Abstract
During the treatment of tuberculosis infection, oxidative stress due to anti-tubercular drugs may result in tissue inflammation. It was suggested that treatment with antioxidant drugs could be beneficial as an adjunct to anti-tuberculosis drug therapy. Recently our group has shown that several C-glycosides are inhibitors of Mycobacterium tuberculosis β-carbonic anhydrases (CAs, EC 4.2.1.1). In an effort to develop novel chemotherapeutic agents against tuberculosis, the anti-tubercular and antioxidant activities of a series of C-glycosides containing the phenol or the methoxyaryl moiety were studied. Many compounds showed inhibition of growth of M. tuberculosis H37Rv strain and good antioxidant ability. A glycomimetic incorporating the 3-hydroxyphenyl moiety showed the best activity profile and therefore this functionality represents lead for the development of novel anti-tubercular agents with dual mechanisms of action.
Introduction
Infection with Mycobacterium tuberculosis and related mycobacteria affects a large fraction of the world population, with an estimated 9.4 million new cases and 1.7 million deaths annuallyCitation1. Furthermore, tuberculosis (TB) infection including both multidrug-resistant tuberculosis (MDR-TB) and drug-resistant tuberculosis (XDR-TB) is a leading cause of death worldwide. The development of novel anti-TB drugs that do not possess cross-resistance with current anti-mycobacterial drugs and have minimal toxicity, is urgently needed due to the increasing incidence of MDR-TB, XDR-TB and TB-HIV co-infection. In the last 20 years, the research on M. tuberculosis has made much progress with the genome decipheredCitation2 and the discovery of possible new drug targets which may lead to the development of compounds possessing a novel mechanism of action, thus helping solve the drug resistance problem mentioned aboveCitation3,Citation4. Among the new such proteins identified after the M. tuberculosis genome was published, there are three β-carbonic anhydrases (CAs, EC 4.2.1.1) which have been cloned, purified and characterized by Jones’ and Supuran’s groups. Several sulfonamide and sulfamate inhibitors of the three mycobacterial carbonic anhydrases have been reportedCitation5.
During the treatment of TB infection, oxidative stress may result in tissue inflammation due to anti-tubercular drugsCitation6. Recently it has been shown that oxidative stress conditions exist in the guinea pig model of TB, similar to what is seen in humans. The systemic and tissue oxidative stress is progressive and correlates with the loss of Nrf2 mediated antioxidant defense mechanisms. Considering the importance of lung lesion progression and the extra-pulmonary dissemination of bacilli in TB pathogenesis, it was suggested that treatment with antioxidant drugs could be beneficial as an adjunct to anti-tuberculosis drug therapy. An added benefit of antioxidant treatment of patients with TB is the reduction of the toxic side effects of anti-tuberculosis drug therapy, mediated in part by the generation of oxygen free radical in the liverCitation7.
These observations led us to propose that the combination of antioxidant activity and mtCAs inhibition might result in novel anti-tubercular agents with dual mechanisms of action. Our strategy towards these dual acting compounds was based on the synthesis of C-glycosides where the carbohydrate moiety is tethered to a phenol or methoxyaryl CA pharmacophore through a carbon chain.
The replacement of the naturally occurring O-glycoside bond by C-glycoside bond is an approach practiced in the synthesis of carbohydrate-containing compounds for the downstream biological applicationsCitation8. This isosteric replacement seeks to enhance stability of the small molecule glycoside toward enzymatic hydrolysis of the glycosidic bond while retaining vital molecular recognition interactions with the biological targetCitation9.
The use of glycomimetics in the design of carbonic anhydrase inhibitors (CAIs) has proven to be a successful approach and now constitutes one of the most attractive ways to develop new generations of effective and selective inhibitorsCitation8,Citation10. Recently one of our groups has applied the “sugar approach” to the preparation of C-cinnamoyl phenols, where the carbohydrate moiety is tethered to a phenol CA pharmacophore through a carbon chainCitation11. These compounds have been tested as inhibitors of the Mycobacterium tuberculosis β-CAs and have shown better inhibitory activity against mtCAs than phenol. Also the anti-tubercular activity of the C-glycosyl phenols was investigated, allowing us to identify the first mtCAs inhibitor with antimycobacterial activityCitation12. These glycosides also showed to be very good inhibitors of Brucella suis CAsCitation13. Very recently we have developed a novel series of C-glycosides containing the methoxyaryl scaffold and investigated them as inhibitors against human and fungal isozymes of carbonic, showing that some of them are potent and highly selective inhibitorsCitation14,Citation15. It should be pointed out that only very recently methoxyphenyl derivatives have been investigated as CAIs because it was considered that they do not bear any moiety normally associated with CA inhibition in their molecules.
