis, crystal structures, characterization and biological studies of nitrile-functionalized silver(I) N-heterocyclic carbene complexes. Inorg Chim Acta 2015;433:35–44
- Haque RA, Ghdhayeb MZ, Budagumpi S, et al Non-symmetrically substituted N-heterocyclic carbene–Ag(I) complexes of benzimidazol-2-ylidenes: synthesis, crystal structures, anticancer activity and transmetallation studies. Inorg Chim Acta 2013;394:519–25
- Akkoç S, Gök Y, Özdemir İ, et al N-Heterocyclic carbene silver complexes: synthesis, characterization and in vitro antimicrobial studies. J Chin Adv Mater Soc 2014;2:20–30
- Gök Y, Akkoç S, Çelikal ÖÖ, et al In vitro antimicrobial studies of naphthalen-1-ylmethyl substituted silver N-heterocyclic carbene complexes. Arab J Chem 2015. [Epub ahead of print]. doi: 10.1016/j.arabjc.2015.04.019
- Gök Y, Akkoç S, Albayrak S, et al N-Phenyl-substituted carbene precursors and their silver complexes: synthesis, characterization and antimicrobial activities. Appl Organomet Chem 2014;28:244–51
- Muskawar PN, Karthikeyan P, Aswar SA, et al NHC–metal complexes based on benzimidazolium moiety for chemical transformation. Arab J Chem 2012. [Epub ahead of print]. doi: 10.1016/j.arabjc.2012.04.040
- Gök Y, Akkoç S, ÖzeroğLu Çelİkal Ö, et al N-functionalized benzimidazol-2-ylidene silver complexes: synthesis, characterization, and antimicrobial studies. Turkish J Chem 2013;37:1007–13
- Gök Y, Akkoç S, Erdoğan H, et al In vitro antimicrobial studies of new benzimidazolium salts and silver N-heterocyclic carbene complexes. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.3109/14756366.2015.1132210
- Armarego WLF, Chai CLL. Chapter 4 – purification of organic chemicals. In: Armarego WLF, Chai CLL, eds. Purification of laboratory chemicals. 6th ed. Oxford: Butterworth-Heinemann; 2009:88–444
- Akkoç S, Gök Y. Synthesis and characterization of 1-phenyl-3-alkylbenzimidazol-2-ylidene salts and their catalytic activities in the Heck and Suzuki cross-coupling reactions. J Coord Chem 2013;66:1396–404
- Akkoç S, Gök Y, İlhan İÖ, et al In situ generation of efficient palladium N-heterocyclic carbene catalysts using benzimidazolium salts for the Suzuki–Miyaura cross-coupling reaction. Curr Org Synth 2016. [Epub ahead of print]. doi: 10.2174/1570179413666151218200334
- Akkoç S, Gök Y, İlhan İÖ, et al N-Methylphthalimide-substituted benzimidazolium salts and PEPPSI Pd–NHC complexes: synthesis, characterization and catalytic activity in carbon–carbon bond-forming reactions. Beilstein J Org Chem 2016;12:81–8
- Clinical and Laboratory Standards Institute. M07-A10: methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard, 10th ed. Vol. M07-A10. Wayne, PA: Clinical and Laboratory Standards Institute; 2015
- Clinical and Laboratory Standards Institute. M27-A3: reference method for broth dilution antifungal susceptibility testing of yeasts; Approved Standard, 3rd ed. Vol. M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2015
- Gök Y, Sarı Y, Akkoç S, et al Antimicrobial studies of N-heterocyclic carbene silver complexes containing benzimidazol-2-ylidene ligand. Inter J Inorg Chem 2014;2014:1–6