The aim of the present work was to study the anti-tubercular and antioxidant activity of a series of C-glycosides that could be useful for the development of novel chemotherapeutic agents against TB.
Materials and methods
Synthesis of C-glycosides
C-glycosides 1–20 were previously described and have been prepared by aldol reaction of aryl aldehydes with per-O-acetylated C-glucosyl or C-galactosyl ketones and subsequent deprotection using triethylamine in methanol/waterCitation11,Citation14.
Anti-mycobacterial activity assay
Bacterial strains and growth media: Mycobacterium tuberculosis strain H37Rv was grown in Middlebrook 7H9 broth (Difco Laboratories, Detroit, MI) supplemented with 1/10 v/v of ADS (a solution containing 50 g/L BSA fraction V, 20 g/L dextrose and 8.1 g/L NaCl), glycerol (1% w/v) herein designated 7H9-ADS-G for short. Tween 80 (0.05% w/v) was added to prevent clumping. When needed, solid media (Middlebrook 7H11) supplemented with ADS (1/10 v/v) and glycerol (1% v/v) was used. Cultures were grown at 37 °C under gentle agitation.
Stock solutions for all the tested compounds were made in DMSO at 10 mg/mL. Working solutions were made by dilution in the above described 7H9-ADS-G medium at a final concentration of 400 μg/mL.
Antimycobacterial activity was determined by a two-fold dilution of the compounds in Middlebrook 7H9-ADS-G medium. For this purpose 96-well plates (Falcon, Cat number 3072, BectonDickinson, Lincoln Park, NJ) were used. The 96-well plates received 100 μL of Middlebrook 7H9 broth and a serial two-fold dilution of the compounds was made directly on the plate. The initial and final drug concentrations tested were 100 and 0.78 μg/mL, respectively. Four compounds were tested in duplicate in each microtiter plate, Rifampicin (from 2 to 0.16 μg/mL; stock solution prepared as a 10 mg/mL solution in methanol) was used as control drug. Two rows were used for growth control (medium and inoculum alone) and sterility control (medium alone). The inoculum was prepared as a 1/25 dilution of a fresh mid-log M. tuberculosis H37Rv suspension (O.D equivalent to Mc Farland 1.0 scale value) made in Middlebrook 7H9-ADS-G. A 100-μL aliquot (containing approximately 106 Colony Forming Units) was used to inoculate the wells except for the row used for sterility testing. Plates were sealed with Parafilm and incubated at 37 °C for seven days. Minimum inhibitory concentration (MIC) was defined as the lowest drug concentration preventing mycobacterial growth as judged by visual inspection.
Antioxidant capacity
Stock solutions of the samples were prepared dissolving 15 mg of the tested compounds in 100 μL DMSO (1 mM) and then homogenizing with absolute methanol until a final volume of 1000 μL. The antioxidant capacity (AC) was determined by two different methods: DPPH and ABTS radical scavenging assaysCitation16. The ABTS assay is based on the ability of samples to quench the stable cation radical 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS•+) with the consequent discoloration of the blue/green solution when compared to the absorbance of a blank. Briefly, 10 μL of sample were added to 1 mL ABTS•+ working solution (absorbance of 0.700 ± 0.02 at 734 nm), incubated for 6 min at room temperature and the reduction of absorbance at 734 nm was recorded in a spectrophotometer (Thermo Scientific, Waltham, MA, Evolution 60S). In the DPPH assay, the capacity of the sample to reduce the stable and purple radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) to the yellow-colored diphenylpicrylhydrazine was determinated. Aliquots of sample (100 μL) were added to test tubes containing 150 μL of 40 mg/L DPPH ethanolic solution. The absorbance reduction at 515 nm was measured until the reaction reached a plateau (60 min). Corresponding blanks were used to determine the stability of the DPPH• solution. For both assays, Trolox was used as antioxidant standard and results were expressed as percentage of radical inhibition per 0.1 mg (for ABTS assay) or 1 mg (for DPPH assay) of tested compounds. Measures were done in triplicate.
Results and discussion
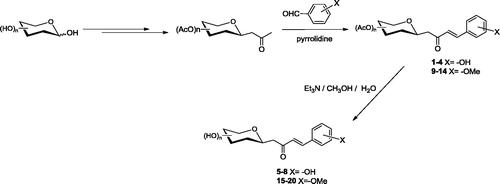
A set of C-cinnamoyl glycosides ( and ) was synthesized as outlined in Scheme 1 and described previously by usCitation11,Citation14. C-cinnamoyl glycosides 1–4 and 9–14 have been prepared by aldol condensation of β-C-glucosyl and β-C-galactosyl ketones with aryl aldehydes at room temperature in the presence of pyrrolidine as catalyst (Scheme 1). The O-acetate protecting groups of the carbohydrate moiety were next removed using triethylamine in methanol/water to afford the deprotected C-glycosides 5–8 and 15–20.
Figure 1. Peracetylated C-glycosides (1–4) and fully deprotected derivatives (5–8) containing the phenol moiety.
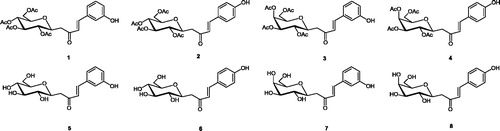
Figure 2. Per-O-acetylated C-glycosides (9–14) and fully deprotected derivatives (15–20) containing the methoxyaryl moiety.
All the glycomimetics prepared have been previously tested against human, bacterial and fungal CAs and showed very good inhibition profilesCitation11–15. The anti-tubercular activity of compounds 1–20 was investigated by using fresh cultures of M. tuberculosis H37Rv grown and tested as previously described. Tests were carried on by duplicate. Our results clearly demonstrated that compounds 1, 2, 6, 7 and 10 displayed growth inhibition activity at concentrations between 50 and 100 μg/mL ().
Table 1. Anti-tubercular activity of C-glycosides 1–20.
Due to their promising anti-tubercular activity, it was advisable to evaluate the antioxidant activity of the compounds we synthesized. We performed DPPH and ABTS radical scavenging assays as they are the most commonly used methods for screening antioxidant activities of the various natural as well as synthetic antioxidants. The results are summarized in and .
Figure 3. Percent inhibition of the DPPH• radical by tested compounds (1 mg). Standard deviation is shown (n = 3).
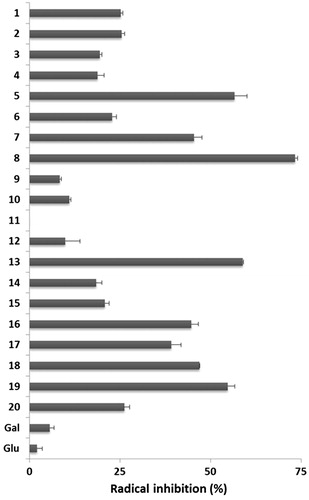
Figure 4. Percent inhibition of the ABTS+• radical by tested compounds (0.1 mg). Standard deviation is shown (n = 3).
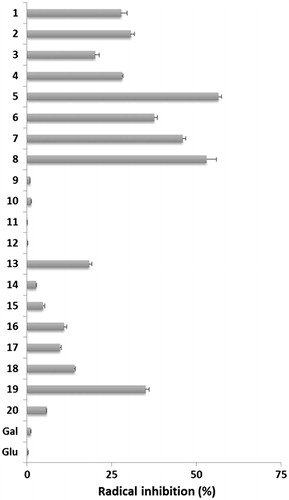
A number of structure–activity relationships (SARs) were identified in this study and are summarized as follows.
The C-glycosides containing the phenol moiety showed good radical scavenging activity. Also the glycomimetics showed better ability compared to the glycosyl ketones used in their preparation, showing that attaching of the phenyl moiety improves its scavenging activity. It should be noted that all deprotected glycomimetics glycosyl exhibited better activity than the per-O-acetylated derivatives. Galactosides 3 and 7, which possess a hydroxyl group at meta position of phenyl ring, showed less antioxidant activity as compared to glucosides 1 and 5, with the same substitution at the aromatic moiety. A closely related situation was found with the glycosides containing a –OH at para position of the phenyl ring, with the exception of compound 8. It is significant to note that the compounds showing anti-tubercular activity also showed a moderate to good radical scavenging activity.
Some C-glycosides incorporating the methoxyaryl moiety showed interesting antioxidant activity. In the 6-methoxynaphtyl series only derivate 17 showed moderate radical scavenging activity. Glicosyl derivatives of veratraldehyde 13, 16 and 19 exhibited good antioxidant ability showing that this moiety could be useful in the design of novel sugar antioxidants. It is interesting to note that galactoside 10, which possesses a very low antioxidant activity, is the only methoxyaryl derivative that showed anti-tubercular activity.
In conclusion, we have investigated the antioxidant and anti-tubercular activities of C-glycosides incorporating the phenol or methoxylaryl moieties. The 3-hydroxyphenyl glycoside (compound 1) exhibited good anti-tubercular and antioxidant activities showing that this moiety therefore represents a lead for the development of novel anti-tubercular agents with dual mechanisms of action.
Declaration of interest
The authors report no declarations of interest. This work was financed in part by the Agencia Nacional de Promoción Científica y Tecnológica (PICT-O GSK0016 and PICT2012-2803), and by UNLP and CONICET (PIP 0701). HRM is a member of Universidad Nacional de Rosario Research Council (CIUNR). A.C., A.B. and P.A.C are members of the Scientific Research Career of CONICET.
References
- Dye C. Doomsday postponed? Preventing and reversing epidemics of drug-resistant tuberculosis. Nat Rev Microbiol 2009;7:81–7
- Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998;393:537–44
- Wilson R, Kumar P, Parashar V, et al. Antituberculosis thiophenes define a requirement for Pks13 in mycolic acid biosynthesis. Nat Chem Biol 2013;9:499–506
- Rivers EC, Mancera RL. New anti-tuberculosis drugs with novel mechanisms of action. Curr Med Chem 2008;15:1956–67
- Nishimori I, Minakuchi T, Maresca A, et al. The β-carbonic anhydrases from Mycobacterium tuberculosis as drug targets. Curr Pharm Des 2010;16:3300–9
- Palanisamy GS, Kirk NM, Ackart DF, et al. Evidence for oxidative stress and defective antioxidant response in guinea pigs with tuberculosis. PLoS One 2011;6:e26254
- Nanashima K, Mawatari T, Tahara N, et al. Genetic variants in antioxidant pathway: risk factors for hepatotoxicity in tuberculosis patients. Tuberculosis 2012;92:253–9
- Colinas PA. Novel glycomimetics: anomeric and N-glycosyl sulfonamides. Curr Org Chem 2012;16:1670–6
- Asensio JL, Cañada FJ, Cheng X, et al. Conformational differences between O- and C-glycosides: the α-O-Man-(1→1)-β-Gal/α-C-Man-(1→1)-β-Gal case-a decisive demonstration of the importance of the exo-anomeric effect on the conformation of glycosides. Chem Eur J 2000;6:1035–41
- Winum JY, Colinas PA, Supuran CT. Glycosidic carbonic anhydrase IX inhibitors: a sweet approach against cancer. Bioorg Med Chem 2013;21:1419–26
- Riafrecha LE, Rodríguez OM, Vullo D, et al. Synthesis of C-cinnamoyl glycosides and their inhibitory activity against mammalian carbonic anhydrases. Bioorg Med Chem 2013;21:1489–94
- Buchieri MV, Riafrecha LE, Rodríguez OM, et al. Inhibition of the β-carbonic anhydrases from Mycobacterium tuberculosis with C-cinnamoyl glycosides: identification of the first inhibitor with anti-mycobacterial activity. Bioorg Med Chem Lett 2013;23:740–3
- Riafrecha LE, Vullo D, Ouahrani-Bettache S, et al. Inhibition of β-carbonic anhydrases from Brucella suis with C-cinnamoyl glycosides incorporating the phenol moiety. J Enzyme Inhib Med Chem 2015;30:1017–20
- Riafrecha LE, Rodríguez OM, Vullo D, et al. Attachment of carbohydrates to methoxyaryl moieties leads to highly selective inhibitors of the cancer associated carbonic anhydrase isoforms IX and XII. Bioorg Med Chem 2014;22:5308–14
- Riafrecha LE, Vullo D, Supuran CT, Colinas PA. C-glycosides incorporating the 6-methoxy-2-naphthyl moiety are selective inhibitors of fungal and bacterial carbonic anhydrases. J Enzyme Inhib Med Chem 2015;30:857–61
- Zaro MJ, Chaves AR, Vicente AR, Concellón A. Distribution, stability and fate of phenolic compounds in white and purple eggplants (Solanum melongena L.). Postharvest Biol Technol 2014;92:70–8